1. Introduction
Several models have been proposed for predicting radiation induced lung disease, e.g., [
1,
2,
3,
4]; however, there remain no clinically verified models that quantitatively predict lung function impairment after radiotherapy (RT) [
5,
6]. The reason for this is that the reported injury rate is highly dependent on the endpoint considered [
7]. In this context, predictive clinical factors for the extent of fibrosis following RT can help in terms of patient selection, while dosimetric parameters can guide plan optimization [
8].
Pulmonary function tests (PFTs) are generally considered to be good surrogate markers for assessing radiation-induced pulmonary impairment [
5] as they can be assessed more objectively than clinical endpoints [
5]. Setup inaccuracies and patient compliance may bias PFTs; a reproducibility within a ±10% margin has been reported [
9]. Nevertheless PFTs are regarded as a clinically established method for assessing lung function [
10].
When comparing the density values of CTs acquired at different time points, it has been shown that an increase in density occurs in areas with a radiation dose of more than 6 Gy [
11]. Although CT density changes after thoracic irradiation are histopathologically correlated with lung fibrosis [
12,
13], it is not completely clear how these changes are related to lung function parameters. A previous study [
7] found only a weak correlation between CT density and PFT dynamics before and after radiation treatment, which led the authors to question the usefulness of lung function testing prior to RT in general.
The aim of the present retrospective study was to correlate the dynamics of carbon monoxide diffusing capacity (DL
) and FEV
, which are the most frequently used lung function parameters in clinic, with those of CT density. In accordance with Ma et al. [
7], we hypothesized that a decline in PFT values would be correlated with an increase in lung tissue density. The focus of the analysis was on differential volumes encompassed by specific isodoses rather than on total lung volume. By clarifying the relation between CT-morphology and lung function dynamics, this study is intended to contribute to better understanding of the development of radiation-induced lung disease.
4. Discussion
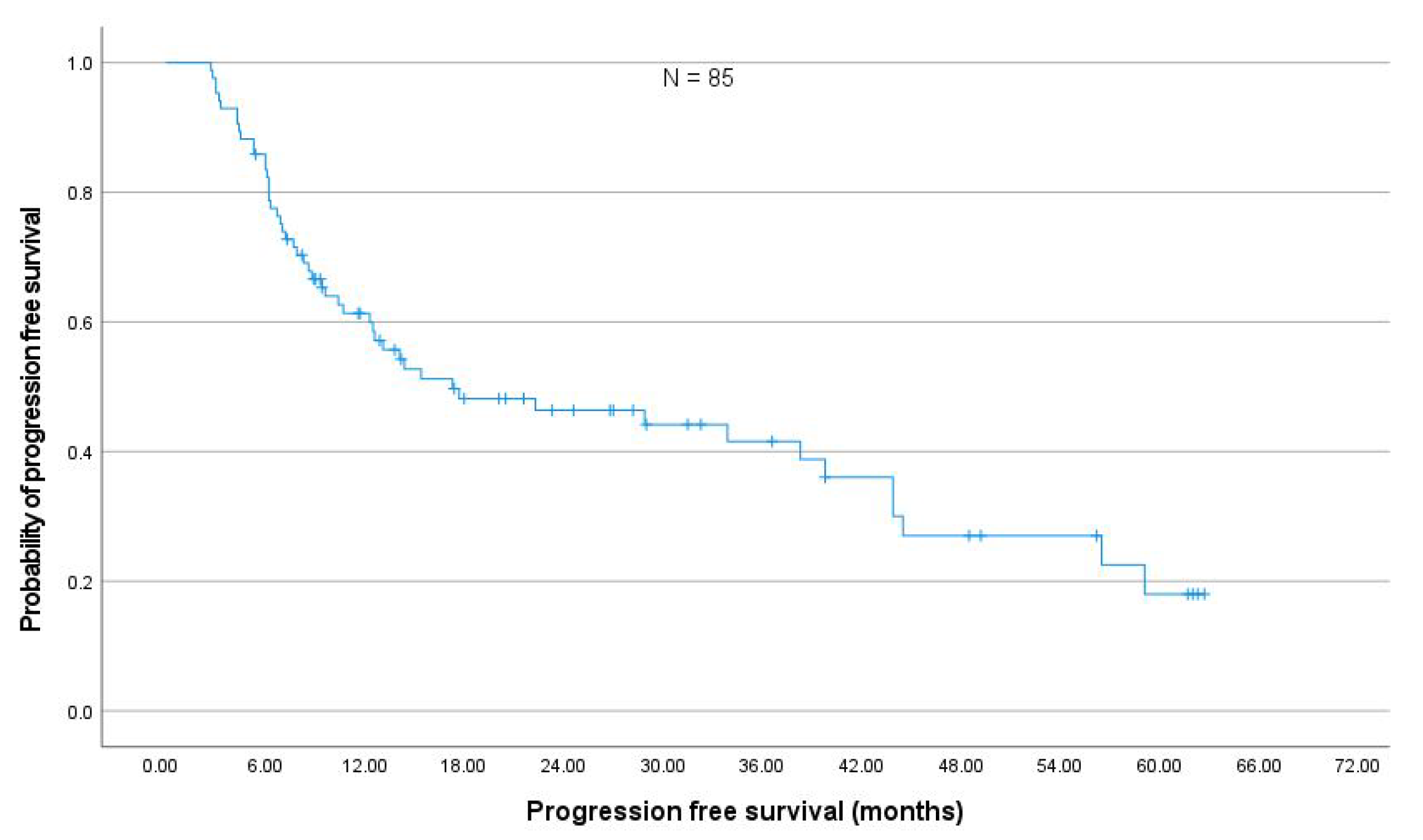
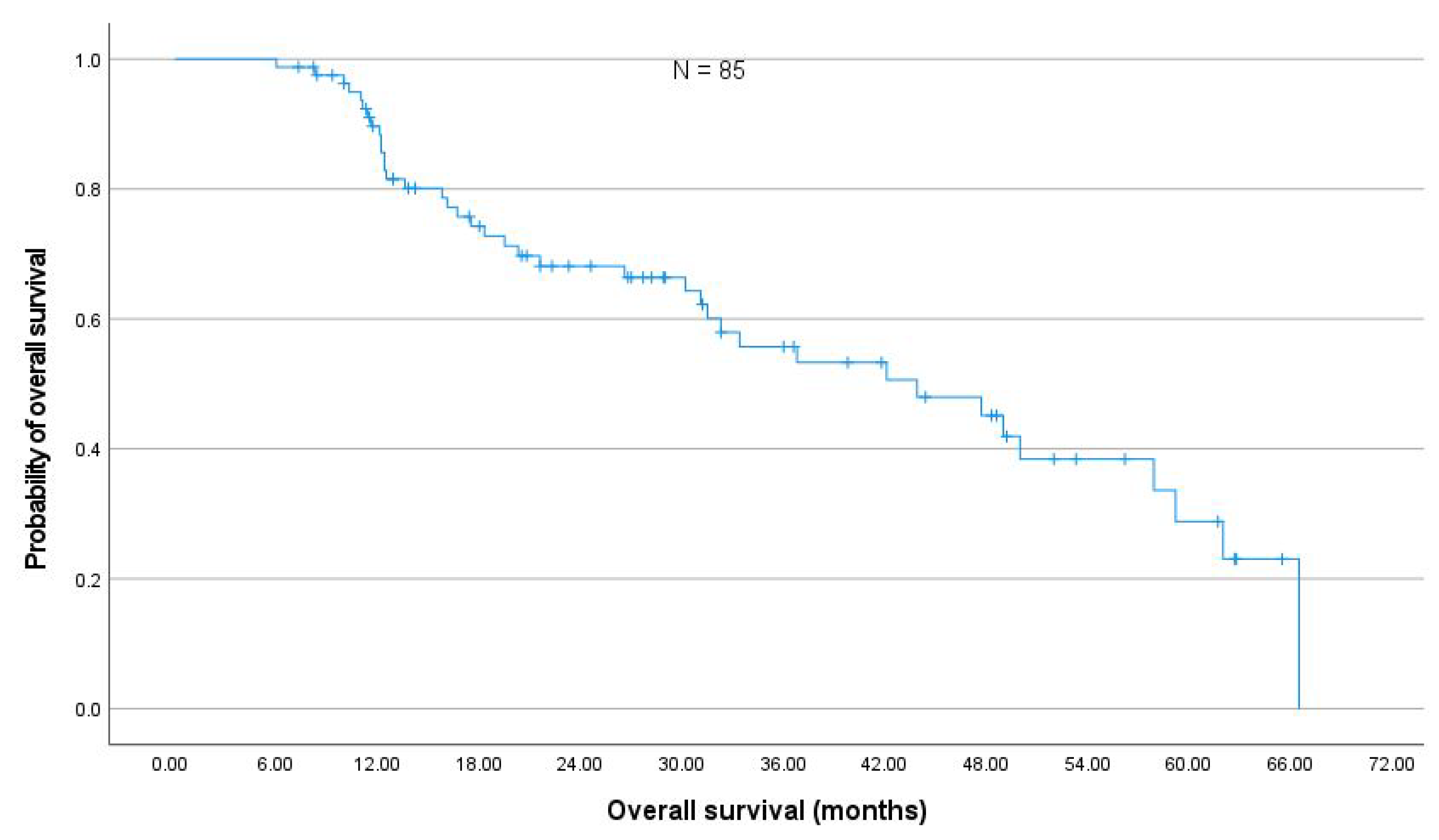
In a representative cohort of 85 NSCLC stage III patients comparable to prospective studies [
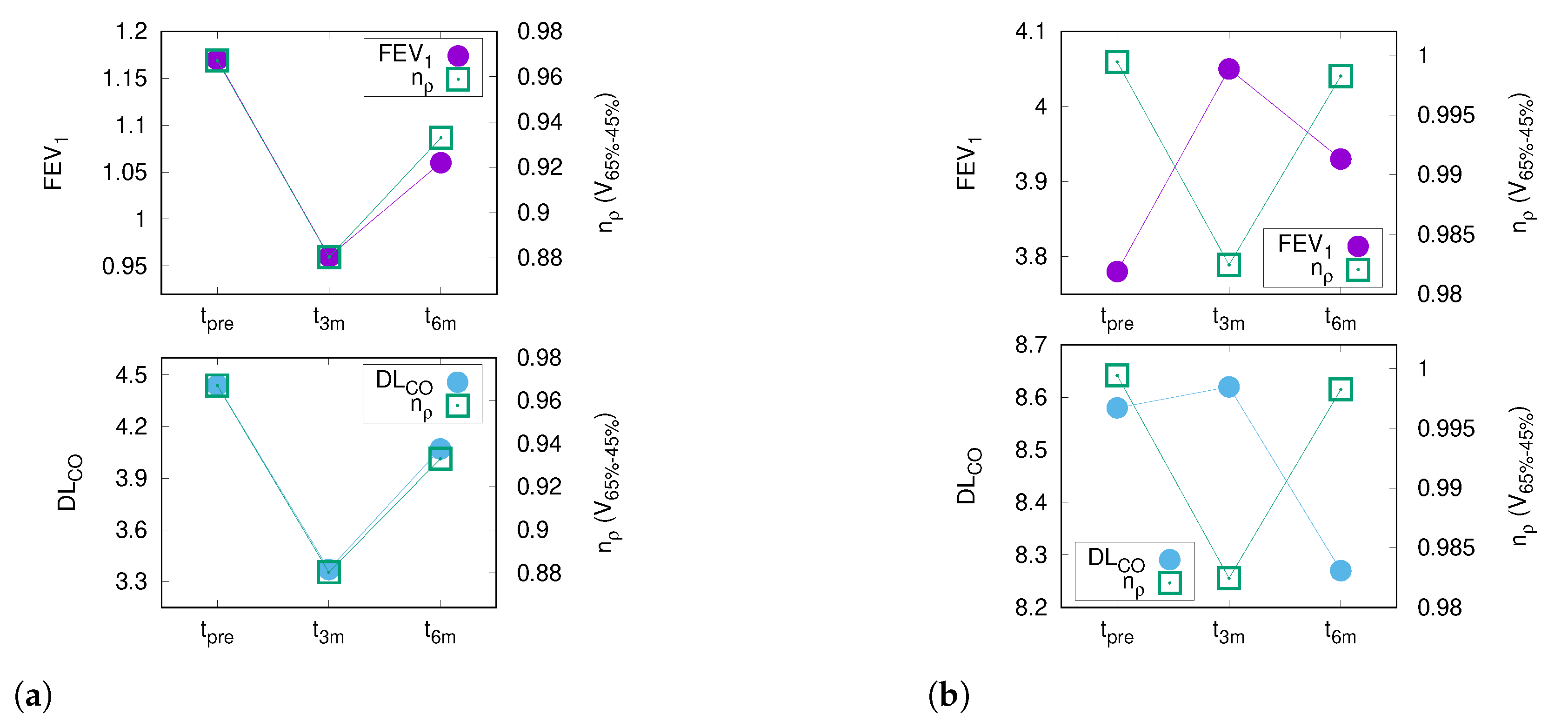
17], the changes in PFT after thoracic RT were moderate. The absolute median decline in FEV
and DL
was less than 5% within six months after the end of RT, which corroborates previously published results by Grambozov et al. [
14] (see
Appendix A Figure A4). The fact that the PFTs were the only significant predictors for pneumonitis in MVA underlines the clinical importance of pre-therapeutic PFTs.
The current study showed a significant correlation between PFTs and CT morphology changes after RT. Of note, the best correlation was found for DL
and the differential lung dose volume between the 65%- and 45%-isodose (V
), which remained significant after Bonferroni correction for multiple testing. In general, the most significant relations (
p < 0.01) between CT morphology and PFT were found for differential volumes excluding the high-dose area. This finding is not counter-intuitive, as post-therapeutic tumor shrinkage followed by the development of fibrosis is an obviously unpredictable physiological process. This is additionally in line with the clinical practice of excluding the PTV/GTV from the delineation of the lungs as organs at risk in order to safely predict the probability of pneumonitis (e.g., [
7,
23,
24,
25]).
Global lung function is represented by PFT parameters such as FEV
and DL
; therefore we hypothesized that regional radiographic density increases, i.e., fibrosis, correlates with PFT decline. Published data on the comparison of PFTs and post-RT fibrosis in the lung are scarce and conflicting. Ma et al., in their cohort of 111 patients, found only a weak correlation between PFTs and CT morphology, with correlation coefficients (CC) between 0.20–0.37 [
7]. Interestingly, FEV
showed higher CCs (0.30–0.37) than DL
(0.17–0.29) [
7]. As 91 patients in this cohort were operated on, the higher CC between FEV
and CT morphology changes can be explained by the fact that a decrease in FEV
primarily represents a reduction of the airways, which is the case when parts of the lung (including bronchi and bronchioli) are surgically removed.
Ma et al. included patients with only one post-therapeutic measurement, while in our analysis dCTs at three well-defined time points were compared. Additionally, these three CTs were acquired in the same position, which significantly enhanced the registration accuracy compared to Ma et al., who co-registered the pCT with one dCT. Furthermore, in contrast to Ma et al. we analyzed differential volumes, which may be a better approach than considering total lung volume. Finally, we used an individualized patient approach; each patient in our study was his/her own control. These methodological differences may explain why the CCs in our study were almost twice as high as in the investigation by Ma et al. (0.57 for DL and 0.47 for FEV). Therefore, we cannot share the viewpoint held by Ma et al. that the validity of PFTs prior to RT is questionable.
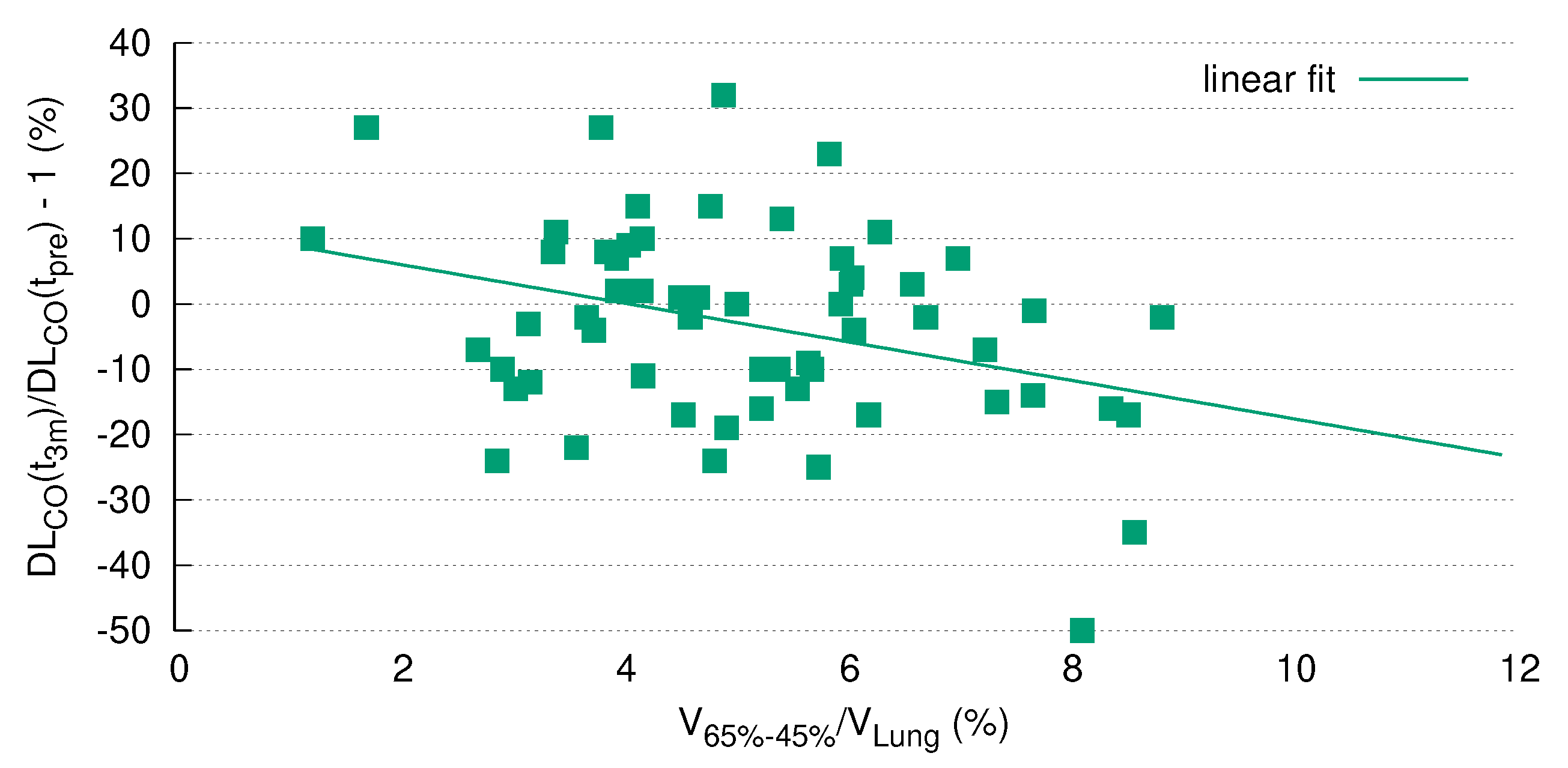
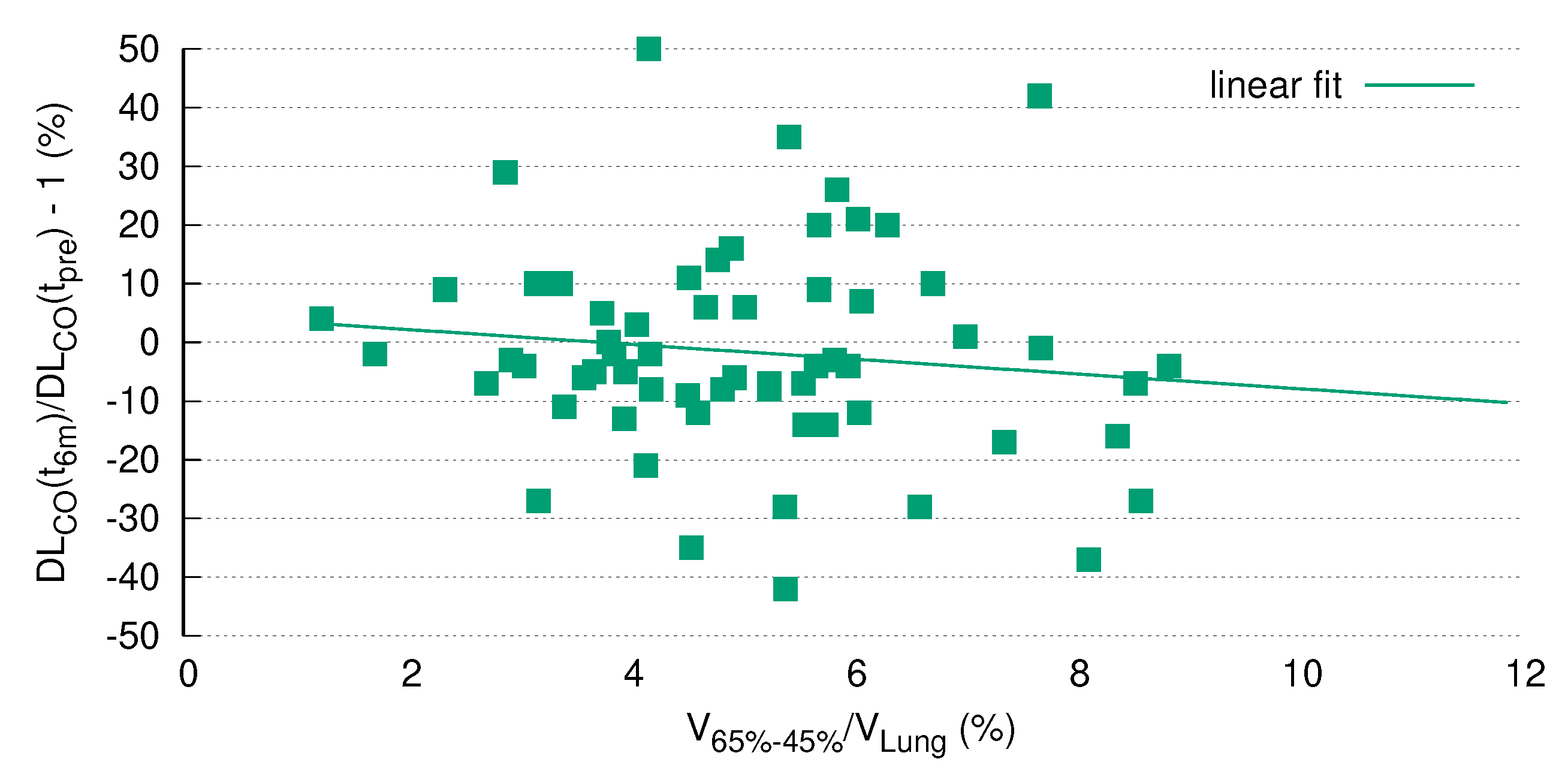
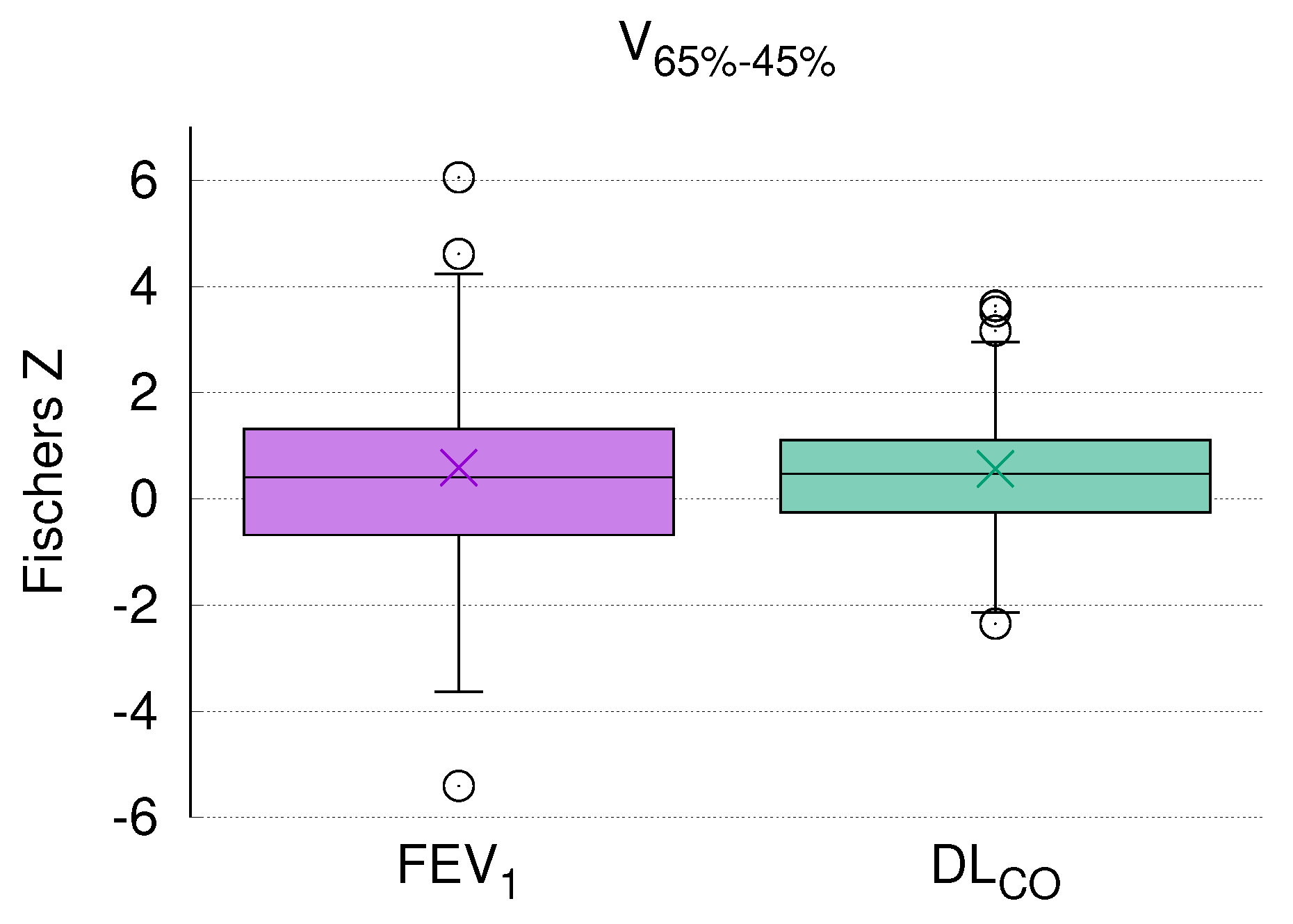
The current analysis clearly demonstrated a highly significant correlation of DL
dynamics with the size of a specific radiation volume. Of note, this correlation remained statistically significant after Bonferroni correction for multiple testing (
Figure 5), which strongly argues in favor of pre-therapeutic PFTs as a prerequisite for safe thoracic RT. Although this issue is a matter of ongoing debate, our results are in line with other studies in the field (see the review by Niezink et al. [
5]).
A retrospective study of 99 patients by Brennan et al. revealed a CC of 0.7 between 4DCT ventilation metrics measured in HU and FEV
[
10], which is on the same order of magnitude as in our study. In this sense, the 4DCT metrics provide additional information on lung function and help with treatment planning to spare critical parts of the lungs in order to retain as much of the vital lung tissue as possible. Major differences of this study compared to ours are the use of 4DCT technology and patient selection with only 60% stage III NSCLC [
10].
As mentioned previously, investigations correlating PFTs and CT density in the context of radiation therapy are scarce. Therefore, data on combined pulmonary fibrosis and emphysema (CPFE) may serve as a model. Individuals with this disease have mild airflow limitation combined with a decline in DL
, which is more pronounced than in patients with idiopathic pulmonary fibrosis (IPF) or emphysema alone [
26]. Both fibrosis and emphysema are areas of low gas exchange; hence, it seems plausible to envisage radiation-induced lung disease as a combination of radiation-induced fibrosis and pre-existing emphysema based on COPD. It is noteworthy that more than half of the patients included in the current analysis had COPD to a certain degree; see
Table 1.
The first analysis in the field, conducted by Heremans et al., dates back 30 years [
19]. In their 45 patients, the authors described a correlation between FEV
, measured airway obstruction, and CT density changes. As opposed to later investigations which differentiated low (LAA) from high attenuation areas (HAA) [
20,
21], this pioneering work by Heremans et al. analyzed total lung density [
19]. In a study by Matsuoka et al. [
20] conducted in 43 CPFE patients, fibrotic and emphysematous areas were defined by HU 0-700 (HAA) and HU <−950 (LAA), respectively. The predictive power of HAA with respect to global lung function measured by DL
was highly significant (
p < 0.0001). These results were corroborated in two other studies [
27,
28] including approximately 20 patients each. The authors argue that the physiological explanation for the pronounced decline in DL
could be a reduction in surface area caused by emphysema and fibrosis, which coincides with our results. Therefore, the percentage of HAA, i.e., fibrotic areas, could be a surrogate for global lung function measured by DL
.
With due caution, the limited reports available thus far together with the data presented here allow us to assume that a relation between global lung function and regional morphologic changes after RT exists. As that in the current study almost 75% of the patients had a positive correlation between CT density and DL
(
Figure 3), it seems that this parameter presents global lung function after RT more accurately than FEV
.
The current study has several obvious limitations. First, PFTs depend on patient compliance and are therefore error-prone. We tried to avoid this bias by measuring lung function at three well defined points of time with each patient as his/her own control. Second, the model-based segmentation algorithm implemented in RayStation
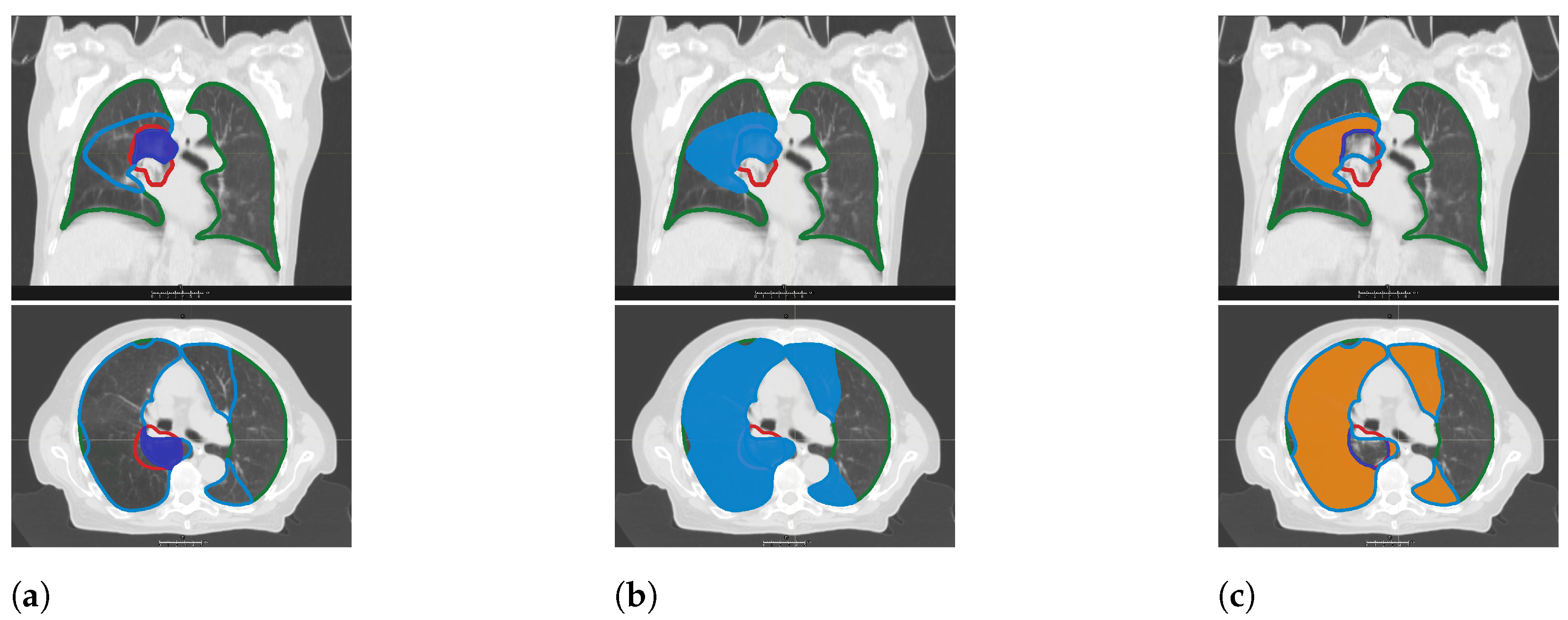
® used for contouring of the lungs on each dCT might lead to uncertainties in areas where the tissue adjacent to the lung has a low electron density. Third, the acquisition of the CT data in different patient positions, with or without contrast medium, and on various types of scanners may be a source of errors. In the current study, three dCTs were matched to the pCT. This represents an inherent systematic error characteristic of all studies in the field. Finally, normalization of HU values to the total lung volume could be a problem if denser areas such as the GTV are included for certain points in time and not for others (which, for instance, reflects tumor retraction after treatment). The relative error due to this type of inaccuracy based on tissue changes in the highest dose region (V
) decreases with increasing size of the volume examined (
Table 3). The focus of the current analysis, however, was placed on the mid-dose levels.
Despite these shortcomings, a significant correlation between CT density changes and DL was found.