[18F]FDG PET/CT in Short-Term Complications of COVID-19: Metabolic Markers of Persistent Inflammation and Impaired Respiratory Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Information and Laboratory Test Results
2.3. Respiratory Function Tests
2.4. PET/CT Data Acquisition
2.5. PET/CT Image Interpretation
2.6. Chest CT and X-ray Image Interpretation
2.7. Statistical Analysis
3. Results
3.1. The [18F]FDG-PET/CT Findings
3.2. Correlation of Volumetric [18F]FDG-PET/CT Parameters with Laboratory Test Results
3.3. Correlation of Volumetric [18F]FDG-PET/CT Parameters with Respiratory Function Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, M.; Chironi, G.; Claessens, Y.E.; Farhad, R.L.; Rouquette, I.; Serrano, B.; Nataf, V.; Hugonnet, F.; Paulmier, B.; Berthier, F.; et al. COVID-19 pneumonia: Relationship between inflammation assessed by whole-body FDG PET/CT and short-term clinical outcome. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Liu, F.; Yen, T.C.; Lan, X. 18F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1281–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capitanio, S.; Nordin, A.J.; Noraini, A.R.; Rossetti, C. PET/CT in nononcological lung diseases: Current applications and future perspectives. Eur. Respir. Rev. 2016, 25, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lütje, S.; Marinova, M.; Kütting, D.; Attenberger, U.; Essler, M.; Bundschuh, R.A. Nuclear medicine in SARS-CoV-2 pandemia: 18F-FDG-PET/CT to visualize COVID-19. Nuklearmedizin 2020, 59, 276–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, S.; Delgado Bolton, R.C.; Kamani, C.H.; Prior, J.O.; Albano, D.; Bertagna, F.; Treglia, G. Role of 2-[18F]FDG as a Radiopharmaceutical for PET/CT in Patients with COVID-19: A Systematic Review. Pharmaceuticals 2020, 13, 377. [Google Scholar] [CrossRef]
- Jin, C.; Luo, X.; Qian, S.; Zhang, K.; Gao, Y.; Zhou, R.; Cen, P.; Xu, Z.; Zhang, H.; Tian, M. Positron emission tomography in the COVID-19 pandemic era. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1–15. [Google Scholar] [CrossRef]
- Mucientes, J.; Calles, L.; Rodríguez, B.; Mitjavila, M. Parameters of metabolic quantification in clinical practice. Is it now time to include them in reports? Rev. Española De Med. Nucl. E Imagen Mol. 2018, 37, 264–270. [Google Scholar] [CrossRef]
- Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected [Internet]. Available online: https://www.who.int/publications/i/item/10665-332299 (accessed on 2 February 2022).
- Paez, D.; Gnanasegaran, G.; Fanti, S.; Bomanji, J.; Hacker, M.; Sathekge, M.; Bom, H.S.; Cerci, J.J.; Chiti, A.; Herrmann, K.; et al. COVID-19 pandemic: Guidance for nuclear medicine departments. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1615–1619. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016, Erratum in Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Río, F.; Calle, M.; Burgos, F.; Casan, P.; Del Campo, F.; Galdiz, J.B.; Giner, J.; Gonz ález-Mangado, N.; Ortega, F.; Puente Maestu, L. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef]
- García Garzón, J.R.; Rodríguez, A.; Cabrera, A. Tomografía por emisión de positrones de cuerpo completo (PET/TAC) con 18F-fluorodesoxiglucosa. Grupo de Trabajo PET, Comité de Procedimientos de la SEMN [Positron emission tomography/computed tomography with 18F-FDG. PET Working Group. Procedures Committee of the Spanish Society of Nuclear Medicine]. Rev. Española De Med. Nucl. 2009, 28, 85–89. (In Spanish) [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Abedi, A.; Balakrishnan, S.; Gholamrezanezhad, A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am. J. Roentgenol. 2020, 215, 87–93. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Camp, J.V.; Wu, A.; Zheng, H.; Kraenzle, J.L.; Biller, A.E.; Vanover, C.D.; Chu, Y.K.; Ng, C.K.; Proctor, M.; et al. Molecular imaging reveals a progressive pulmonary inflammation in lower airways in ferrets infected with 2009 H1N1 pandemic influenza virus. PLoS ONE 2012, 7, e40094. [Google Scholar] [CrossRef] [Green Version]
- Chefer, S.; Thomasson, D.; Seidel, J.; Reba, R.C.; Bohannon, J.K.; Lackemeyer, M.G.; Bartos, C.; Sayre, P.J.; Bollinger, L.; Hensley, L.E.; et al. Modeling [(18)F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res. 2015, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Colandrea, M.; Gilardi, L.; Travaini, L.L.; Fracassi, S.L.V.; Funicelli, L.; Grana, C.M. 18F-FDG PET/CT in asymptomatic patients with COVID-19: The submerged iceberg surfaces. Jpn. J. Radiol. 2020, 38, 1007–1011. [Google Scholar] [CrossRef]
- Johnson, L.N.; Vesselle, H. COVID-19 in an asymptomatic patient undergoing FDG PET/CT. Radiol. Case Rep. 2020, 15, 1809–1812. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, J.; Chen, L.; Fu, C.; Kang, Y.; Zhang, W.; Fakhri, G.E.; Gu, J.; Shao, F.; Wang, M. Inflammatory response in lungs and extrapulmonary sites detected by [18F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2531–2542. [Google Scholar] [CrossRef]
- Scarlattei, M.; Baldari, G.; Silva, M.; Bola, S.; Sammartano, A.; Migliari, S.; Graziani, T.; Cidda, C.; Sverzellati, N.; Ruffini, L. Unknown SARS-CoV-2 pneumonia detected by PET/CT in patients with cancer. Tumori J. 2020, 106, 325–332. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Feng, X.; Li, S.; Sun, Q.; Zhu, J.; Chen, B.; Xiong, M.; Cao, G. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 301. [Google Scholar] [CrossRef]
- Sayah, W.; Berkane, I.; Guermache, I.; Sabri, M.; Lakhal, F.Z.; Rahali, S.Y.; Djidjeli, A.; Merah, F.; Belaid, B.; Berkani, L.; et al. Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19. Cytokine 2021, 141, 155428. [Google Scholar] [CrossRef]
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Ko, F.W.; Chan, M.C.; Chan, D.P.; Tong, M.W.; et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Park, W.B.; Jun, K.I.; Kim, G.; Choi, J.P.; Rhee, J.Y.; Cheon, S.; Lee, C.H.; Park, J.S.; Kim, Y.; Joh, J.S.; et al. Correlation between Pneumonia Severity and Pulmonary Complications in Middle East Respiratory Syndrome. J. Korean Med. Sci. 2018, 33, e169. [Google Scholar] [CrossRef]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef]
- You, J.; Zhang, L.; Ni-Jia-Ti, M.Y.; Zhang, J.; Hu, F.; Chen, L.; Dong, Y.; Yang, K.; Zhang, B.; Zhang, S. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J. Infect. 2020, 81, e150–e152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef] [PubMed]
- van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin. Infect. Dis. 2020, 73, e1089–e1098. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yang, B.; Jiang, N.; Fu, W.; He, X.; Zhou, Y.; Ma, W.L.; Wang, X. Three-month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. J. Korean Med. Sci. 2020, 35, e418. [Google Scholar] [CrossRef] [PubMed]
- Casali, M.; Lauri, C.; Altini, C.; Bertagna, F.; Cassarino, G.; Cistaro, A.; Erba, A.P.; Ferrari, C.; Mainolfi, C.G.; Palucci, A.; et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: A guide for image acquisition and interpretation. Clin. Transl. Imaging 2021, 9, 299–339. [Google Scholar] [CrossRef] [PubMed]
- Alonso Sanchez, J.; García Prieto, J.; Galiana Morón, A.; Pilkington-Woll, J.P. PET/CT of COVID-19 as an Organizing Pneumonia. Clin. Nucl. Med. 2020, 45, 642–643. [Google Scholar] [CrossRef] [PubMed]

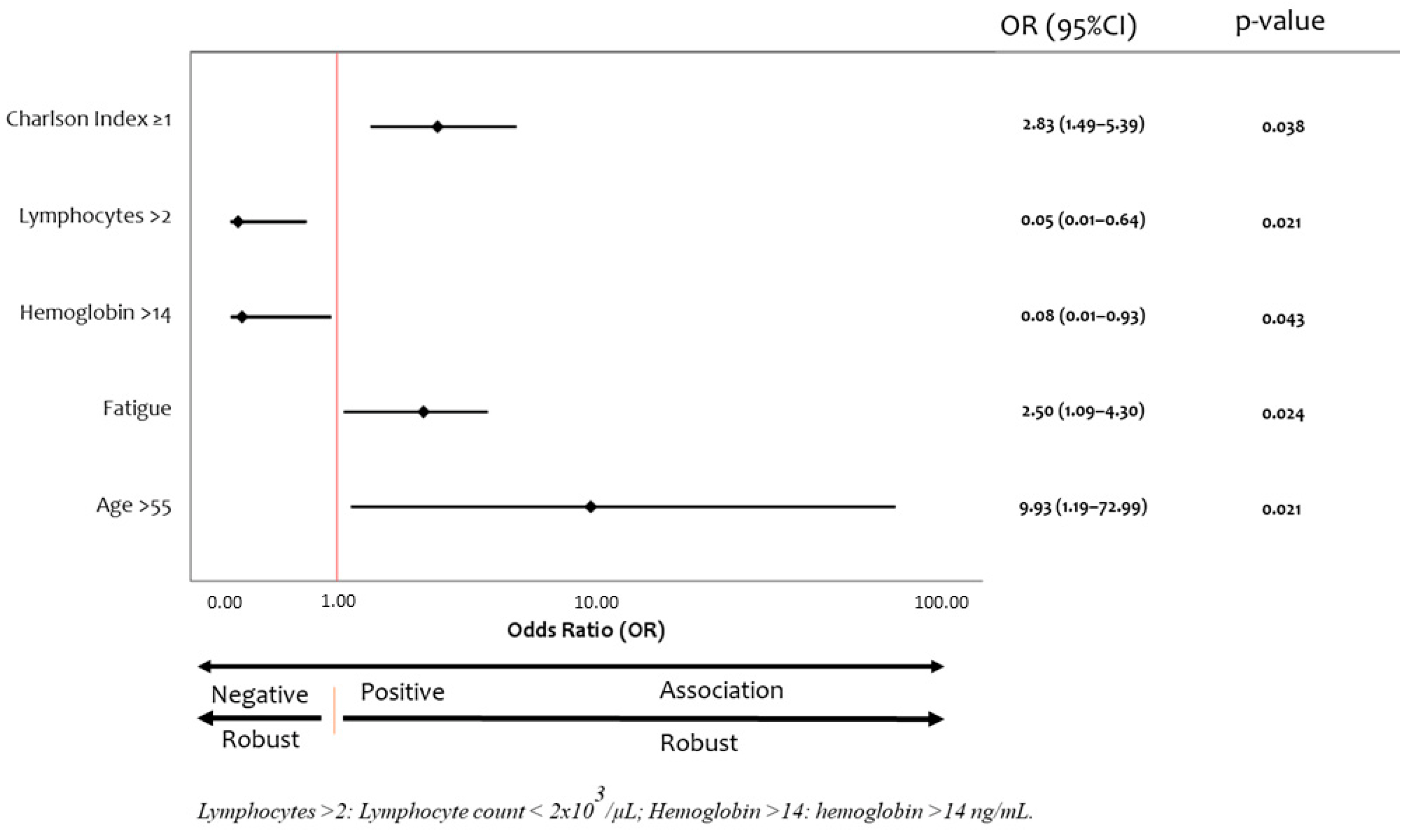
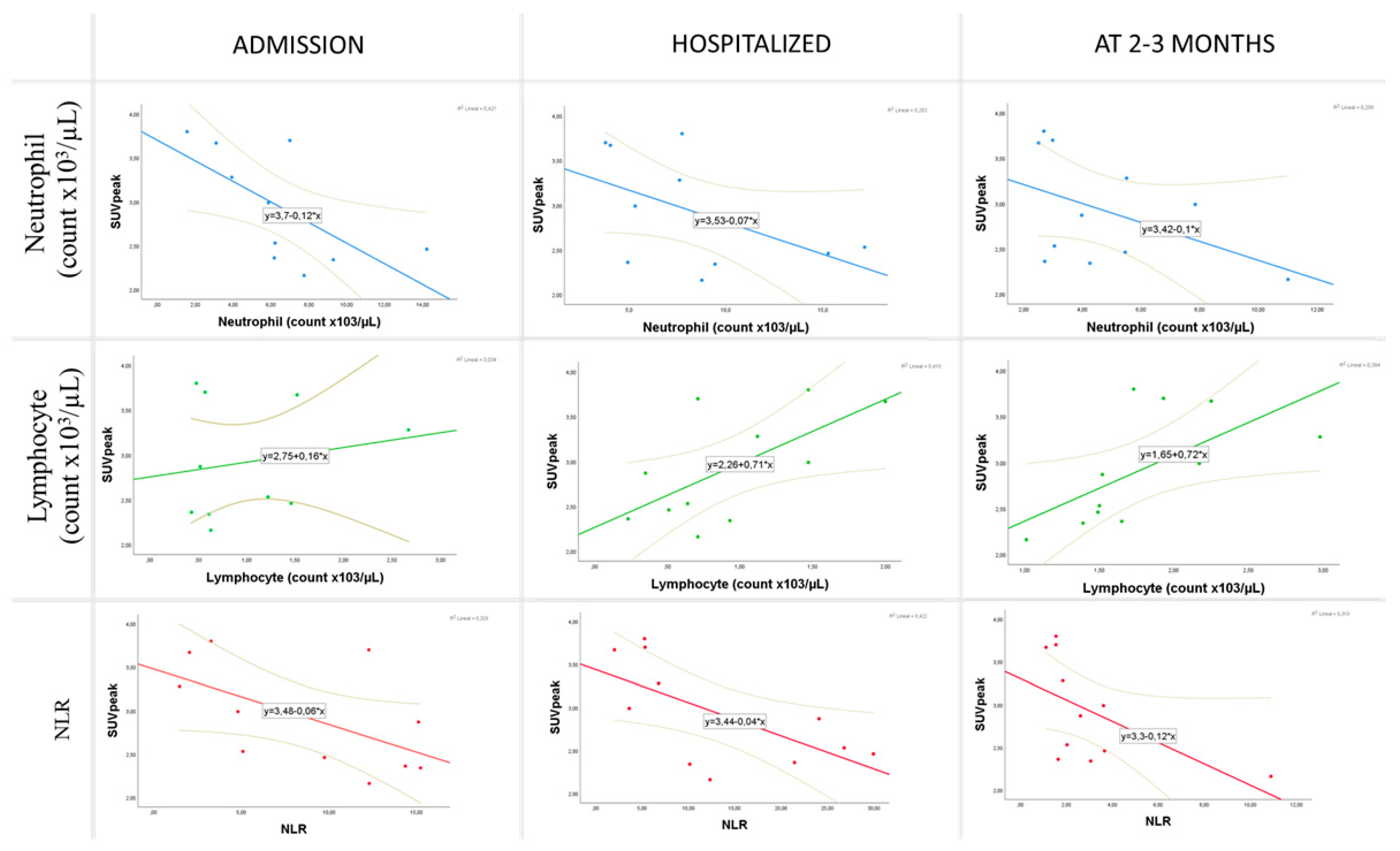
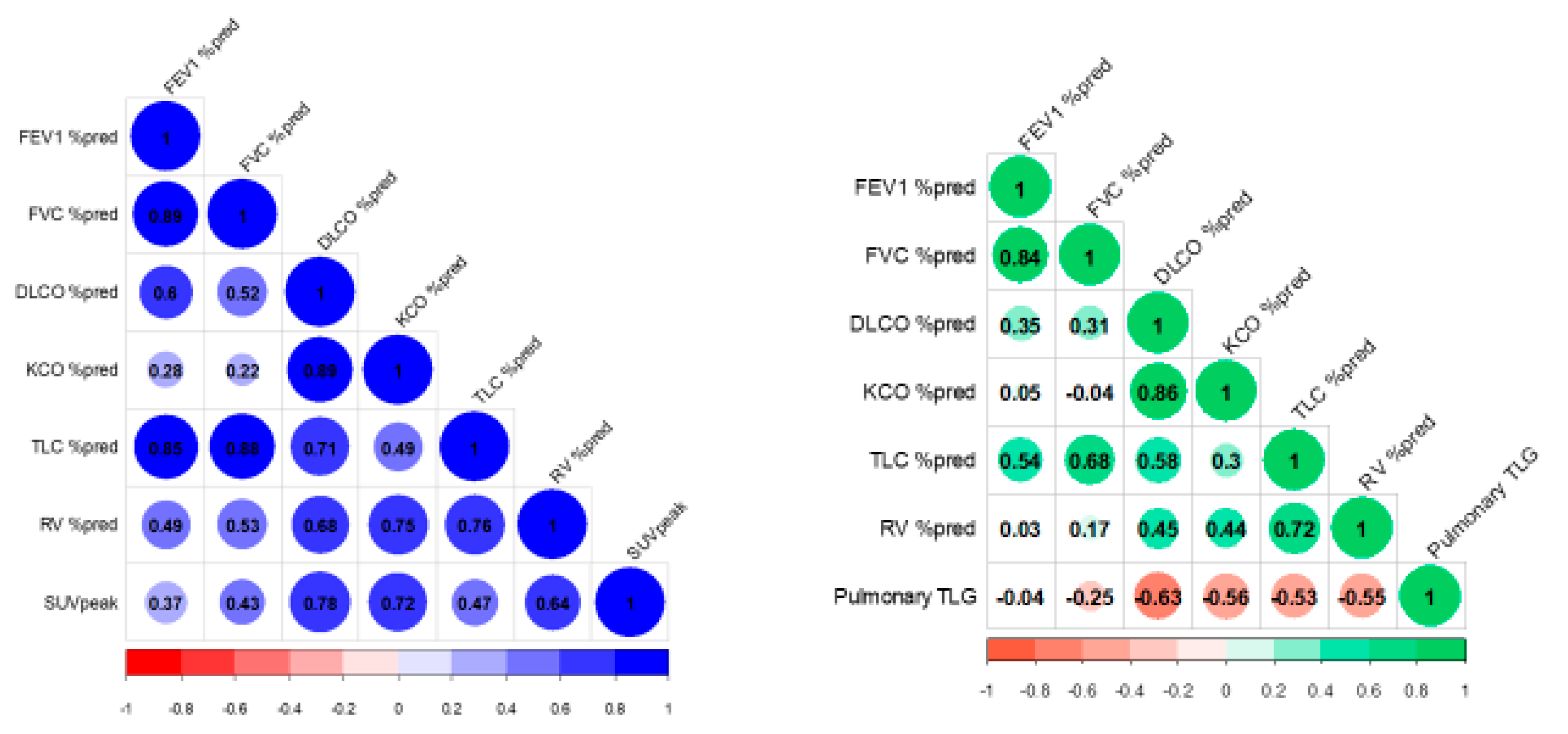
| Clinical Characteristics (n) | Mean ± SD or n (%) |
|---|---|
| Age (years) | 55.85 ± 9.28 |
| Gender (Male) | 12 (60) |
| BMI (kg/m2) | 34.11 ± 7.23 |
| Comorbidities | |
| Former or current smoking habit | 2 (10) |
| Hypertension | 5 (25) |
| Diabetes | 3 (15) |
| Hyperlipidemia | 2 (10) |
| Atrial fibrillation | 2 (10) |
| Asthma | 3 (15) |
| Charlson Comorbidity index | 1.60 ± 1.14 |
| Charlson Comorbidity index ≥ 2 | 9 (45) |
| Clinical characteristics at admission | |
| Fever | 17 (85) |
| Dyspnea | 15 (75) |
| Irritative cough | 16 (80) |
| Fatigue | 14 (70) |
| Myalgia | 11 (55) |
| Anosmia/Ageusia | 2 (10) |
| Digestive symptoms | 9 (45) |
| Headache | 3 (15) |
| ARDS (PaO2/FIO2 < 300 mmHg) | 14 (70) |
| Blood oxygen saturation | 90.90 ± 5.33 |
| Laboratory test results at admission | |
| Hemoglobin (g/dL) | 14.86 ± 1.84 |
| White blood cell (count ×103/µL) | 7.68 ± 3.11 |
| Neutrophil (count ×103/µL) | 6.28 ± 3.16 |
| Lymphocyte (count ×103/µL) | 0.99 ± 0.57 |
| NLR | 8.36 ± 5.86 |
| Platelet (count ×103/µL) | 206.65 ± 54.49 |
| Ferritin (ng/mL) | 1327.81 ± 1402.58 |
| C-reactive protein (mg/L) | 81.20 ± 54.61 |
| LDH (U/L) | 398.15 ± 113.80 |
| AST(U/L) | 49.25 ± 38.60 |
| ALT(U/L) | 48.30 ± 46.36 |
| Albumin (g/dL) | 3.92 ± 0.50 |
| D-dimer (mg/L) | 0.73 ± 0.52 |
| Characteristics of Hospitalization | |
| Hospital stay (days) | 16.70 ± 11.99 |
| Pneumonia (chest X-ray) | 19 (95) |
| ICU admission | 10 (50) |
| Invasive mechanical ventilation | 5 (25) |
| Bolus therapy with glucocorticoid | 14 (60) |
| Antiviral therapy | 5 (25) |
| Selective inhibitors of pro-inflammatory cytokines | 6 (30) |
| Variable | SUVPeak | Pulmonary TLG | |||
|---|---|---|---|---|---|
| Spearman’s rho | p-Value | Spearman’s rho | p-Value | ||
| Admission | Hemoglobin (g/dL) | −0.664 | 0.026 | ||
| Neutrophil count | −0.764 | 0.006 | |||
| Lymphocyte count | 0.636 | 0.035 | |||
| NLR | −0.664 | 0.026 | |||
| Hospital stay | Neutrophil count | −0.700 | 0.016 | ||
| Lymphocyte count | 0.618 | 0.043 | |||
| NLR | −0.627 | 0.039 | |||
| IL-6 | 0.624 | 0.010 | |||
| C-reactive protein | 0.618 | 0.004 | |||
| PCT | 0.570 | 0.049 | |||
| LDH | 0.445 | 0.049 | |||
| Troponin | 0.883 | 0.002 | |||
| Fibrinogen | 0.635 | 0.015 | |||
| D-dimer | 0.674 | 0.001 | |||
| Short-term follow-up | Neutrophil count | −0.679 | 0.022 | ||
| Lymphocyte count | 0.791 | 0.004 | |||
| NLR | −0.727 | 0.011 | |||
| Variable | SUVpeak | Pulmonary TLG | ||
|---|---|---|---|---|
| Spearman’s rho | p-Value | Spearman’s rho | p-Value | |
| DLCO% pred | 0.782 | 0.008 | −0.628 | 0.005 |
| KCO% pred | 0.721 | 0.019 | −0.564 | 0.014 |
| TLC% pred | 0.467 | 0.174 | −0.532 | 0.023 |
| RV% pred | 0.636 | 0.048 | −0.554 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triviño-Ibáñez, E.M.; Jiménez-Rodríguez, B.M.; Rudolphi-Solero, T.; García-Rivero, E.Y.; Rodríguez-Fernández, A.; Llamas-Elvira, J.M.; Gómez-Río, M.; Morales-García, C. [18F]FDG PET/CT in Short-Term Complications of COVID-19: Metabolic Markers of Persistent Inflammation and Impaired Respiratory Function. Diagnostics 2022, 12, 835. https://doi.org/10.3390/diagnostics12040835
Triviño-Ibáñez EM, Jiménez-Rodríguez BM, Rudolphi-Solero T, García-Rivero EY, Rodríguez-Fernández A, Llamas-Elvira JM, Gómez-Río M, Morales-García C. [18F]FDG PET/CT in Short-Term Complications of COVID-19: Metabolic Markers of Persistent Inflammation and Impaired Respiratory Function. Diagnostics. 2022; 12(4):835. https://doi.org/10.3390/diagnostics12040835
Chicago/Turabian StyleTriviño-Ibáñez, Eva María, Beatriz María Jiménez-Rodríguez, Teodoro Rudolphi-Solero, Encarnación Yolanda García-Rivero, Antonio Rodríguez-Fernández, José Manuel Llamas-Elvira, Manuel Gómez-Río, and Concepción Morales-García. 2022. "[18F]FDG PET/CT in Short-Term Complications of COVID-19: Metabolic Markers of Persistent Inflammation and Impaired Respiratory Function" Diagnostics 12, no. 4: 835. https://doi.org/10.3390/diagnostics12040835






