Successful Full-Term Delivery via Selective Ectopic Embryo Reduction Accompanied by Uterine Cerclage in a Heterotopic Cesarean Scar Pregnancy: A Case Report and Literature Review
Abstract
:1. Introduction
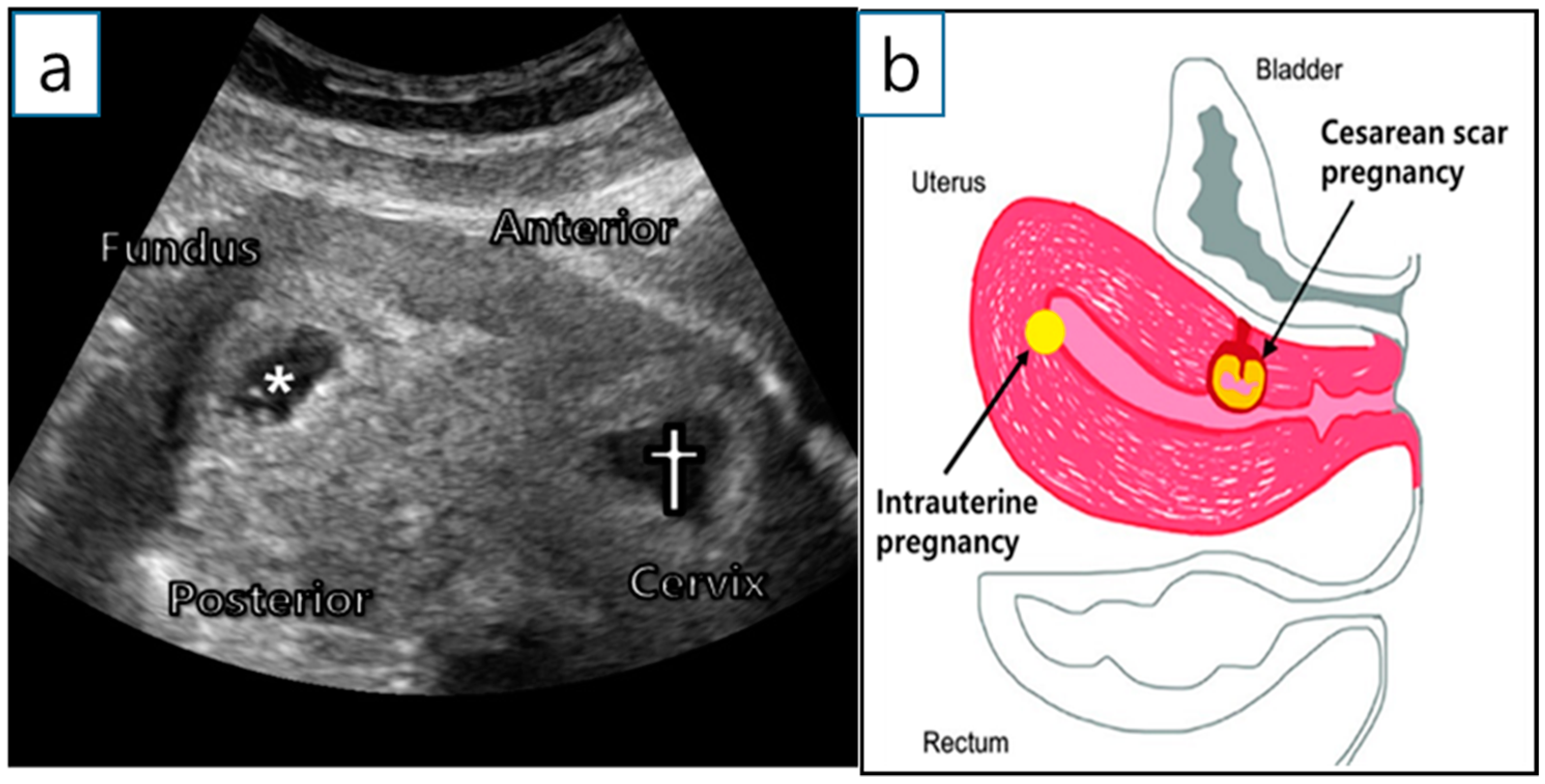
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.N.; Chen, C.K.; Wang, H.S.; Chiueh, H.Y.; Soong, Y.K. Successful management of heterotopic cesarean scar pregnancy combined with intrauterine pregnancy after in vitro fertilization-embryo transfer. Fertil. Steril. 2007, 88, 706.e13–706.e16. [Google Scholar] [CrossRef]
- Yu, H.; Luo, H.; Zhao, F.; Liu, X.; Wang, X. Successful selective reduction of a heterotopic cesarean scar pregnancy in the second trimester: A case report and review of the literature. BMC Pregnancy Childbirth 2016, 16, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugurlucan, F.G.; Bastu, E.; Dogan, M.; Kalelioglu, I.; Alanya, S.; Has, R. Management of cesarean heterotopic pregnancy with transvaginal ultrasound-guided potassium chloride injection and gestational sac aspiration, and review of the literature. J. Minim. Invasive Gynecol. 2012, 19, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Szychowski, J.M.; Owen, J.; Hankins, G.; Iams, J.D.; Sheffield, J.S.; Perez-Delboy, A.; Wing, D.A.; Guzman, E.R.; Consortium, V.U.T. Suture type and ultrasound-indicated cerclage efficacy. J. Matern. Fetal Neonatal Med. 2012, 25, 2287–2290. [Google Scholar] [CrossRef]
- Bernard, L.; Pereira, L.; Berghella, V.; Rust, O.; Mittal, S.; Daly, S.; Vaarasmaki, M.; Cotter, A.; Gomez, R.; Prasertcharoensuk, W.; et al. Effect of suture material on outcome of cerclage in women with a dilated cervix in the 2nd trimester: Results from the expectant management compared to physical exam-indicated cerclage (EM-PEC) international cohort study. Am. J. Obstet. Gynecol. 2006, 195, S103. [Google Scholar] [CrossRef]
- Kim, M.L.; Jun, H.S.; Kim, J.Y.; Seong, S.J.; Cha, D.H. Successful full-term twin deliveries in heterotopic cesarean scar pregnancy in a spontaneous cycle with expectant management. J. Obstet. Gynaecol. Res. 2014, 40, 1415–1419. [Google Scholar] [CrossRef]
- Lincenberg, K.R.; Behrman, E.R.; Bembry, J.S.; Kovac, C.M. Uterine Rupture with Cesarean Scar Heterotopic Pregnancy with Survival of the Intrauterine Twin. Case Rep. Obstet. Gynecol. 2016, 2016, 6832094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Cha, H.H.; Han, A.R.; Lee, S.G.; Seong, W.J. Heterotopic pregnancy after a single embryo transfer. Obstet. Gynecol. Sci. 2016, 59, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.D.; Weng, Y.; Wang, C.F.; Huang, L.L.; Zhu, X.M. Research on the expression of integrin β3 and leukaemia inhibitory factor in the decidua of women with cesarean scar pregnancy. BMC Pregnancy Childbirth 2017, 17, 84. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, B.C.; Hwang, J.L.; Pan, H.S.; Huang, S.C.; Chen, C.Y.; Chen, P.H. Heterotopic Caesarean scar pregnancy combined with intrauterine pregnancy successfully treated with embryo aspiration for selective embryo reduction: Case report. Hum. Reprod. 2004, 19, 285–287. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Sehgal, A.; Huria, A.; Mehra, R. Secondary abdominal pregnancy and its associated diagnostic and operative dilemma: Three case reports. J. Med. Case Rep. 2009, 3, 7382. [Google Scholar] [CrossRef] [Green Version]
- Demirel, L.C.; Bodur, H.; Selam, B.; Lembet, A.; Ergin, T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: Case report. Fertil. Steril. 2009, 91, 1293.e5–1293.e7. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Fernandez, H.; Chauveaud, A.; Doumerc, S.; Frydman, R. Successful management of a heterotopic Caesarean scar pregnancy: Potassium chloride injection with preservation of the intrauterine gestation: Case report. Hum. Reprod. 2003, 18, 189–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazicioglu, H.F.; Turgut, S.; Madazli, R.; Aygün, M.; Cebi, Z.; Sönmez, S. An unusual case of heterotopic twin pregnancy managed successfully with selective feticide. Ultrasound Obstet. Gynecol. 2004, 23, 626–627. [Google Scholar] [CrossRef]
- Taşkin, S.; Taşkin, E.A.; Ciftçi, T.T. Heterotopic cesarean scar pregnancy: How should it be managed? Obstet. Gynecol. Surv. 2009, 64, 690–695; quiz 697. [Google Scholar] [CrossRef]
- Wang, C.J.; Tsai, F.; Chen, C.; Chao, A. Hysteroscopic management of heterotopic cesarean scar pregnancy. Fertil. Steril. 2010, 94, 1529.e15–1529.e18. [Google Scholar] [CrossRef]
- Gupta, R.; Vaikousi, E.; Whitlow, B. Heterotopic caesarean section scar pregnancy. J. Obstet. Gynaecol. 2010, 30, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Litwicka, K.; Greco, E.; Prefumo, F.; Fratelli, N.; Scarselli, F.; Ferrero, S.; Iammarrone, E.; Frusca, T. Successful management of a triplet heterotopic caesarean scar pregnancy after in vitro fertilization-embryo transfer. Fertil. Steril. 2011, 95, 291.e1–291.e3. [Google Scholar] [CrossRef]
- Dueñas-Garcia, O.F.; Young, C. Heterotopic cesarean scar pregnancy associated with a levonorgestrel-releasing intrauterine device. Int. J. Gynaecol. Obstet. 2011, 114, 153–154. [Google Scholar] [CrossRef]
- Bai, X.X.; Gao, H.J.; Yang, X.F.; Dong, M.Y.; Zhu, Y.M. Expectant management of heterotopic cesarean scar pregnancy. Chin. Med. J. 2012, 125, 1341–1344. [Google Scholar]
- Uysal, F.; Uysal, A. Spontaneous heterotopic cesarean scar pregnancy: Conservative management by transvaginal sonographic guidance and successful pregnancy outcome. J. Ultrasound Med. 2013, 32, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.W.; Shek, N.W.; Li, R.H.; Chu, F.; Pun, T.C. Management of heterotopic cesarean scar pregnancy by repeated transvaginal ultrasonographic-guided aspiration with successful preservation of normal intrauterine pregnancy and complicated by arteriovenous malformation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, R.; Krätschell, R.; Henrich, W.; David, M. Operative Therapy for Heterotopic Scar Pregnancy and Successful Birth of the Intrauterine Foetus—Case Report and Review of the Literature. Geburtshilfe Frauenheilkd 2015, 75, 384–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czuczwar, P.; Stępniak, A.; Woźniak, A.; Woźniak, S.; Paszkowski, T. Successful treatment of spontaneous heterotopic caesarean scar pregnancy by local potassium chloride injection with preservation of the intrauterine pregnancy. Ginekol. Pol. 2016, 87, 727. [Google Scholar] [CrossRef] [Green Version]
- Miyague, A.H.; Chrisostomo, A.P.; Costa, S.L.; Nakatani, E.T.; Kondo, W.; Gomes, C.C. Treatment of heterotopic caesarean scar pregnancy complicated with post termination increase in size of residual mass and morbidly adherent placenta. J. Clin. Ultrasound 2018, 46, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.H.; Andrzejewski, J.; Murnane, A.; Lang, C. Surgical Management of a Heterotopic Cesarean Scar Pregnancy with Preservation of an Intrauterine Pregnancy. Obstet. Gynecol. 2016, 128, 613–616. [Google Scholar] [CrossRef]
- Vikhareva, O.; Nedopekina, E.; Herbst, A. Normal vaginal delivery at term after expectant management of heterotopic caesarean scar pregnancy: A case report. J. Med. Case Rep. 2018, 12, 179. [Google Scholar] [CrossRef]
- Tymon-Rosario, J.; Chuang, M. Selective Reduction of a Heterotopic Cesarean Scar Pregnancy Complicated by Septic Abortion. Case Rep. Obstet. Gynecol. 2018, 2018, 6478589. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Y.; Zhou, Y.; Qian, Y.; Luo, J.M.; Huang, X.F.; Zhang, X.M. Management of heterotopic cesarean scar pregnancy with preservation of intrauterine pregnancy: A case report. World J. Clin. Cases 2021, 9, 6428–6434. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, H.; Lin, G.; Xiang, S.; Qin, J.; Gong, F.; Li, X. Heterotopic Cesarean Scar Pregnancy: An Analysis of 20 Cases Following in vitro Fertilization-Embryo Transfer. J. Ultrasound Med. 2021, 40, 2239–2249. [Google Scholar] [CrossRef]
- Authreya, A.J.; Agrawal, P.; Makam, A. Ultrasound-guided procedures in the management of heterotopic caesarean scar pregnancy—A review of case reports and case series. Australas. J. Ultrasound Med. 2021, 24, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Laing-Aiken, Z.; Robson, D.; Wu, J. Surgical management of first-trimester bleeding in a heterotopic caesarean scar pregnancy: A case report and review of literature. Case Rep. Womens Health 2020, 27, e00209. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Z.; Yin, Q.; Xu, Y.; Ma, Y.; Zhang, Q.; Yu, Y. Heterotopic cesarean scar pregnancy: Diagnosis, treatment, and prognosis. J. Ultrasound Med. 2014, 33, 1533–1537. [Google Scholar] [CrossRef] [PubMed]



| Reference | Conception/Previous CS (n) | Diagnosis Modality/GW/Symptoms or Event | Cardiac Activity of CSP/IUP | Management/GW | RGT | Antenatal Event | Pregnancy Outcome |
|---|---|---|---|---|---|---|---|
| Salomon [13], 2003 | ART/1 | TVUS/8/None | Yes/Yes | US-guided intervention (KCL injection)/NM | Persistent | PROM | CS at 36 GW, live female, 2800 g, RGT excision during CS |
| Yazicioglu [14], 2004 | Spontaneous/1 | TVUS/6+2/VB | Yes/Yes | US-guided intervention (KCL injection)/7+2 | Spontaneously disappeared | PROM | CS at 30 GW, live male, 1530 g, RGT detachment without complications |
| Hsieh [10], 2004 | ART (twin IUPs + CSP)/2 | TVUS/6/VB | Yes/Yes | US-guided intervention (EA)/NM | Spontaneously disappeared | Preterm labor | CS at 32 GW |
| Wang [1], 2007 | ART/3 | TVUS/7/None | Yes/Yes | US-guided intervention (KCL injection)/NM | Persistent | Preterm labor | CS at 35 GW, live male, 1820 g, bilateral internal iliac artery ligation due uterine bleeding, RGT excision during CS |
| Demirel [12], 2009 | N/M/1 | TVUS/6+5/VB | Yes/Yes | Surgical intervention (laparoscopy)/NM | Removed | None | CS at 38 GW, live singleton |
| Taşkin [15], 2009 | N/M/1 | TVUS/8+4/VB | Yes/Yes | US-guided intervention (KCL injection)/9 | Persistent | Preterm labor | CS at 34 GW, live female, 2310 g, RGT excision during CS |
| Wang [16], 2010 | ART/1 | TVUS/7/VB | Yes/Yes | Surgical intervention (hysteroscopy)/7 | Removed | None | CS at 39 GW, live male, 3250g |
| Gupta [17], 2010 | ART/4 | TVUS/6+1/None | Yes/Yes | US-guided intervention (EA)/6+3 | Persistent | None | Termination at 12 GW due to trisomy 13 |
| Litwicka [18], 2011 | ART/1 | TVUS/6/None | Yes/Yes | US-guided intervention (KCl + MTX injection)/8 | Persistent | Placental abruption | CS at 36 GW, 1990 g male, Miller syndrome |
| Dueñas-Garcia and Young [19], 2011 | Spontaneous/3 | TVUS, MRI/5/None | Yes/Yes | MTX + leucovorin (used for abortion)/NM | NM | ||
| Ugurlucan [3], 2012 | ART/1 | TVUS/6/VB | Yes/Yes | US-guided intervention (KCl injection + EA)/NM | None | None | CS at 38 GW, live singleton, subtotal hysterectomy due to postpartum bleeding |
| Bai [20], 2012 | ART/1 | TVUS/7+6/VB | Yes/Yes | Expectant | Persistent | CSP miscarriageat 8+4 GW, VB and protruding RGT | CS at 36+4 GW due to preterm labor, live male, 2950 g |
| Uysal [21], 2013 | Spontaneous/2 | TVUS/8/None | Yes/Yes | US-guided intervention (KCL injection)/NM | Persistent | Preterm labor | CS at 35 GW, live female, 2480 g, incomplete uterine rupture, RGT excision during CS |
| Lui [22], 2014 | ART/1 | TVUS/5/VB | Yes/Yes | US-guided intervention (repeated EA)/NM | Persistent | None | CS at 37 GW, live female, 2660 g, RGT with AVM, selective UAE |
| Kim [6], 2014 | Spontaneous/2 | TVUS/5+5/None | Yes/Yes | Expectant | Persistent | None | CS at 37+2 GW, live twins, bladder adhesion, placenta accreta, bilateral uterine artery ligation |
| Armbrust [23], 2015 | ART/2 | TVUS/7/None | Yes/Yes | Surgical intervention (laparotomy)/NM | None | None | CS at 37+2 GW, live singleton, 2895 g |
| Yu [2], 2016 | ART/1 | TVUS/11/None | Yes/Yes | US-guided intervention (KCl)/16+4 | Persistent | PPT, accreta | CS at 37+6 GW, live male, 2890 g, profuse vascularization with bladder adhesion, RGT excision during CS |
| Czuczwar [24], 2016 | NM/1 | TVUS/6/None | Yes/Yes | US-guided intervention (KCl injection)/7 | None | None | CS at 37 GW, live male, 3060 g |
| Lincenberg [7], 2016 | NM/3 | TVUS/10+2/AP, intraperitoneal hemorrhage | Yes/Yes | Surgical intervention (laparoscopy, laparotomy)/10+2 | Persistent | Uterine rupture | CS at 23+1 GW, live female, 423 g |
| Vetter [26], 2016 | Spontaneous/1 | TVUS/5/VB | Yes/Yes (too early) | Surgical intervention (laparotomy)/NM | None | None | CS at 37+1 GW, live female, 3479 g |
| Miyague [25], 2018 | NM/1 | TVUS, MRI/6/None | Yes/Yes | US-guided intervention (combined KCL injection + EA)/NM | Growth with vascularity | RGT growth and AVM and MAP formation | Hysterectomy |
| Vikhareva [27], 2018 | NM/1 | TVUS/13/None | None/Yes | Expectant | Disappeared at 18 GW | None | VD at 39 GW, live male, 2985 g |
| Tymon-Rosario [28], 2018 | NM/2 | TVUS/NM/None | Yes/Yes | US-guided intervention (KCL injection)/10+6 | N/M | Septic shock | Hysterectomy after UAE, D&C |
| Ashwini J Authreya [31], 2021 | ART/1 | TVUS/7+6/None | Yes/Yes | US-guided intervention (KCL injection)/NM | None | None | CS at 38 GW, a term healthy baby |
| Zheng-Yun Chen [29], 2021 | Spontaneous/1 | TVUS/8/None | Yes/Yes | Hyperosmolar glucose injection and EA/8+2, transcervical D&C | Disappeared at 20 GW | Vaginal bleeding | CS at 34+2 GW, a healthy baby PROM |
| Ouyang [30], 2021 | ART/1 | TVUS/6/None | Yes/Yes | Abortion (D&C) | D&C + UAE | ||
| Ouyang [30], 2021 | ART/1 | TVUS/6/VB | Yes/Yes | US-guided intervention (KCL injection)/NM | Persistent | Vaginal bleeding | IUP miscarriage at 14 GW |
| Ouyang [30], 2021 | ART/1 | TVUS/6+2/VB | Yes/Yes | Expectant/8 | Persistent | Hysteroscopic excision of the CSP due to placenta accreta at 8 GW | |
| Ouyang [30], 2021 | ART/1 | TVUS/6/None | Yes/None | HIFU/7 | Persistent | Hysteroscopic removal of RGT | Miscarriage of IUP at 7 GW |
| Ouyang [30], 2021 | ART/1 | TVUS/5+5/VB | Yes/Yes | D&C/13 | NM | IUP and CSP miscarriage at 13 GW | |
| Ouyang [30], 2021 | ART/1 | TVUS/6/VB | Yes/Yes | Expectant | Disappeared at 20 GW | CSP miscarriage at 13 GW | CS at 29 GW, live female, 1300 g |
| Ouyang [30], 2021 | ART/1 | TVUS/6/VB | Yes/Yes | Expectant | NM | None | CS at 40 GW, live two females, 2900 g and 2200 g |
| Ouyang [30], 2021 | ART/1 | TVUS/5+5/VB | Yes/Yes | Expectant | NM | IUP miscarriage at 20 GW | CS at 36 GW, live female (CSP), 3000 g |
| Ouyang [30], 2021 | ART/1 | TVUS/6/VB | Yes/Yes | Expectant | NM | IIOC | Induced abortion at 22 GW |
| Ouyang [30], 2021 | ART/2 | TVUS/6/None | Yes/Yes | Expectant | Persistent | CSP miscarriage at 10 GW | CS at 37 GW, live male, 2600 g |
| Ouyang [30], 2021 | ART/1 | TVUS/6/None | None/Yes | Expectant | Disappeared at 22 GW | PROM | CS at 36 GW, live female, 2900 g |
| Ouyang [30], 2021 | ART/1 | TVUS/6+5/VB,AP | None/Yes | Expectant | Persistent | None | CS at 39 GW, live female 3900 g |
| Ouyang [30], 2021 | ART/1 | TVUS/6/VB | None/Yes | Expectant | Persistent | Placental abruption | CS at 24 GW |
| Ouyang [30], 2021 | ART/4 | TVUS/8+5/VB | None/Yes | Expectant | Disappeared at 16 GW | None | CS at 39 GW, live singleton, 2900 g |
| Ouyang [30], 2021 | ART/1 | TVUS/6+2/VB | None/Yes | Expectant | Persistent | Complete placenta previa | Emergency CS at 35 GW, live male, 2600 g |
| Ouyang [30], 2021 | ART/1 | TVUS/7+1/AP | None/Yes | Expectant | Persistent | PPROM | Induced abortion at 24 GW) |
| Ouyang [30], 2021 | ART/1 | TVUS/6/None | None/Yes | Expectant | Disappeared at 24 GW | None | CS at 39 GW, live male, 3150 g |
| Ouyang [30], 2021 | ART/1 | TVUS/5+1/VB | None/Yes | Abortion (D&C and UAE at 7 GW) | Removed | ||
| Ouyang [30], 2021 | ART/1 | TVUS/4+6/AP | Yes/Yes | Expectant | NM | IUP miscarriage at 13 GW | Hysteroscopic removal of IUP |
| Ouyang [30], 2021 | ART/1 | TVUS/11/None | None/Yes | Expectant | None | Uterine rupture at 12 GW | Laparotomy repair |
| Laing-Aiken [32], 2020 | Spontaneous/1 | TVUS/9/VB | Yes/Yes | Surgical intervention (D&C, laparotomy)/9 | Removed | PPROM | CS at 28+1 GW, live male, 1200 g, bilateral uterine artery ligation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Koh, J.H.; Lee, J.; Sim, Y.; Lee, S.-H.; Lee, S.-J.; Ahn, J.-W.; Roh, H.-J.; Kim, J.S. Successful Full-Term Delivery via Selective Ectopic Embryo Reduction Accompanied by Uterine Cerclage in a Heterotopic Cesarean Scar Pregnancy: A Case Report and Literature Review. Diagnostics 2022, 12, 762. https://doi.org/10.3390/diagnostics12030762
Kim H, Koh JH, Lee J, Sim Y, Lee S-H, Lee S-J, Ahn J-W, Roh H-J, Kim JS. Successful Full-Term Delivery via Selective Ectopic Embryo Reduction Accompanied by Uterine Cerclage in a Heterotopic Cesarean Scar Pregnancy: A Case Report and Literature Review. Diagnostics. 2022; 12(3):762. https://doi.org/10.3390/diagnostics12030762
Chicago/Turabian StyleKim, Hyoeun, Ji Hye Koh, Jihee Lee, Yeongeun Sim, Sang-Hun Lee, Soo-Jeong Lee, Jun-Woo Ahn, Hyun-Jin Roh, and Jeong Sook Kim. 2022. "Successful Full-Term Delivery via Selective Ectopic Embryo Reduction Accompanied by Uterine Cerclage in a Heterotopic Cesarean Scar Pregnancy: A Case Report and Literature Review" Diagnostics 12, no. 3: 762. https://doi.org/10.3390/diagnostics12030762







