An Evolution Gaining Momentum—The Growing Role of Artificial Intelligence in the Diagnosis and Treatment of Spinal Diseases
Abstract
1. Introduction
2. Typical Forms of Artificial Intelligence Currently Applied in Spine Care
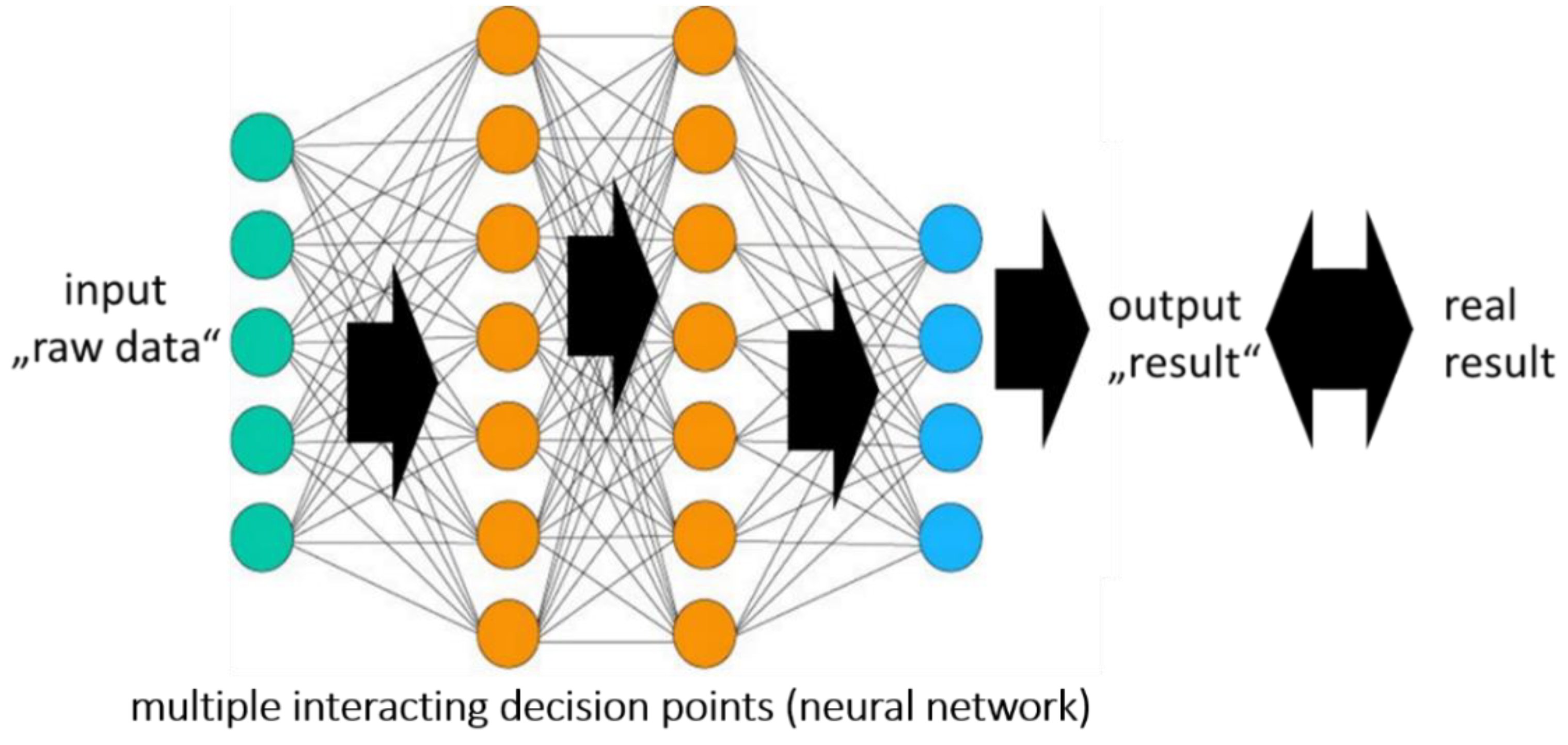
2.1. Artificial Intelligence, Machine Learning, and Neural Network
2.2. Neural Networks and the “Black Box”
2.3. Major Forms of Machine Learning Applied in Spine Care
2.3.1. Supervised Learning
2.3.2. Reinforcement Learning
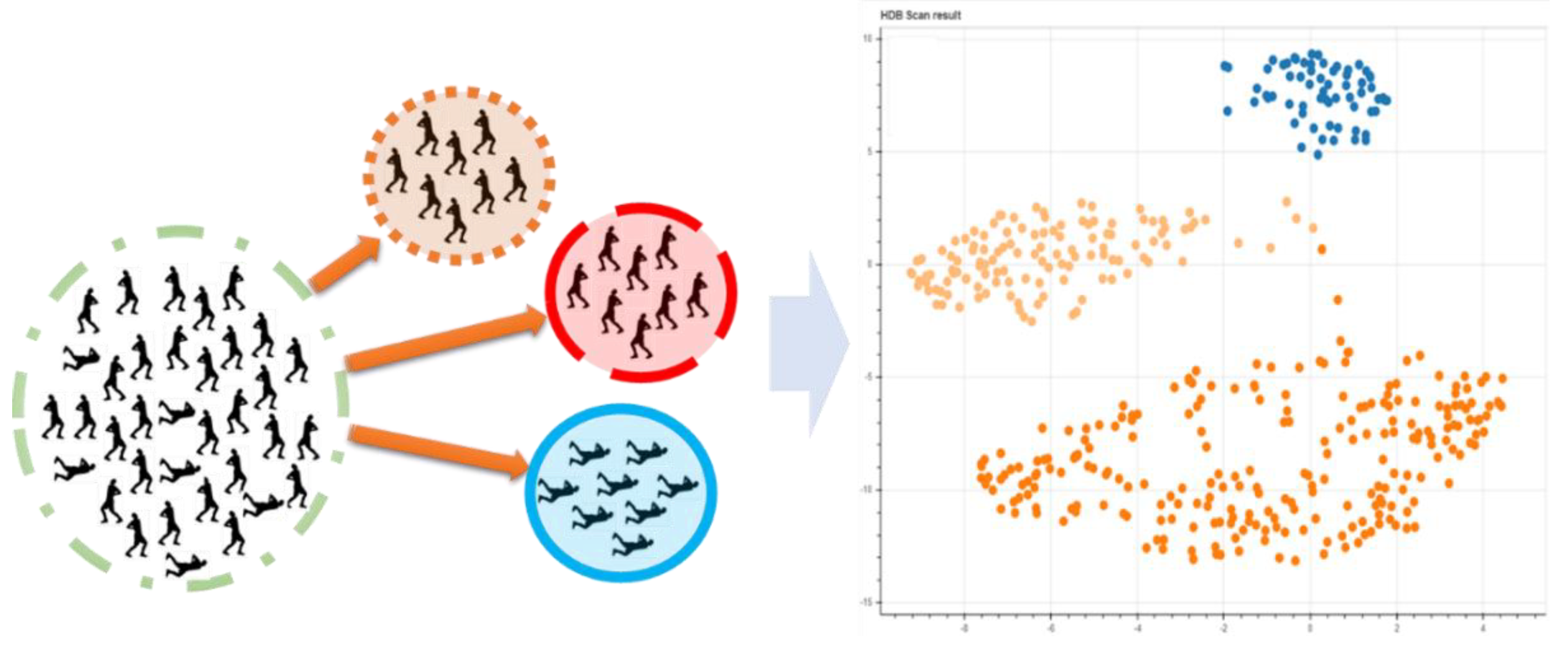
2.3.3. Unsupervised Learning
3. AI Applications in the Diagnosis and Treatment of Spinal Disorders
3.1. Diagnostic Imaging
3.2. Robotics
3.3. Biomechanical Spine Assessement
3.4. Prediction of Diagnosis and Outcome
4. AI in Spine Care Future
4.1. Data Quality and Verification
4.2. Database Repositories for Use in Spine Therapy
4.3. Advantages of AI in the Future of Spine Diagnostics and Treatment
4.4. Ethical Issues of AI in Spine Diagnostics and Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McCarthy, J.; Minsky, M.L.; Rochester, N.; Shannon, C.E. A proposal for the dartmouth summer research project on artificial intelligence, 31 August 1955. AI Mag. 2006, 27, 12. [Google Scholar]
- Fenn, J.; LeHong, H. Hype Cycle for Emerging Technologies; Gartner: Stamford, CT, USA, 2011. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Galbusera, F.; Casaroli, G.; Bassani, T. Artificial intelligence and machine learning in spine research. JOR Spine 2019, 2, e1044. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiong, T.; Xu, D.; Zhou, S.K.; Xu, Z.; Chen, M.; Park, J.; Grbic, S.; Tran, T.D.; Chin, S.P.; et al. Deep Image-To-Image Recurrent Network with Shape Basis for Automatic Vertebra Labeling in Large-Scale 3D CT Volumes. U.S. Patent No. 10,366,491, 30 July 2019. [Google Scholar]
- Galbusera, F.; Niemeyer, F.; Wilke, H.-J.; Bassani, T.; Casaroli, G.; Anania, C.; Costa, F.; Brayda-Bruno, M.; Sconfienza, L.M. Fully automated radiological analysis of spinal disorders and deformities: A deep learning approach. Eur. Spine J. 2019, 28, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Cheung, J.P.Y.; Wong, K.K.; Dokos, S.; Li, S.; Choy, R.W.; To, S.; Li, R.J.; Zhang, T. An artificial intelligence powered platform for auto-analyses of spine alignment irrespective of image quality with prospective validation. EClinicalMedicine 2022, 43, 101252. [Google Scholar] [CrossRef]
- Guermazi, A.; Tannoury, C.; Kompel, A.J.; Murakami, A.M.; Ducarouge, A.; Gillibert, A.; Li, X.; Tournier, A.; Lahoud, Y.; Jarraya, M.; et al. Improving radiographic fracture recognition performance and efficiency using artificial intelligence. Radiology 2021, 302, 210937. [Google Scholar] [CrossRef]
- Galbusera, F.; Cina, A.; Bassani, T.; Panico, M.; Sconfienza, L.M. Automatic diagnosis of spinal disorders on radiographic images: Leveraging existing unstructured datasets with natural language processing. Glob. Spine J. 2021, 11, 21925682211026910. [Google Scholar] [CrossRef]
- Chianca, V.; Cuocolo, R.; Gitto, S.; Albano, D.; Merli, I.; Badalyan, J.; Cortese, M.C.; Messina, C.; Luzzati, A.; Parafioriti, A.; et al. Radiomic machine learning classifiers in spine bone tumors: A multi-software, multi-scanner study. Eur. J. Radiol. 2021, 137, 109586. [Google Scholar] [CrossRef]
- Karandikar, P.; Massaad, E.; Hadzipasic, M.; Kiapour, A.; Joshi, R.S.; Shankar, G.M.; Shin, J.H. Machine learning applications of surgical imaging for the diagnosis and treatment of spine disorders: Current state of the art. Neurosurgery 2022, 90, 372–382. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, X.; Liu, C.; Kuang, X.; Hu, Y. Automatic lumbar spine tracking based on siamese convolutional network. J. Digit. Imaging 2019, 33, 423–430. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, K.R.; Kim, S.; Park, J.Y. Deep learning application in spinal implant identification. Spine 2021, 46, E318–E324. [Google Scholar] [CrossRef]
- Niemeyer, F.; Galbusera, F.; Tao, Y.; Kienle, A.; Beer, M.; Wilke, H.-J. A deep learning model for the accurate and reliable classification of disc degeneration based on MRI data. Investig. Radiol. 2021, 56, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lafage, R.; Ang, B.; Alshabab, B.S.; Elysee, J.; Lovecchio, F.; Weissman, K.; Kim, H.J.; Schwab, F.; Lafage, V. Predictive model for selection of upper treated vertebra using a machine learning approach. World Neurosurg. 2021, 146, e225–e232. [Google Scholar] [CrossRef] [PubMed]
- Ulivieri, F.M.; Rinaudo, L.; Messina, C.; Piodi, L.P.; Capra, D.; Lupi, B.; Meneguzzo, C.; Sconfienza, L.M.; Sardanelli, F.; Giustina, A.; et al. Bone strain index predicts fragility fracture in osteoporotic women: An artificial intelligence-based study. Eur. Radiol. Exp. 2021, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ryu, H.; Kim, S.W.; Oh, J.K.; Kim, T.H. Prediction of recurrence in pyogenic vertebral osteomyelitis by artificial neural network using time-series data of c-reactive protein: A retrospective cohort study of 704 patients. Spine 2021, 46, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- de Vries, B.C.S.; Hegeman, J.H.; Nijmeijer, W.; Geerdink, J.; Seifert, C.; Groothuis-Oudshoorn, C.G.M. Comparing three machine learning approaches to design a risk assessment tool for future fractures: Predicting a subsequent major osteoporotic fracture in fracture patients with osteopenia and osteoporosis. Osteoporos. Int. 2021, 32, 437–449. [Google Scholar] [CrossRef]
- Hornung, A.L.; Hornung, C.M.; Mallow, G.M.; Barajas, J.N.; Espinoza Orias, A.A.; Galbusera, F.; Wilke, H.J.; Colman, M.; Phillips, F.M.; An, H.S.; et al. Artificial intelligence and spine imaging: Limitations, regulatory issues and future direction. Eur. Spine J. 2022, 1–15. [Google Scholar] [CrossRef]
- Rasouli, J.J.; Shao, J.; Neifert, S.; Gibbs, W.N.; Habboub, G.; Steinmetz, M.P.; Benzel, E.; Mroz, T.E. Artificial intelligence and robotics in spine surgery. Glob. Spine J. 2021, 11, 556–564. [Google Scholar] [CrossRef]
- Jiang, N.; Luk, K.D.-K.; Hu, Y. A machine learning-based surface electromyography topography evaluation for prognostic prediction of functional restoration rehabilitation in chronic low back pain. Spine 2017, 42, 1635–1642. [Google Scholar] [CrossRef]
- Zhang, J.; Lockhart, T.E.; Soangra, R. Classifying lower extremity muscle fatigue during walking using machine learning and inertial sensors. Ann. Biomed. Eng. 2014, 42, 600–612. [Google Scholar] [CrossRef]
- Xie, N.; Wilson, P.J.; Reddy, R. Use of machine learning to model surgical decision-making in lumbar spine surgery. Eur. Spine J. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Abd, M.A.; Taing, A.; Tsai, C.T.; Vrionis, F.D.; Engeberg, E.D. Robotic replica of a human spine uses soft magnetic sensor array to forecast intervertebral loads and posture after surgery. Sensors 2021, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, F.; Arjmand, N.; Nasrabadi, A. Coupled artificial neural networks to estimate 3D whole-body posture, lumbosacral moments, and spinal loads during load-handling activities. J. Biomech. 2020, 102, 109332. [Google Scholar] [CrossRef]
- van Hooff, M.L.; van Loon, J.; van Limbeek, J.; de Kleuver, M. The nijmegen decision tool for chronic low back pain. Development of a clinical decision tool for secondary or tertiary spine care specialists. PLoS ONE 2014, 9, e104226. [Google Scholar] [CrossRef]
- Mann, N.H., 3rd; Brown, M.D. Artificial intelligence in the diagnosis of low back pain. Orthop. Clin. N. Am. 1991, 22, 303–314. [Google Scholar] [CrossRef]
- Coupé, V.M.; van Hooff, M.L.; de Kleuver, M.; Steyerberg, E.W.; Ostelo, R.W. Decision support tools in low back pain. Best Pract. Res. Clin. Rheumatol. 2016, 30, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Sivaganesan, A.; Asher, A.L.; Devin, C.J. Prediction model for outcome after low-back surgery: Individualized likelihood of complication, hospital readmission, return to work, and 12-month improvement in functional disability. Neurosurg. Focus 2015, 39, E13. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Cizik, A.M.; Hamilton, D.; Chapman, J.R. Predicting surgical site infection after spine surgery: A validated model using a prospective surgical registry. Spine J. 2014, 14, 2112–2117. [Google Scholar] [CrossRef]
- Mallow, G.M.; Siyaji, Z.K.; Galbusera, F.; Espinoza-Orias, A.A.; Giers, M.; Lundberg, H.; Ames, C.; Karppinen, J.; Louie, P.K.; Phillips, F.M.; et al. Intelligence-based spine care model: A new era of research and clinical decision-making. Glob. Spine J. 2021, 11, 135–145. [Google Scholar] [CrossRef]
- Gutman, M.J.; Schroeder, G.D.; Murphy, H.; Flanders, A.E.; Vaccaro, A.R. Artificial intelligence in spine care. Clin. Spine Surg. 2021, 34, 121–124. [Google Scholar] [CrossRef]
- Durand, W.M.; Daniels, A.H.; Hamilton, D.K.; Passias, P.; Kim, H.J.; Protopsaltis, T.; LaFage, V.; Smith, J.S.; Shaffrey, C.; Gupta, M.; et al. Artificial intelligence models predict operative versus nonoperative management of patients with adult spinal deformity with 86% accuracy. World Neurosurg. 2020, 141, e239–e253. [Google Scholar] [CrossRef]
- Ames, C.P.; Smith, J.S.; Pellisé, F.; Kelly, M.; Alanay, A.; Acaroglu, E.; Pérez-Grueso, F.J.S.; Kleinstück, F.; Obeid, I.; Vila-Casademunt, A. Artificial intelligence based hierarchical clustering of patient types and intervention categories in adult spinal deformity surgery: Towards a new classification scheme that predicts quality and value. Spine 2019, 44, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; Lafage, R.; Hamilton, D.K.; Passias, P.G.; Kim, H.J.; Protopsaltis, T.; Lafage, V.; Smith, J.S.; Shaffrey, C.; Gupta, M.; et al. Artificial intelligence clustering of adult spinal deformity sagittal plane morphology predicts surgical characteristics, alignment, and outcomes. Eur. Spine J. 2021, 30, 2157–2166. [Google Scholar] [CrossRef]
- Joshi, R.S.; Lau, D.; Ames, C.P. Artificial intelligence for adult spinal deformity: Current state and future directions. Spine J. 2021, 21, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Harada, G.K.; Siyaji, Z.K.; Mallow, G.M.; Hornung, A.L.; Hassan, F.; Basques, B.A.; Mohammed, H.A.; Sayari, A.J.; Samartzis, D.; An, H.S. Artificial intelligence predicts disk re-herniation following lumbar microdiscectomy: Development of the “RAD” risk profile. Eur. Spine J. 2021, 30, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Wirries, A.; Geiger, F.; Hammad, A.; Oberkircher, L.; Blumcke, I.; Jabari, S. Artificial intelligence facilitates decision-making in the treatment of lumbar disc herniations. Eur. Spine J. 2020, 30, 2176–2184. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Quddusi, A.; Klukowska, A.M.; Schröder, M.L. Initial classification of low back and leg pain based on objective functional testing: A pilot study of machine learning applied to diagnostics. Eur. Spine J. 2020, 29, 1702–1708. [Google Scholar] [CrossRef]
- Pedersen, C.F.; Andersen, M.O.; Carreon, L.Y.; Eiskjaer, S. Applied machine learning for spine surgeons: Predicting outcome for patients undergoing treatment for lumbar disc herniation using PRO data. Glob. Spine J. 2020, 10, 2192568220967643. [Google Scholar] [CrossRef]
- Massaad, E.; Ha, Y.; Shankar, G.M.; Shin, J.H. Clinical prediction modeling in intramedullary spinal tumor surgery. Acta Neurochir. Suppl. 2022, 134, 333–339. [Google Scholar] [CrossRef]
- Wang, K.Y.; Suresh, K.V.; Puvanesarajah, V.; Raad, M.; Margalit, A.; Jain, A. Using Predictive Modeling and Machine Learning to Identify Patients Appropriate for Outpatient Anterior Cervical Fusion and Discectomy. Spine 2021, 46, 665–670. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.R.; Li, W.; Fan, T.; Zhao, J.; Kang, M.; Dong, R.; Qu, Y. Prediction of the risk of C5 palsy after posterior laminectomy and fusion with cervical myelopathy using a support vector machine: An analysis of 184 consecutive patients. J. Orthop. Surg. Res. 2021, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Devana, S.K.; Lee, C.; Bugarin, A.; Lord, E.L.; Shamie, A.N.; Park, D.Y.; van der Schaar, M.; SooHoo, N.F. Prediction of major complications and readmission after lumbar spinal fusion: A machine learning-driven approach. World Neurosurg. 2021, 152, e227–e234. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.; Shah, S.; Newton, P. Machine learning predicts the 3d outcomes of adolescent idiopathic scoliosis surgery using patient-surgeon specific parameters. Spine 2021, 46, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.L.; Neifert, S.N.; Oermann, E.K.; Gilligan, J.T.; Rothrock, R.J.; Yuk, F.J.; Gal, J.S.; Nistal, D.A.; Caridi, J.M. Application of cooperative game theory principles to interpret machine learning models of nonhome discharge following spine surgery. Spine 2021, 46, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kuris, E.O.; Veeramani, A.; McDonald, C.L.; DiSilvestro, K.J.; Zhang, A.S.; Cohen, E.M.; Daniels, A.H. Predicting readmission after anterior, posterior, and posterior interbody lumbar spinal fusion: A neural network machine learning approach. World Neurosurg. 2021, 151, e19–e27. [Google Scholar] [CrossRef]
- Khan, O.; Badhiwala, J.H.; Akbar, M.A.; Fehlings, M.G. Prediction of worse functional status after surgery for degenerative cervical myelopathy: A machine learning approach. Neurosurgery 2021, 88, 584–591. [Google Scholar] [CrossRef]
- Karhade, A.V.; Bongers, M.E.R.; Groot, O.Q.; Cha, T.D.; Doorly, T.P.; Fogel, H.A.; Hershman, S.H.; Tobert, D.G.; Srivastava, S.D.; Bono, C.M.; et al. Development of machine learning and natural language processing algorithms for preoperative prediction and automated identification of intraoperative vascular injury in anterior lumbar spine surgery. Spine J. 2021, 21, 1635–1642. [Google Scholar] [CrossRef]
- Ogink, P.T.; Groot, O.Q.; Karhade, A.V.; Bongers, M.E.R.; Oner, F.C.; Verlaan, J.J.; Schwab, J.H. Wide range of applications for machine-learning prediction models in orthopedic surgical outcome: A systematic review. Acta Orthop. 2021, 92, 526–531. [Google Scholar] [CrossRef]
- Wirries, A.; Geiger, F.; Hammad, A.; Redder, A.; Oberkircher, L.; Ruchholtz, S.; Bluemcke, I.; Jabari, S. Combined artificial intelligence approaches analyzing 1000 conservative patients with back pain-a methodological pathway to predicting treatment efficacy and diagnostic groups. Diagnostics 2021, 11, 1934. [Google Scholar] [CrossRef]
- Ibrahim, H.; Liu, X.; Denniston, A.K. Reporting guidelines for artificial intelligence in healthcare research. Clin. Exp. Ophthalmol. 2021, 49, 470–476. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Watanabe, Y.; Madani, A.; Hashimoto, D.A.; Meireles, O.R.; Takeshita, N.; Mori, K.; Ito, M. Artificial intelligence for computer vision in surgery: A call for developing reporting guidelines. Ann. Surg. 2021, 275, e609–e611. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Lancet Digit. Health 2020, 2, e537–e548. [Google Scholar] [CrossRef]
- Bazoukis, G.; Hall, J.; Loscalzo, J.; Antman, E.M.; Fuster, V.; Armoundas, A.A. The inclusion of augmented intelligence in medicine: A framework for successful implementation. Cell Rep. Med. 2022, 3, 100485. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Karhade, A.V.; Park, H.Y.; Sheppard, W.L.; Macyszyn, L.J.; Everson, R.G.; Shamie, A.N.; Park, D.Y.; Schwab, J.H.; Hornicek, F.J. Updated external validation of the SORG machine learning algorithms for prediction of ninety-day and one-year mortality after surgery for spinal metastasis. Spine J. 2021, 21, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Aschauer-Wallner, S.; Mattiassich, G.; Aigner, L.; Resch, H. The austrian spinal cord injury study: A registry for patients living with a traumatic spinal cord injury. Spinal Cord Ser. Cases 2017, 3, 17076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebastian, A.S. Database research in spine surgery. Clin. Spine Surg. 2016, 29, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.L.; Knightly, J.; Mummaneni, P.V.; Alvi, M.A.; McGirt, M.J.; Yolcu, Y.U.; Chan, A.K.; Glassman, S.D.; Foley, K.T.; Slotkin, J.R. Quality outcomes database spine care project 2012–2020: Milestones achieved in a collaborative North American outcomes registry to advance value-based spine care and evolution to the American spine registry. Neurosurg. Focus 2020, 48, E2. [Google Scholar] [CrossRef]
- Groot, O.Q.; Bindels, B.J.J.; Ogink, P.T.; Kapoor, N.D.; Twining, P.K.; Collins, A.K.; Bongers, M.E.R.; Lans, A.; Oosterhoff, J.H.F.; Karhade, A.V.; et al. Availability and reporting quality of external validations of machine-learning prediction models with orthopedic surgical outcomes: A systematic review. Acta Orthop. 2021, 92, 385–393. [Google Scholar] [CrossRef]
- Saravi, B.; Hassel, F.; Ülkümen, S.; Zink, A.; Shavlokhova, V.; Couillard-Despres, S.; Boeker, M.; Obid, P.; Lang, G.M. Artificial intelligence-driven prediction modeling and decision making in spine surgery using hybrid machine learning models. J. Pers. Med. 2022, 12, 509. [Google Scholar] [CrossRef]
- Cerrato, P.; Halamka, J. The Digital Reconstruction of Healthcare: Transitioning from Brick and Mortar to Virtual Care; HIMSS Publishing: Chicago, IL, USA, 2021. [Google Scholar]
- Etzel, C.M.; Veeramani, A.; Zhang, A.S.; McDonald, C.L.; DiSilvestro, K.J.; Cohen, E.M.; Daniels, A.H. Supervised machine learning for predicting length of stay after lumbar arthrodesis: A comprehensive artificial intelligence approach. J. Am. Acad. Orthop. Surg. 2021, 30, 125–132. [Google Scholar] [CrossRef]
- Berjano, P.; Langella, F.; Ventriglia, L.; Compagnone, D.; Barletta, P.; Huber, D.; Mangili, F.; Licandro, G.; Galbusera, F.; Cina, A.; et al. The influence of baseline clinical status and surgical strategy on early good to excellent result in spinal lumbar arthrodesis: A machine learning approach. J. Pers. Med. 2021, 11, 1377. [Google Scholar] [CrossRef]
- Wiens, J.; Saria, S.; Sendak, M.; Ghassemi, M.; Liu, V.X.; Doshi-Velez, F.; Jung, K.; Heller, K.; Kale, D.; Saeed, M. Do no harm: A roadmap for responsible machine learning for health care. Nat. Med. 2019, 25, 1337–1340. [Google Scholar] [CrossRef]
- Kedra, J.; Gossec, L. Big data and artificial intelligence: Will they change our practice? Jt. Bone Spine 2020, 87, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Santacatterina, M.; Pennicooke, B.; Shahrestani, S.; Ballatori, A.M.; Orrico, K.O.; Burke, J.F.; Manley, G.T.; Tarapore, P.E.; Huang, M.C.; et al. Does state malpractice environment affect outcomes following spinal fusions? A robust statistical and machine learning analysis of 549,775 discharges following spinal fusion surgery in the United States. Neurosurg. Focus 2020, 49, E18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Grabowski, M.M.; Habboub, G.; Mroz, T.E. The impact of artificial intelligence on quality and safety. Glob. Spine J. 2020, 10, 99S–103S. [Google Scholar] [CrossRef] [PubMed]
- Cartolovni, A.; Tomicic, A.; Lazic Mosler, E. Ethical, legal, and social considerations of AI-based medical decision-support tools: A scoping review. Int. J. Med. Inform. 2022, 161, 104738. [Google Scholar] [CrossRef]
- Gijsberts, C.M.; Groenewegen, K.A.; Hoefer, I.E.; Eijkemans, M.J.; Asselbergs, F.W.; Anderson, T.J.; Britton, A.R.; Dekker, J.M.; Engström, G.; Evans, G.W. Race/ethnic differences in the associations of the framingham risk factors with carotid IMT and cardiovascular events. PLoS ONE 2015, 10, e0132321. [Google Scholar] [CrossRef]
- Char, D.S.; Shah, N.H.; Magnus, D. Implementing machine learning in health care—Addressing ethical challenges. N. Engl. J. Med. 2018, 378, 981. [Google Scholar] [CrossRef]
- Seyyed-Kalantari, L.; Zhang, H.; McDermott, M.; Chen, I.Y.; Ghassemi, M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat. Med. 2021, 27, 2176–2182. [Google Scholar] [CrossRef]
- James, C.A.; Wachter, R.M.; Woolliscroft, J.O. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirries, A.; Geiger, F.; Oberkircher, L.; Jabari, S. An Evolution Gaining Momentum—The Growing Role of Artificial Intelligence in the Diagnosis and Treatment of Spinal Diseases. Diagnostics 2022, 12, 836. https://doi.org/10.3390/diagnostics12040836
Wirries A, Geiger F, Oberkircher L, Jabari S. An Evolution Gaining Momentum—The Growing Role of Artificial Intelligence in the Diagnosis and Treatment of Spinal Diseases. Diagnostics. 2022; 12(4):836. https://doi.org/10.3390/diagnostics12040836
Chicago/Turabian StyleWirries, Andre, Florian Geiger, Ludwig Oberkircher, and Samir Jabari. 2022. "An Evolution Gaining Momentum—The Growing Role of Artificial Intelligence in the Diagnosis and Treatment of Spinal Diseases" Diagnostics 12, no. 4: 836. https://doi.org/10.3390/diagnostics12040836
APA StyleWirries, A., Geiger, F., Oberkircher, L., & Jabari, S. (2022). An Evolution Gaining Momentum—The Growing Role of Artificial Intelligence in the Diagnosis and Treatment of Spinal Diseases. Diagnostics, 12(4), 836. https://doi.org/10.3390/diagnostics12040836





