Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas
Abstract
:1. Introduction
2. Results
2.1. Rates of Cells Positive for NY-ESO-1 and MAGE-A4
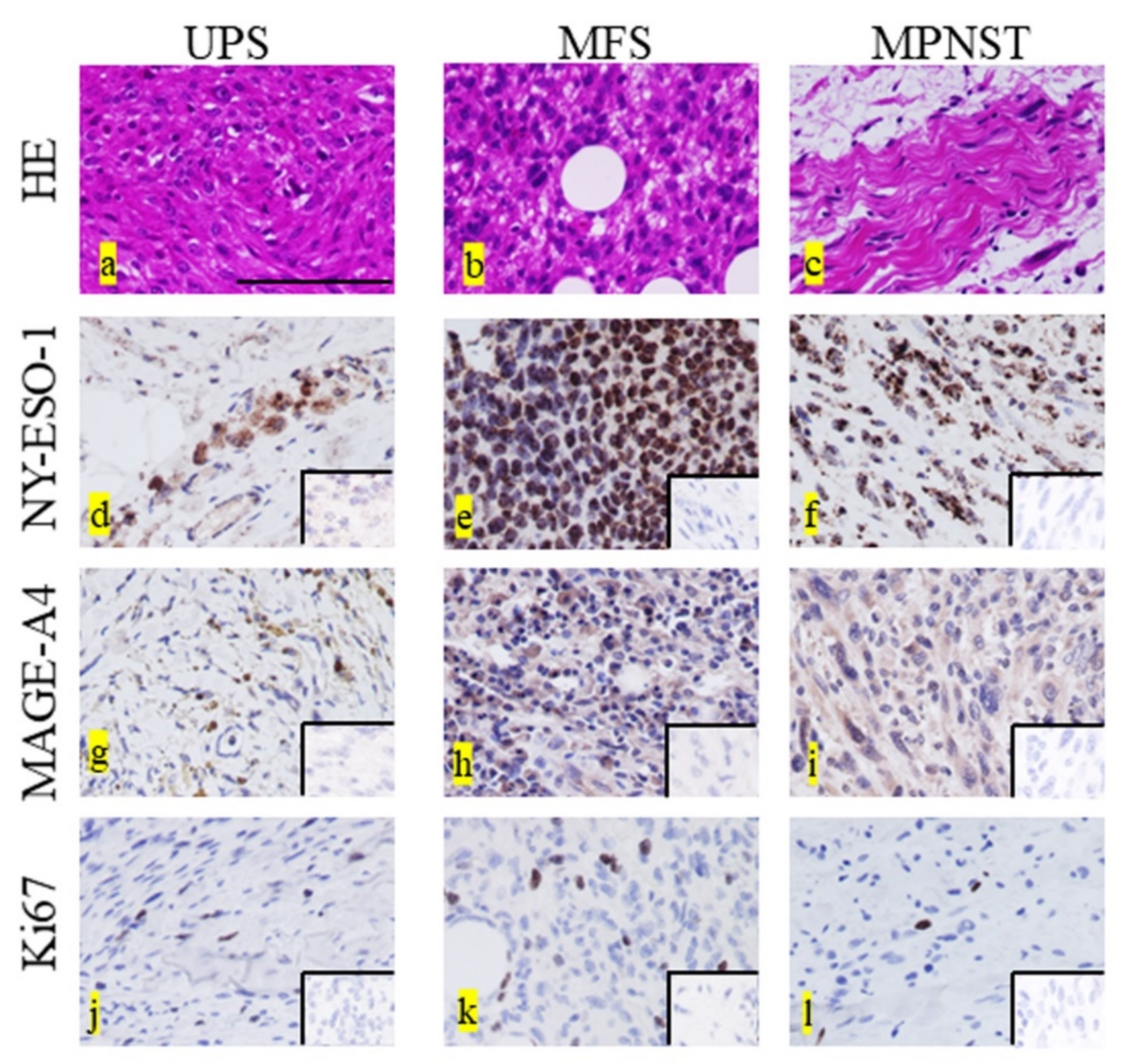
2.2. Immunohistochemical Staining for Each Marker
2.3. Correlation between the Expression Rates of Two CTAs
2.4. Correlation between the Expression of NY-ESO-1 or MAGE-A4 and Age
2.5. Correlation between the Expression of NY-ESO-1 or MAGE-A4 and Tumor Size
2.6. Correlation between the Expression of NY-ESO-1 or MAGE-A4 and Histological Grade
2.7. Correlation between the Expression of NY-ESO-1 or MAGE-A4 and Maximum Standardized Uptake Value
2.8. Expression of NY-ESO-1 or MAGE-A1 in Continuously Disease-Free Cases or Those with No Evidence of Disease in Comparison with Alive with Disease or Died of Other Cause Cases
3. Discussion
Limitations
4. Materials and Methods
4.1. Immunohistochemical Staining
4.2. Immunohistochemical Positive Cell Rate
4.3. Immunohistochemical Positive Case Rate of Each Marker
4.4. Statical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher, C.D.; Lazar, A.J.; Baldini, E.H.; Messiou, C.; Blay, J.Y.; Pollock, R.E.; Gronchi, A.; Singer, S. Soft Tissue and Bone Tumours. In WHO Classification of Tumors, 5th ed.; Antonescu, C.R., Ed.; IARC Publications: Lyon, France, 2020; Volume 5, pp. 6–12. [Google Scholar]
- Penel, N.; Coindre, J.M.; Giraud, A.; Terrier, P.; Ranchere-Vince, D.; Collin, F.; Guellec, S.L.E.; Bazille, C.; Lae, M.; de Pinieux, G.; et al. Presentation and outcome of frequent and rare sarcoma histologic subtypes: A study of 10,262 patients with localized visceral/soft tissue sarcoma managed in reference centers. Cancer 2018, 124, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-tissue sarcomas in adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Mizusawa, J.; Fukuda, H.; Araki, N.; Chuman, H.; Takahashi, M.; Ozaki, T.; Hiruma, T.; Tsuchiya, H.; Morioka, H.; et al. Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcomas in the extremities (JCOG0304). Jpn. J. Clin. Oncol. 2015, 45, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Chugh, R.; Zhao, L.; Lucas, D.R.; Biermann, J.S.; Zalupski, M.M.; Feng, M.; Wong, S.L.; Jacobson, J.; Zyczynski, L.; et al. A randomized open-label phase II study of neo/adjuvant doxorubicin and ifosfamide versus gemcidabine and docetaxel in patients with localized, high risk, soft tissue sarcoma. Eur. J. Cancer 2015, 51, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (Palette): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Stefanovski, P.D.; Bidoli, E.; De Paoli, A.; Buonadonna, A.; Boz, G.; Libra, M.; Morassut, S.; Rossi, C.; Carbone, A.; Frustaci, S. Prognostic factors in soft tissue sarcomas: A study of 395 patients. Eur. J. Surg. Oncol. 2002, 28, 153–164. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Gure, A.O.; Jungbluth, A.A.; Old, L.J.; Chen, Y.T. Cancer/testis antigens: An expanding family of targets for cancer immunotherapy. Immunol. Rev. 2002, 188, 22–32. [Google Scholar] [CrossRef]
- Burgess, M.; Tawbi, H. Immunotherapeutic approaches to sarcoma. Curr. Treat. Options Oncol. 2015, 16, 26. [Google Scholar] [CrossRef]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 based immunotherapy of cancer: Current perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef]
- Weon, J.L.; Potts, P.R. The MAGE protein family and cancer. Curr. Opin. Cell Biol. 2015, 37, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Wada, H.; Yamasaki, M.; Miyata, H.; Nishikawa, H.; Sato, E.; Kageyama, S.; Shiku, H.; Mori, M.; Doki, Y. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine 2014, 32, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; de Graaff, M.A.; Ingram, D.R.; Lim, S.; Lev, D.C.; Briaire-de Bruijn, I.H.; Somaiah, N.; Bovée, J.V.; Lazar, A.J.; Nielsen, T.O. NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod. Pathol. 2015, 28, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Kageyama, S.; Miyahara, Y.; Ishikawa, T.; Ueda, S.; Soga, N.; Naota, H.; Mukai, K.; Harada, N.; Ikeda, H.; et al. MAGE-A4, NY-ESO-1 and SAGE mRNA expression rates and co-expression relationships in solid tumours. BMC Cancer 2020, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, T.; Matsumine, A.; Kageyama, S.; Asanuma, K.; Matsubara, T.; Nakamura, T.; Iino, T.; Ikeda, H.; Shiku, H.; Sudo, A. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol. Lett. 2019, 17, 3937–3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemminger, J.A.; Iwenofu, O.H. NY-ESO-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms. Mod. Pathol. 2013, 26, 1204–1210. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.F.; Brasseur, F.; Hulsbergen-van de Kaa, C.A.; van de Rakt, M.W.; Figdor, C.G.; Adema, G.J.; Hoogerbrugge, P.M.; Coulie, P.G.; de Vries, I.J. Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int. J. Cancer 2007, 120, 67–74. [Google Scholar] [CrossRef]
- Resnick, M.B.; Sabo, E.; Kondratev, S.; Kerner, H.; Spagnoli, G.C.; Yakirevich, E. Cancer-testis antigen expression in uterine malignancies with an emphasis on carcinosarcomas and papillary serous carcinomas. Int. J. Cancer 2002, 101, 190–195. [Google Scholar] [CrossRef]
- Lou, Y.; Wan, W.; Wu, Z.; Yang, J.; Xu, K.; Huang, Q.; Liu, T.; Wei, H.; Yang, X.; Xiao, J. Prognostic factors for patients with undifferentiated high grade pleomorphic sarcoma of the spine. Spine 2019, 44, E539–E548. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Miceli, R.; Grosso, F.; Fiore, M.; Puma, E.; Pennacchioli, E.; Barisella, M.; Sangalli, C.; Mariani, L.; Casali, P.G.; et al. Myxofibrosarcoma: Prognostic factors and survival in a series of patients treated at a single institution. Ann. Surg. Oncol. 2011, 18, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xu, L.; Zhao, Z.; Xu, S.; Zhang, X.; Liu, T.; Zhang, S.; Yu, S. Clinicopathological features and prognosis of malignant peripheral nerve sheath tumor: A retrospective study of 159 cases from 1999 to 2016. Oncotarget 2017, 8, 104785–104795. [Google Scholar] [CrossRef]
- Shurell, E.; Vergara-Lluri, M.E.; Li, Y.; Crompton, J.G.; Singh, A.; Bernthal, N.; Wu, H.; Eilber, F.C.; Dry, S.M. Comprehensive adipocytic and neurogenic tissue microarray analysis of NY-ESO-1 expression—A promising immunotherapy target in malignant peripheral nerve sheath tumor and liposarcoma. Oncotarget 2016, 7, 72860–72867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iura, K.; Kohashi, K.; Ishii, T.; Maekawa, A.; Bekki, H.; Otsuka, H.; Yamada, Y.; Yamamoto, H.; Matsumoto, Y.; Iwamoto, Y.; et al. MAGEA4 expression in bone and soft tissue tumors: Its utility as a target for immunotherapy and diagnostic marker combined with NY-ESO-1. Virchows Arch. 2017, 471, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Robbins, P.F.; Raffeld, M.; Aung, P.P.; Tsokos, M.; Rosenberg, S.A.; Miettinen, M.M.; Lee, C.C. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: Significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod. Pathol. 2012, 25, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Shukla, N.K.; Deo, S.V.S.; Agarwala, S.; Sharma, D.N.; Sharma, M.C.; Bakhshi, S. Prognostic factors affecting survival in metastatic soft tissue sarcoma: An analysis of 110 patients. Clin. Transl. Oncol. 2016, 18, 310–316. [Google Scholar] [CrossRef]
- Coindre, J.M.; Terrier, P.; Bui, N.B.; Bonichon, F.; Collin, F.; Le Doussal, V.; Mandard, A.M.; Vilain, M.O.; Jacquemier, J.; Duplay, H.; et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J. Clin. Oncol. 1996, 14, 869–877. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, S.E.; Choi, Y.L.; Seo, S.W.; Sung, K.S.; Koo, H.H.; Choi, J.Y. Prognostic value of 18F-FDG PET/CT in patients with soft tissue sarcoma: Comparisons between metabolic parameters. Skelet. Radiol. 2014, 43, 641–648. [Google Scholar] [CrossRef]
- Lahat, G.; Dhuka, A.R.; Lahat, S.; Lazar, A.J.; Lewis, V.O.; Lin, P.P.; Feig, B.; Cormier, J.N.; Hunt, K.K.; Pisters, P.W.; et al. Complete soft tissue sarcoma resection is a viable treatment option for select elderly patients. Ann. Surg. Oncol. 2009, 16, 2579–2586. [Google Scholar] [CrossRef]
- Chen, S.; Huang, W.; Luo, P.; Cai, W.; Yang, L.; Sun, Z.; Zheng, B.; Yan, W.; Wang, C. Undifferentiated pleomorphic sarcoma: Long-term follow-up from a large institution. Cancer Manag. Res. 2019, 11, 10001–10009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodyla, M.; Demircioglu, A.; Schaarschmidt, B.M.; Bertram, S.; Morawitz, J.; Bauer, S.; Podleska, L.; Rischpler, C.; Forsting, M.; Herrmann, K.; et al. Evaluation of the predictive potential of 18F-FDG PET and DWI data sets for relevant prognostic parameters of primary soft-tissue sarcomas. Cancers 2021, 13, 2753. [Google Scholar] [CrossRef] [PubMed]
- Dewan, V.; Darbyshire, A.; Sumathi, V.; Jeys, L.; Grimer, R. Prognostic and survival factors in myxofibrosarcomas. Sarcoma 2012, 2012, 830879. [Google Scholar] [CrossRef] [PubMed]
- Fuglø, H.M.; Jørgensen, S.M.; Loft, A.; Hovgaard, D.; Petersen, M.M. The diagnostic and prognostic value of ¹⁸F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.; Hirose, T.; Scheithauer, B.W.; Schild, S.E.; Gunderson, L.L. Malignant peripheral nerve sheath tumor: Analysis of treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 351–360. [Google Scholar] [CrossRef]
- Martin, E.; Coert, J.H.; Flucke, U.E.; Slooff, W.M.; Ho, V.K.Y.; van der Graaf, W.T.; van Dalen, T.; van de Sande, M.A.J.; van Houdt, W.J.; Grünhagen, D.J.; et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur. J. Cancer 2020, 124, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.; Shamim, S.A.; Rastogi, S.; Upadhyay, D.M.R.; Pandey, A.K.; Kumar, R. Role of 18F-FDG PET/computed tomography in prognostication and management of malignant peripheral nerve sheath tumors. Nucl. Med. Commun. 2020, 41, 924–932. [Google Scholar] [CrossRef]
- Schietinger, A.; Greenberg, P.D. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2014, 35, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Ito, T.; Akagi, M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur. J. Histochem. 2021, 65, 3203. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef] [Green Version]



| Positive Cell Rate (%) | |
|---|---|
| NY-ESO-1 | |
| 0–10 | 12 |
| 10–50 | 7 |
| ≥50 | 3 |
| MAGE-A4 | |
| 0–10 | 8 |
| 10–50 | 4 |
| ≥50 | 10 |
| Ki67 | |
| 0–10 | 5 |
| 10–50 | 17 |
| ≥50 | 0 |
| Molecules | NY-ESO-1 | MAGE-A4 | ||
|---|---|---|---|---|
| Outcome | CDF or NED | AWD or DOD | CDF or NED | AWD or DOD |
| Average (%) | 23.3 | 0.69 | 34.7 | 55.7 |
| S.D. | 31.5 | 1.35 | 34.1 | 43.0 |
| p-value | 0.13 | 0.26 |
| Factor | Patients, n |
|---|---|
| Age (years) | |
| >70 | 9 |
| ≤70 | 13 |
| Sex | |
| Male | 13 |
| Female | 9 |
| Tumor site | |
| Arms | 4 |
| Legs | 9 |
| Trunk | 9 |
| Histological type | |
| MFS | 9 |
| UPS | 10 |
| MPNST | 3 |
| Histological grade | |
| Grade 1 | 2 |
| Grade 2 | 8 |
| Grade 3 | 12 |
| Tumor size (cm) | |
| <5 | 8 |
| 5–10 | 11 |
| >10 | 3 |
| SUV-max | |
| <5 | 4 |
| 5–10 | 4 |
| >10 | 5 |
| Treatment | |
| Wide resection, Flap | 4 |
| Wide resection, Post operative radition | 3 |
| Wide resection | 10 |
| Wide resection, skin graft | 2 |
| Marginal resection | 2 |
| Radiation | 1 |
| Recurrence | |
| (+) | 8 |
| (−) | 14 |
| Metastasis | |
| (+) | 5 |
| (−) | 17 |
| Outcome | |
| CDF | 11 |
| NED | 6 |
| AWD | 3 |
| DOD | 2 |
| Follow-up period (years) | |
| <3 | 8 |
| ≥3 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, K.; Nishimura, S.; Ito, T.; Akagi, M. Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas. Diagnostics 2022, 12, 733. https://doi.org/10.3390/diagnostics12030733
Hashimoto K, Nishimura S, Ito T, Akagi M. Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas. Diagnostics. 2022; 12(3):733. https://doi.org/10.3390/diagnostics12030733
Chicago/Turabian StyleHashimoto, Kazuhiko, Shunji Nishimura, Tomohiko Ito, and Masao Akagi. 2022. "Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas" Diagnostics 12, no. 3: 733. https://doi.org/10.3390/diagnostics12030733






