Factors Influencing Mammographic Density in Asian Women: A Retrospective Cohort Study in the Northeast Region of Peninsular Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Population
2.2. Breast Cancer Data
2.3. Study Design and Patient Selection
2.4. Statistical Analysis and Software
2.4.1. Missing Data Handling
2.4.2. Logistic Regression
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolou-Ghamari, Z. Prevalence of Breast Cancer in Isfahan Province, Iran. Women’s Health Bull. 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.K.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Kim, J.Y.; Ahn, J.S.; Park, W.; Yu, J.; Park, Y.H. Breast cancer epidemiology of the working-age female population reveals significant implications for the South Korean economy. J. Breast Cancer 2018, 21, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Sinaga, E.S.; Ahmad, R.A.; Shivalli, S.; Hutajulu, S.H. Age at diagnosis predicted survival outcome of female patients with breast cancer at a tertiary hospital in Yogyakarta, Indonesia. Pan Afr. Med. J. 2018, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Cao, M.; Li, H.; He, S.; Chen, W. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin. J. Cancer Res. 2020, 32, 129–139. [Google Scholar] [CrossRef]
- Yap, Y.S.; Lu, Y.S.; Tamura, K.; Lee, J.E.; Ko, E.Y.; Park, Y.H.; Cao, A.Y.; Lin, C.H.; Toi, M.; Wu, J.; et al. Insights into Breast Cancer in the East vs the West: A Review. JAMA Oncol. 2019, 5, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manan, A.; Basri, H.; Kaur, N.; Abdul RAhman, S.Z.; Amir, P.N.; Ali, N.; Raman, S.; Bahtiar, B.; Mustafa Ramdzuan, N.S.; Syed Soffian, S.S.; et al. Malaysia National Cancer Registry Report 2012–2016; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2019.
- Youn, H.J.; Han, W. A review of the epidemiology of breast cancer in Asia: Focus on risk factors. Asian Pac. J. Cancer Prev. 2020, 21, 867–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. In Breast Cancer Metastasis and Drug Resistance: Challenges and Progress; Ahmad, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 9–29. ISBN 978-3-030-20301-6. [Google Scholar]
- Lee, C.I.; Chen, L.E.; Elmore, J.G. Risk-based Breast Cancer Screening: Implications of Breast Density. Med. Clin. N. Am. 2017, 101, 725–741. [Google Scholar] [CrossRef]
- Bond-Smith, D.; Stone, J. Methodological challenges and updated findings from a meta-analysis of the association between mammographic density and breast cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef]
- Bell, R.J.; Evans, J.; Fox, J.; Pridmore, V. Using an automated measure of breast density to explore the association between ethnicity and mammographic density in Australian women. J. Med. Imaging Radiat. Oncol. 2019, 63, 183–189. [Google Scholar] [CrossRef]
- Nazari, S.S.; Mukherjee, P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018, 25, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Bell, R.J. Mammographic density and breast cancer screening. Climacteric 2020, 23, 460–465. [Google Scholar] [CrossRef]
- Park, B.; Cho, H.M.; Lee, E.H.; Song, S.; Suh, M.; Choi, K.S.; Kang, B.J.; Ko, K.; Yi, A.; Jung, H.K.; et al. Does breast density measured through population-based screening independently increase breast cancer risk in asian females? Clin. Epidemiol. 2018, 10, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Assi, V.; Warwick, J.; Cuzick, J.; Duffy, S.W. Clinical and epidemiological issues in mammographic density. Nat. Rev. Clin. Oncol. 2012, 9, 33–40. [Google Scholar] [CrossRef]
- Gardezi, S.J.S.; Elazab, A.; Lei, B.; Wang, T. Breast cancer detection and diagnosis using mammographic data: Systematic review. J. Med. Internet Res. 2019, 21, e14464. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Little, R.J.A. A Test of Missing Completely at Random for Multivariate Data with Missing Values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Tierney, N.; Cook, D.; McBain, M.; Fay, C. Naniar: Data Structures, Summaries, and Visualisations for Missing Data, R package version 0.6.1. 2021. Available online: https://cran.r-project.org/web/packages/naniar/index.html (accessed on 5 March 2022).
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- van Buuren, S. Flexible Imputation of Missing Data, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018; ISBN 9780429492259. [Google Scholar]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; ISBN 9780471722144. [Google Scholar]
- Box, G.E.P.; Tidwell, P.W. Transformation of the Independent Variables. Technometrics 1962, 4, 531–550. [Google Scholar] [CrossRef]
- Duffy, S.W.; Morrish, O.W.E.; Allgood, P.C.; Black, R.; Gillan, M.G.C.; Willsher, P.; Cooke, J.; Duncan, K.A.; Michell, M.J.; Dobson, H.M.; et al. Mammographic density and breast cancer risk in breast screening assessment cases and women with a family history of breast cancer. Eur. J. Cancer 2018, 88, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanders, J.O.P.; Bakker, M.F.; Veldhuis, W.B.; Peeters, P.H.M.; van Gils, C.H. The effect of weight change on changes in breast density measures over menopause in a breast cancer screening cohort. Breast Cancer Res. 2015, 17, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engmann, N.J.; Scott, C.; Jensen, M.R.; Winham, S.J.; Ma, L.; Brandt, K.R.; Mahmoudzadeh, A.; Whaley, D.H.; Hruska, C.B.; Wu, F.F.; et al. Longitudinal changes in volumetric breast density in healthy women across the menopausal transition. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1324–1331. [Google Scholar] [CrossRef]
- Sung, H.; Ren, J.; Li, J.; Pfeiffer, R.M.; Wang, Y.; Guida, J.L.; Fang, Y.; Shi, J.; Zhang, K.; Li, N.; et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. npj Breast Cancer 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.Y.; Guo, S.; Cui, M.K.; Zheng, Y.F.; Liao, Z.X.; Zhang, Q.; Piao, H.Z. Influential factors and prediction model of mammographic density among Chinese women. Medicine 2021, 100, e26586. [Google Scholar] [CrossRef]
- Vourtsis, A.; Berg, W.A. Breast density implications and supplemental screening. Eur. Radiol. 2019, 29, 1762–1777. [Google Scholar] [CrossRef]
- Pape, R.; Spuur, K.; Umo, P. Exploring correlations between the breast density of the women of Papua New Guinea and breast cancer risk factors. Radiography 2019, 25, e79–e87. [Google Scholar] [CrossRef]
- Rajaram, N.; Mariapun, S.; Eriksson, M.; Tapia, J.; Kwan, P.Y.; Ho, W.K.; Harun, F.; Rahmat, K.; Czene, K.; Taib, N.A.M.; et al. Differences in mammographic density between Asian and Caucasian populations: A comparative analysis. Breast Cancer Res. Treat. 2017, 161, 353–362. [Google Scholar] [CrossRef]
- Reimers, L.L.; Goldberg, M.; Tehranifar, P.; Michels, K.B.; Cohn, B.A.; Flom, J.D.; Wei, Y.; Cirillo, P.; Terry, M.B. Benign breast disease and changes in mammographic breast density. Breast Cancer Res. 2021, 23, 49. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Pfeiffer, R.M.; Patel, D.A.; Linville, L.; Brinton, L.A.; Gierach, G.L.; Yang, X.R.; Papathomas, D.; Visscher, D.; Mies, C.; et al. Terminal duct lobular unit involution of the normal breast: Implications for breast cancer etiology. J. Natl. Cancer Inst. 2014, 106, dju286. [Google Scholar] [CrossRef] [Green Version]
- Gierach, G.L.; Patel, D.A.; Pfeiffer, R.M.; Figueroa, J.D.; Linville, L.; Papathomas, D.; Johnson, J.M.; Chicoine, R.E.; Herschorn, S.D.; Shepherd, J.A.; et al. Relationship of terminal duct lobular unit involution of the breast with area and volume mammographic densities. Cancer Prev. Res. 2016, 9, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. J. Natl. Cancer Inst. 2014, 106, dju255. [Google Scholar] [CrossRef]
- Shamsi, U.; Afzal, S.; Shamsi, A.; Azam, I.; Callen, D. Factors associated with mammographic breast density among women in Karachi Pakistan. BMC Womens Health 2021, 21, 438. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Gu, R.; Hu, Y.; Liu, F.; Yun, M.; Xiao, Q.; Wu, M.; Liu, Q.; Su, F. Influence of factors on mammographic density in premenopausal Chinese women. Eur. J. Cancer Prev. 2016, 25, 306–311. [Google Scholar] [CrossRef]
- Azam, S.; Sjölander, A.; Eriksson, M.; Gabrielson, M.; Czene, K.; Hall, P. Determinants of mammographic density change. JNCI Cancer Spectr. 2019, 3, pkz004. [Google Scholar] [CrossRef] [Green Version]
- Masala, G.; Assedi, M.; Sera, F.; Ermini, I.; Occhini, D.; Castaldo, M.; Pierpaoli, E.; Caini, S.; Bendinelli, B.; Ambrogetti, D.; et al. Can dietary and physical activity modifications reduce breast density in postmenopausal women? The DAMA study, a randomized intervention trial in Italy. Cancer Epidemiol. Biomark. Prev. 2019, 28, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Brand, J.S.; Czene, K.; Eriksson, L.; Trinh, T.; Bhoo-Pathy, N.; Hall, P.; Celebioglu, F. Influence of lifestyle factors on mammographic density in postmenopausal women. PLoS ONE 2013, 8, e81876. [Google Scholar] [CrossRef]
- Ekpo, E.U.; Brennan, P.C.; Mello-Thoms, C.; McEntee, M.F. Relationship between Breast Density and Selective Estrogen-Receptor Modulators, Aromatase Inhibitors, Physical Activity, and Diet: A Systematic Review. Integr. Cancer Ther. 2016, 15, 127–144. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.S.; Bertrand, K.A.; Lajous, M.; Tamimi, R.M.; Torres, G.; López-Ridaura, R.; Romieu, I. Reproductive and lifestyle risk factors and mammographic density in Mexican women. Ann. Epidemiol. 2015, 25, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Trinh, T.; Christensen, S.E.; Brand, J.S.; Cuzick, J.; Czene, K.; Sjölander, A.; Bälter, K.; Hall, P. Background risk of breast cancer influences the association between alcohol consumption and mammographic density. Br. J. Cancer 2015, 113, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Madley-Dowd, P.; Hughes, R.; Tilling, K.; Heron, J. The proportion of missing data should not be used to guide decisions on multiple imputation. J. Clin. Epidemiol. 2019, 110, 63–73. [Google Scholar] [CrossRef] [Green Version]
| Variables | Non-Dense n (%) | Dense n (%) | Missing Values n (%) |
|---|---|---|---|
| Age (years) 1 | 55.5 (9.6) | 49.9 (8.3) | 3 (0.3) |
| Age at menarche (years) 1 | 13.1 (1.5) | 13.1 (1.5) | 97 (8.9) |
| Weight (kg) 1 | 65.9 (12.7) | 60.9 (12.3) | 263 (24.0) |
| Height (cm) 1 | 155.2 (6.1) | 155.3 (6.5) | 692 (63.0) |
| Body mass index 1 | 27.7 (5.7) | 25.6 (5.2) | 696 (64.0) |
| Number of children 1 | 4 (2.5) | 3 (2.6) | 529 (48.0) |
| Race | 34 (3.1) | ||
| Others | 9 (1.5) | 8 (1.7) | |
| Chinese | 56 (9.6) | 77 (16.2) | |
| Malay | 517 (88.8) | 390 (82.1) | |
| Menopause status | 0 (0.0) | ||
| No | 215 (36.3) | 309 (61.9) | |
| Yes | 377 (63.7) | 190 (38.1) | |
| Family history | 520 (48.0) | ||
| No | 249 (81.1) | 204 (77.3) | |
| Yes | 58 (18.9) | 60 (22.7) | |
| BC-HR | 51 (4.7) | ||
| No | 374 (65.7) | 310 (65.8) | |
| Yes | 195 (34.3) | 161 (34.2) | |
| TAHBSO | 70 (6.4) | ||
| No | 498 (89.1) | 409 (88.5) | |
| Yes | 61 (10.9) | 53 (11.5) | |
| BI-RADS classification | 0 (0.0) | ||
| 1 | 93 (15.7) | 52 (10.4) | |
| 2 | 379 (64.0) | 288 (57.7) | |
| 3 | 59 (10.0) | 78 (15.6) | |
| 4 | 23 (3.9) | 44 (8.8) | |
| 5 | 32 (5.4) | 30 (6.0) | |
| 6 | 6 (1.0) | 7 (1.4) | |
| Diagnosis | 0 (0.0) | ||
| Normal | 124 (20.9) | 106 (21.2) | |
| Benign | 444 (75.0) | 368 (73.7) | |
| Malignant | 24 (4.1) | 25 (5.0) |
| Variables | OR | 95% (CI) | p-Value |
|---|---|---|---|
| Age | 0.93 | 0.92, 0.95 | <0.001 |
| Age at menarche | 1.02 | 0.94, 1.11 | 0.643 |
| Weight | 0.97 | 0.95, 0.98 | <0.001 |
| Height | 1.01 | 0.99, 1.04 | 0.313 |
| Body mass index | 0.91 | 0.88, 0.94 | <0.001 |
| Number of children | 0.88 | 0.82, 0.94 | <0.001 |
| Race | |||
| Others | - | - | |
| Chinese | 1.57 | 0.57, 4.35 | 0.383 |
| Malay | 0.84 | 0.32, 2.20 | 0.720 |
| Menopause status | |||
| No | - | - | |
| Yes | 0.35 | 0.27, 0.45 | <0.001 |
| Family history | |||
| No | - | - | |
| Yes | 1.26 | 0.87, 1.83 | 0.227 |
| BC-HR | |||
| No | - | - | |
| Yes | 0.98 | 0.76, 1.27 | 0.880 |
| TAHBSO | |||
| No | - | - | |
| Yes | 1.04 | 0.71, 1.54 | 0.831 |
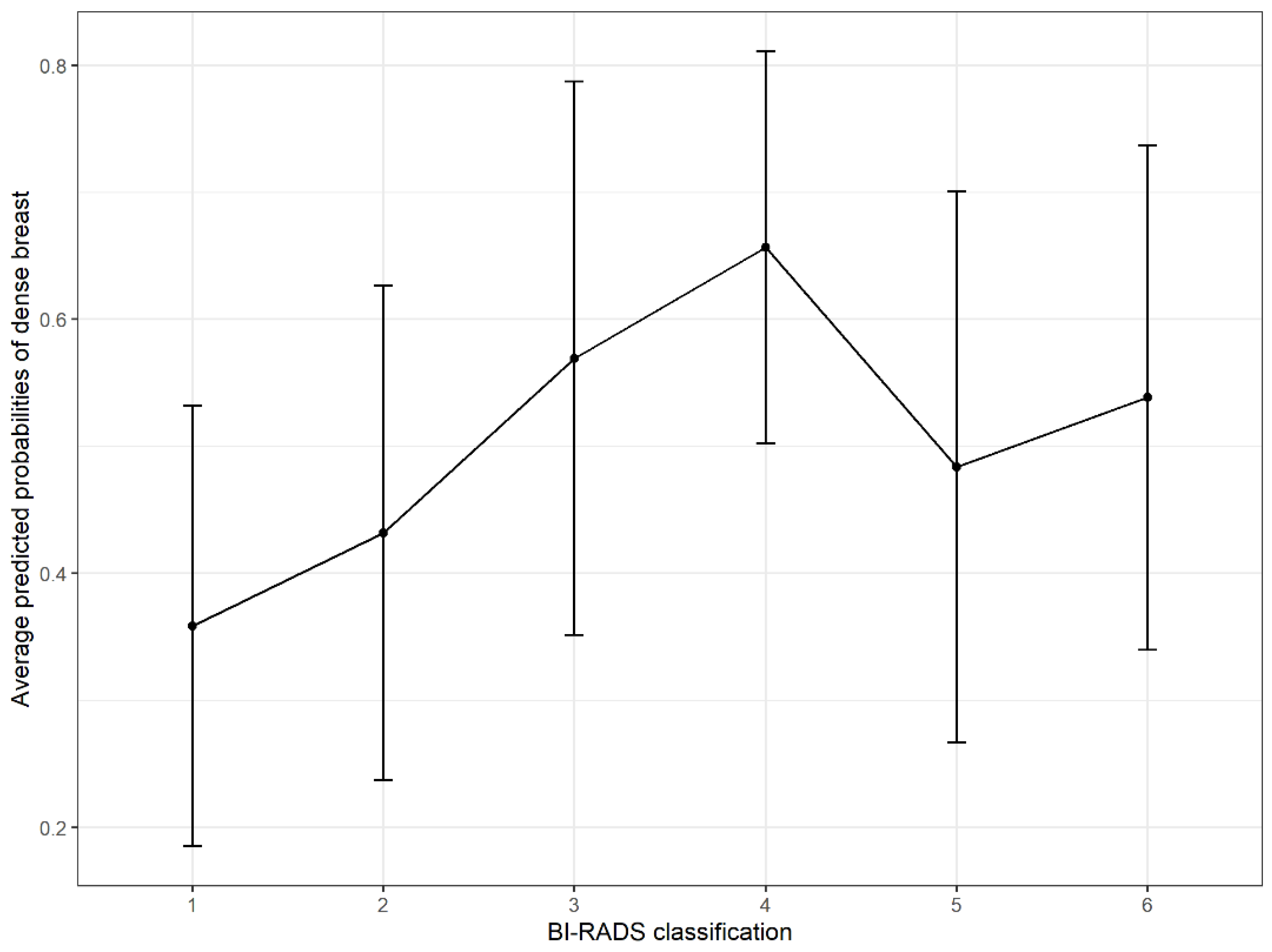
| BI-RADS classification | |||
| 1 | - | - | |
| 2 | 1.36 | 0.94, 1.97 | 0.107 |
| 3 | 2.36 | 1.46, 3.82 | <0.001 |
| 4 | 3.42 | 1.86, 6.29 | <0.001 |
| 5 | 1.68 | 0.92, 3.07 | 0.093 |
| 6 | 2.09 | 0.67, 6.55 | 0.207 |
| Diagnosis | |||
| Normal | - | - | |
| Benign | 0.97 | 0.72, 1.30 | 0.837 |
| Malignant | 1.22 | 0.66, 2.26 | 0.530 |
| Variables | OR | 95% (CI) | p-Value |
|---|---|---|---|
| Age | 0.94 | 0.92, 0.96 | <0.001 |
| Number of children | 0.88 | 0.81, 0.96 | 0.003 |
| Body mass index | 0.88 | 0.85, 0.92 | <0.001 |
| Menopause status | |||
| No | - | - | |
| Yes | 0.59 | 0.42, 0.82 | 0.002 |
| BI-RADS classification | |||
| 1 | - | - | |
| 2 | 1.87 | 1.22, 2.84 | 0.004 |
| 3 | 3.25 | 1.86, 5.66 | <0.001 |
| 4 | 3.75 | 1.88, 7.46 | <0.001 |
| 5 | 2.46 | 1.21, 5.02 | 0.013 |
| 6 | 2.50 | 0.65, 9.56 | 0.180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanis, T.M.; Arifin, W.N.; Haron, J.; Wan Abdul Rahman, W.F.; Ruhaiyem, N.I.R.; Abdullah, R.; Musa, K.I. Factors Influencing Mammographic Density in Asian Women: A Retrospective Cohort Study in the Northeast Region of Peninsular Malaysia. Diagnostics 2022, 12, 860. https://doi.org/10.3390/diagnostics12040860
Hanis TM, Arifin WN, Haron J, Wan Abdul Rahman WF, Ruhaiyem NIR, Abdullah R, Musa KI. Factors Influencing Mammographic Density in Asian Women: A Retrospective Cohort Study in the Northeast Region of Peninsular Malaysia. Diagnostics. 2022; 12(4):860. https://doi.org/10.3390/diagnostics12040860
Chicago/Turabian StyleHanis, Tengku Muhammad, Wan Nor Arifin, Juhara Haron, Wan Faiziah Wan Abdul Rahman, Nur Intan Raihana Ruhaiyem, Rosni Abdullah, and Kamarul Imran Musa. 2022. "Factors Influencing Mammographic Density in Asian Women: A Retrospective Cohort Study in the Northeast Region of Peninsular Malaysia" Diagnostics 12, no. 4: 860. https://doi.org/10.3390/diagnostics12040860









