The Role of Urinary VEGF in Observational Studies of BPS/IC Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
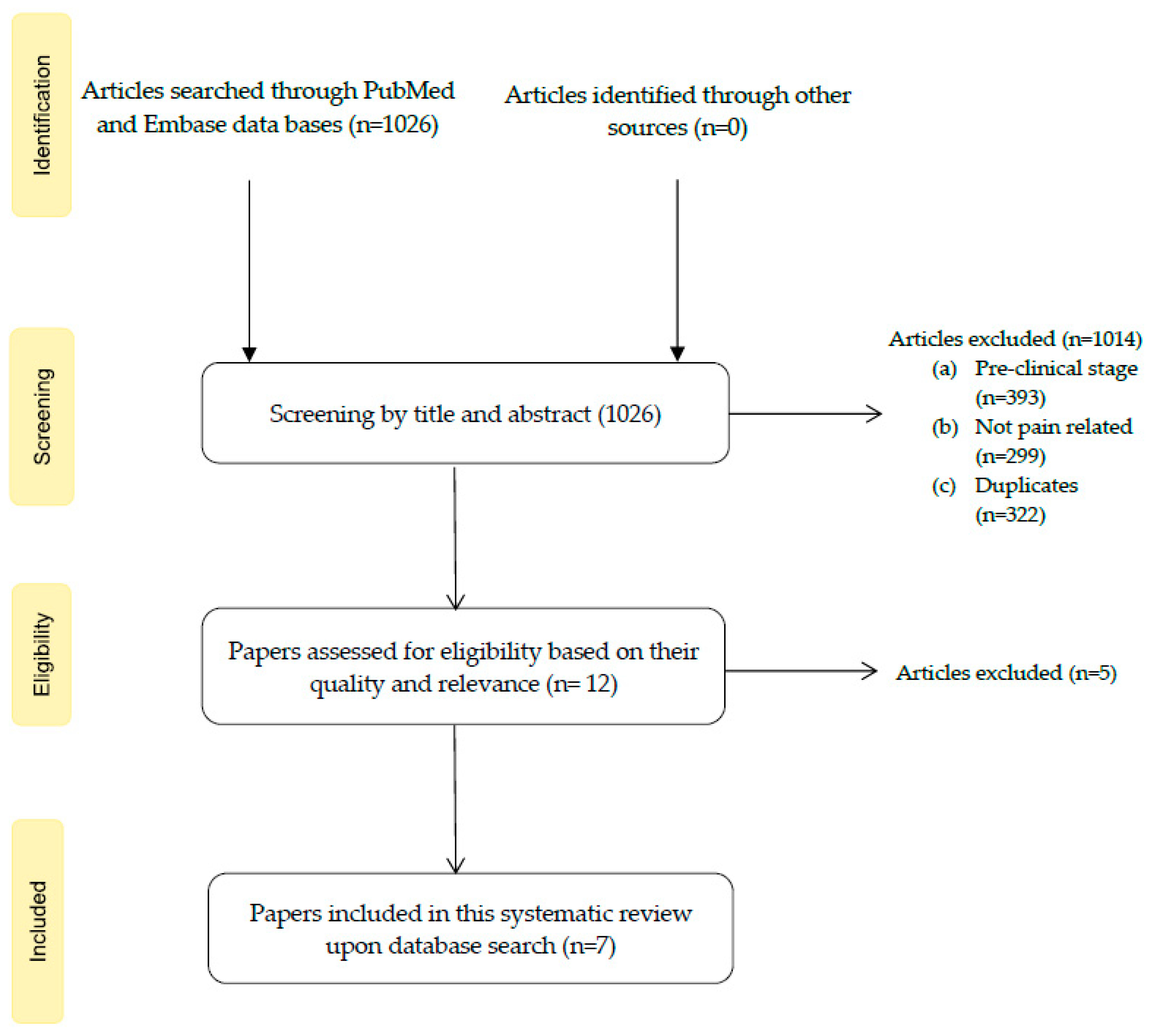
3.1. Study Selection
3.2. Studies’ Overall Characteristics
3.3. Individual Characteristics, Findings and Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Title | Selection | Comparability | Results | Final Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts Based on the Design or Analysis | Assessment of Outcome | Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | ||
| Association of Longitudinal Changes in Symptoms and Urinary Biomarkers in Patients with Urological Chronic Pelvic Pain Syndrome: A MAPP Research Network Study | (1) | - | (1) | - | (2) | (1) | (1) | (1) | 7/9 |
| Relationship of Pain Catastrophizing With Urinary Biomarkers in Women With Bladder Pain Syndrome | Not enough data in study´s abstract for quality assessment | ||||||||
| Title | Selection | Comparability | Results | Final Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case Definition Adequate | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls Based on the Design or Analysis | Ascertainment for Exposure | Same Method of Ascertainment in Cases/Controls | Non-Response Rate | ||
| Identification of novel non-invasive biomarkers of urinary chronic pelvic pain syndrome: findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network | (1) | - | (1) | (1) | (2) | (1) | (1) | (1) | 8/9 |
| Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder | (1) | (1) | - | - | (2) | (1) | (1) | (1) | 7/9 |
| Angiogenesis in bladder tissues is strongly correlated with urinary frequency and bladder pain in patients with interstitial cystitis/bladder pain syndrome | (1) | (1) | - | - | (1) | (1) | (1) | (1) | 6/9 |
| Molecular Taxonomy of Interstitial Cystitis/Bladder Pain Syndrome Based on Whole Transcriptome Profiling by Next-Generation RNA Sequencing of Bladder Mucosal Biopsies | (1) | (1) | (1) | (1) | (1) | (1) | - | (1) | 7/9 |
| Title | Relationship of Bladder Pain with Clinical and Urinary Markers of Neuroinflammation in Women with Urinary Urgency without Urinary Incontinence | ||
|---|---|---|---|
| Yes | No | Not Applicable | |
| Were the criteria for inclusion in the sample clearly defined? | (1) | ||
| Were the study subjects and the setting described in detail? | (1) | ||
| Was the exposure measured in a valid and reliable way? | (1) | ||
| Were objective, standard criteria used for measurement of the condition? | (1) | ||
| Were any confounding factors identified? | (1) | ||
| Were strategies to deal with confounding factors stated? | (1) | ||
| Were the outcomes measured in a valid and reliable way? | (1) | ||
| Was appropriate statistical analysis used? | (1) | ||
| Total | 8/8 | ||
| Overall Appraisal | Included  | Excluded  | Seek further information  |
Appendix B
| Title | Author Year | Study Design | Sample Size | Sample Characteristics | Study Aim | Quality Assessment |
|---|---|---|---|---|---|---|
| Identification of novel non-invasive biomarkers of urinary chronic pelvic pain syndrome: findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network | A. Dagher 2017 | Case-control | 491 | UCPPS: 120 male and 139 female participants (Mean age = 43.3 y); Race: 229 white and 10 black; Ethnicity: 19 Hispanic and 239 Non-Hispanic HC group: 50 male and 75 female participants (Mean age = 42.5 y); Race: 90 white and 16 black; Ethnicity: 8 Hispanic and 117 Non-Hispanic PC group: 32 male and 75 female participants (Mean age = 41.6 y); Race: 76 white and 12 black; Ethnicity: 10 Hispanic and 96 Non-Hispanic | To compare urinary levels of VEGF/VEGF-R1 and NGAL, MMP-9/NGAL complex between UCPPS, patients and HC and PC patients while correlating them with symptom severity | 8/9 |
| Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder | A. Furuta 2018 | Case-control | 88 | OAB: 28 patients (7 male; 21 female); Mean age (57.7 y); BPS: 30 patients (9 male; 21 female); Mean age (60.2 y); IC: 30 patients (9 male; 21 female); Mean age (63.5 y) | To investigate the contributing factors of chronic inflammation in IC patients | 7/9 |
| Angiogenesis in bladder tissues is strongly correlated with urinary frequency and bladder pain in patients with interstitial cystitis/bladder pain | A. Furuta 2019 | Case-control | 36 | Control: 12 females (Mean age 56.7 y); BPS: 12 females (Mean age 53.3 y); IC: 12 females (Mean age 57.0 y) | To clarify the correlation among bladder inflammation, angiogenesis, fibrosis and urothelial denudation using immsunohistochemistry, OSSI, OSPI and VAS pain scores | 6/9 |
| Molecular Taxonomy of Interstitial Cystitis/Bladder Pain Syndrome Based on Whole Transcriptome Profiling by Next-Generation RNA Sequencing of Bladder Mucosal Biopsies | Y. Akiyama 2019 | Case-control | 42 | IC: 12 patients (Mean age: 70.5 y) BPS: 11 patients (Mean age: 58.8 y) Hypersensitive bladder: 10 patients (Mean age: 63.5 y) Control group: 9 patients (Mean age: 64.6 y) | To perform transcriptome of BPS/IC subtypes and non-BPS/IC subtypes controls, providing the basis of a molecular taxonomy of BPS/IC, and explore biological pathways specifically affected in each subtype | 7/9 |
| Association of Longitudinal Changes in Symptoms and Urinary Biomarkers in Patients with Urological Chronic Pelvic Pain Syndrome: A MAPP Research Network Study | R. Roy 2021 | Prospective cohort | 216 | UCPPS patients: 116 female and 100 male patients | To analyze a series of non-invasive biomarkers for their ability to objectively monitor the longitudinal clinical status of UCPPS patients | 7/9 |
| Relationship of Bladder Pain with Clinical and Urinary Markers of Neuroinflammation in Women with Urinary Urgency Without Urinary Incontinence | A. Soriano 2021 | Cross-sectional | 101 | NBP (Mean age = 48.9): Caucasian: 32 patients; African American: 4 patients; Other: 2 patients BP (Mean age = 44.6): Caucasian: 55 patients; African American: 5 patients; Other: 2 patients | To assess if bladder pain is associated with presence of neurogenic inflammation in the bladder wall and neuroinflammatory biomarkers in the urine in women with urinary urgency without incontinence | 8/8 |
| Relationship of Pain Catastrophizing With Urinary Biomarkers in Women With Bladder Pain Syndrome | A. Soriano 2021 | Cohort | 62 | Women who met AUA criteria of bladder pain syndrome | To assess greater pain catastrophizing with higher urinary biomarkers (VEGF and BDNF) levels in women with bladder pain syndrome | No quality assessment possible |
| Title | VEGF/VEGF-R1 Levels in Groups | VEGF/VEGF-R1 Levels’ Relations with Symptoms | Questionnaire Scores | Limitations |
|---|---|---|---|---|
| Identification of novel non-invasive biomarkers of urinary chronic pelvic pain syndrome: findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network | VEGF concentration: Females: (mean UCPPS = 3.72, mean HC = 3.46; p = 0.0076) Males: (mean UCPPS = 1.78, mean HC = 0.98, p < 0.0001) VEGF-R1 concentration: Males: (mean UCPPS = −0.33, mean HC = −1.25, p = 0.004) | Relation between VEGF concentrations and Pain severity: Females: β = 1.280, p = 0.0127; Males: β = −0.692, p = 0.0627 Urinary severity: Females: β = 1.125, p = 0.0378; Males: β = −1.070, p = 0.0126 Relation between VEGF-R1 concentrations and Pain severity: Females: β = 0.366, p = 0.2198; Males: β = −0.257, p = 0.2638 Urinary severity: Females: β = 0.117, p = 0.7092; Males: β = 0.038 p = 0.8851 | - | Only UCPPS with more severe urologic pain were included |
| Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder | VEGF levels: OAB (Mean 19.5 ± 7.5) BPS (Mean 69.7 ± 40.1) * IC (Mean 48.7 ± 28.1) * | - | OAB (OSSI-1.1 ± 1.1; OSPI: 0.6 ± 1.1; VAS: 0.4 ± 0.9) IC (OSSI: 10.3 ± 5.5 *; OSPI: 9.0 ± 4.8 *; VAS: 5.5 ± 3.3 *) BPS (OSSI: BPS:8.1 ± 4.4 *; OSPI: 7.6 ± 4.9 *; VAS: 4.8 ± 3.3 *) | Small sample size Previous bladder hydrodistension history might influence results |
| Angiogenesis in bladder tissues is strongly correlated with urinary frequency and bladder pain in patients with interstitial cystitis/bladder pain | VEGF expression levels in immunohistochemical staining: Increase in IC (p < 0.01) and BPS (p < 0.01) vs. controls | - | Control (OSSI-0.9 ± 0.9; OSPI: 0.0 ± 0.0; VAS: 0.1 ± 0.2) IC (OSSI: 15.9 ± 2.6 **; OSPI: 15.0 ± 1.2 **; VAS: 9.4 ± 0.9 **); BPS (OSSI:9.3 ± 4.7 **; OSPI: 8.5 ± 4.7 **; VAS: 5.9 ± 2.1 **) | Small sample size |
| Molecular Taxonomy of Interstitial Cystitis/Bladder Pain Syndrome Based on Whole Transcriptome Profiling by Next-Generation RNA Sequencing of Bladder Mucosal Biopsies | VEGF expression in bladder biopsies: BPS: Median 0.014; 99% CI 0.013–0.018 HSB: Median 0.016; 99% CI 0.012–0.024 Controls: Median 0.014; 99% CI 0.014–0.018 IC: Median 0.412; 99% CI 0.035–0.048, p = 0.002 vs. BPS; p = 0.004 vs. HSB; p = 0.007 vs. controls | VEGF expression in IC patients correlated with: - Storage symptoms (ρ = 0.47; p < 0.01); - Voiding symptoms (ρ = 0.40; p = 0.02) - Daytime frequency (ρ = 0.35; p = 0.03) - Nocturia (ρ = 0.40; p = 0.01) - Decrease in hydrodistension (ρ = −0.46; p < 0.01) | OSSI: (IC: 15.8 ± 3.7; BPS: 12.3 ± 3.0; HSB: 9.8 ± 4.8; control: 3.3 ± 1.4) OSPI: (IC: 13.3 ± 2.1; BPS: 12.4 ± 2.3; HSB: 10.7 ± 2.6; control: 1.8 ± 2.0) VAS pain score: (IC: 8.2 ± 2.3; BPS: 5.7 ± 3.3; HSB: 4.9 ± 2.4; control: 0) Significant differences (p < 0.01) between all groups within each questionnaire | Small sample size Use of some inadequate biopsy samples |
| Association of Longitudinal Changes in Symptoms and Urinary Biomarkers in Patients with Urological Chronic Pelvic Pain Syndrome: A MAPP Research Network Study | Relation between VEGF levels and symptom improvement: Pain severity: OR = 0.78 (p = 0.03) Urinary severity: OR = 1.06 (p = 0.62) Relation between VEGF-R1 levels and symptom improvement: Pain severity: OR = 0.88 (p = 0.06) Urinary severity: OR = 0.92 (p = 0.23) | Suboptimal reliability data concerning some of the biomarkers | ||
| Relationship of Bladder Pain with Clinical and Urinary Markers of Neuroinflammation in Women with Urinary Urgency Without Urinary Incontinence | VEGF levels association with: - Urgency: β 0.09, 95% CI −0.12–0.29 (p = 0.39) - Bladder pain: β 0.04, 95% CI 0.001–0.07 (p = 0.04) - Neuropathic pain: β 0.10 95% CI -0.15-0.35 (p = 0.42) | NBP: (F-GUPI: 12.3 ± 6.1; OSSI: 6.7 ± 3.4; OSPI: 4.9 ± 3.2; PainDETECT: 5.1 ± 4.8) BP: (F-GUPI: 28.1 ± 6.9 ***; OSSI: 9.8 ± 4.9 ***; OSPI: 8.7 ± 4.2 ***; PainDETECT: 13.3 ± 8.6 ***) | Small sample size No healthy patients group control | |
| Relationship of Pain Catastrophizing with Urinary Biomarkers in Women with Bladder Pain Syndrome | Lower VEGF levels: Higher pain catastrophizing (p = 0.03) Higher VEGF levels: Bladder pain (p = 0.01) | Higher catastrophizing scores: worse urinary symptoms and quality of life, greater pelvic and neuropathic pain scores (all p < 0.01) | Not reported in abstract |
References
- Berry, S.H.; Elliott, M.N.; Suttorp, M.; Bogart, L.M.; Stoto, M.A.; Eggers, P.; Nyberg, L.; Clemens, J.Q. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. J. Urol. 2011, 186, 540–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogart, L.M.; Berry, S.H.; Clemens, J.Q. Symptoms of Interstitial Cystitis, Painful Bladder Syndrome and Similar Diseases in Women: A Systematic Review. J. Urol. 2007, 177, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Ueda, T.; Tomoe, H.; Lin, A.T.; Kuo, H.-C.; Lee, M.-H.; Oh, S.-J.; Kim, J.C.; Lee, K.-S. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int. J. Urol. 2016, 23, 542–549. [Google Scholar] [CrossRef]
- Dell, J.R.; Mokrzycki, M.L.; Jayne, C.J. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.M.; Schulte, A.R.; Herndon, C.M. Interstitial Cystitis: An Update on the Disease Process and Treatment. J. Pain Palliat. Care Pharmacother. 2018, 32, 49–58. [Google Scholar] [CrossRef]
- Fall, M.; Baranowski, A.P.; Elneil, S.; Engeler, D.; Hughes, J.; Messelink, E.J.; Oberpenning, F.; Williams, A.C.D.C. EAU Guidelines on Chronic Pelvic Pain. Eur. Urol. 2010, 57, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, V.; Moldwin, R. Addressing quality of life in the patient with interstitial cystitis/bladder pain syndrome. Asian J. Urol. 2016, 4, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Luo, Y.; Hanno, P.M.; Maeda, D.; Homma, Y. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int. J. Urol. 2020, 27, 491–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homma, Y.; Akiyama, Y.; Tomoe, H.; Furuta, A.; Ueda, T.; Maeda, D.; Lin, A.T.; Kuo, H.-C.; Lee, M.-H.; Oh, S.-J.; et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2020, 27, 578–589. [Google Scholar] [CrossRef]
- Van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Faculty Opinions recommendation of Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2007, 53, 60–67. [Google Scholar] [CrossRef]
- Whitmore, K.E.; Fall, M.; Sengiku, A.; Tomoe, H.; Logadottir, Y.; Kim, Y.H. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2019, 26, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, M.; Saito, R.; Ogawa, O.; Yoshimura, N.; Ueda, T. Possible Mechanisms inducing Glomerulations in Interstitial Cystitis: Relationship between Endoscopic Findings and Expression of Angiogenic Growth Factors. J. Urol. 2004, 172, 945–948. [Google Scholar] [CrossRef]
- Towner, R.A.; Wisniewski, A.B.; Wu, D.H.; Van Gordon, S.B.; Smith, N.; North, J.C.; McElhaney, R.; Aston, C.E.; Shobeiri, S.A.; Kropp, B.P.; et al. A Feasibility Study to Determine Whether Clinical Contrast Enhanced Magnetic Resonance Imaging can Detect Increased Bladder Permeability in Patients with Interstitial Cystitis. J. Urol. 2016, 195, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Homma, T.; Igawa, Y.; Seki, S.; Ishizuka, O.; Imamura, T.; Akahane, S.; Homma, Y.; Nishizawa, O. CXCR3 Binding Chemokine and TNFSF14 Over Expression in Bladder Urothelium of Patients With Ulcerative Interstitial Cystitis. J. Urol. 2010, 183, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.T.; Yoshimura, N.; Tyagi, V.; Jacobs, B.; Leng, W.; Tyagi, P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J. Urol. 2012, 31, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Towner, R.A.; Smith, N.; Saunders, D.; Lerner, M.; Greenwood-Van Meerveld, B.; Hurst, R.E. Assessing bladder hyper-permeability biomarkers in vivo using molecularly-targeted MRI. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 57–65. [Google Scholar]
- Ruiz de Almodovar, C.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef]
- Saban, R. Angiogenic factors, bladder neuroplasticity and interstitial cystitis-new pathobiological insights. Transl Androl Urol. 2015, 4, 555–562. [Google Scholar]
- Lee, J.-D.; Lee, M.-H. Increased Expression of Hypoxia-inducible Factor-1α and Vascular Endothelial Growth Factor Associated With Glomerulation Formation in Patients With Interstitial Cystitis. Urology 2011, 78, 971.e11–971.e15. [Google Scholar] [CrossRef]
- Saban, M.R.; Davis, C.A.; Avelino, A.; Cruz, F.; Maier, J.; Bjorling, D.E.; Sferra, T.J.; Hurst, R.E.; Saban, R. VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG. BMC Physiol. 2011, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Claesson-Welsh, L. VEGF receptor signal transduction—A brief update. Vasc. Pharmacol. 2016, 86, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Valizadeh, M.R.; Hassanshahi, G.; Khorramdelazad, H. Expression of vascular endothelial growth factor and its receptors in infertile men with varicocele. J. Reprod. Immunol. 2020, 140, 103131. [Google Scholar] [CrossRef] [PubMed]
- Saban, R.; Saban, M.R.; Maier, J.; Fowler, B.; Tengowski, M.; Davis, C.A.; Wu, X.-R.; Culkin, D.J.; Hauser, P.; Backer, J.; et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am. J. Physiol. Physiol. 2008, 295, F1613–F1623. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, M. VEGF-VEGFR System as a Target for Suppressing Inflammation and other Diseases. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 135–144. [Google Scholar] [CrossRef]
- Failla, C.M.; Carbo, M.; Morea, V. Positive and Negative Regulation of Angiogenesis by Soluble Vascular Endothelial Growth Factor Receptor-1. Int. J. Mol. Sci. 2018, 19, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Lasserson, T.; Chandler, J.; Tovey, D.; Thomas, J.; Flemyng, E.C.R. Methodological Expectations of Cochrane Intervention Reviews (MECIR). 2022. Available online: https://community.cochrane.org/sites/default/files/uploads/MECIR%20PRINTED%20BOOKLET%20FINAL%20v1.01.pdf (accessed on 28 March 2022).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000. [Google Scholar]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Porritt, K.; Gomersall, J.; Lockwood, C. JBI’s systematic reviews: Study selection and critical appraisal. AJN Am. J. Nurs. 2014, 114, 47–52. [Google Scholar] [CrossRef]
- Dagher, A.; Curatolo, A.; Sachdev, M.; Stephens, A.J.; Mullins, C.; Landis, J.R.; van Bokhoven, A.; El-Hayek, A.; Froehlich, J.; Briscoe, A.C.; et al. Identification of novel non-invasive biomarkers of urinary chronic pelvic pain syndrome (UCPPS): Findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) research network. BJU Int. 2017, 120, 130. [Google Scholar] [CrossRef]
- Kind, T.; Cho, E.; Park, T.D.; Deng, N.; Liu, Z.; Lee, T.; Fiehn, O.; Kim, J. Interstitial Cystitis-Associated Urinary Metabolites Identified by Mass-Spectrometry Based Metabolomics Analysis. Sci. Rep. 2016, 6, 39227. [Google Scholar] [CrossRef] [Green Version]
- Furuta, A.; Suzuki, Y.; Igarashi, T.; Koike, Y.; Kimura, T.; Egawa, S.; Yoshimura, N. Angiogenesis in bladder tissues is strongly correlated with urinary frequency and bladder pain in patients with interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2019, 26, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, Y.; Maeda, D.; Katoh, H.; Morikawa, T.; Niimi, A.; Nomiya, A.; Sato, Y.; Kawai, T.; Goto, A.; Fujimura, T.; et al. Molecular Taxonomy of Interstitial Cystitis/Bladder Pain Syndrome Based on Whole Transcriptome Profiling by Next-Generation RNA Sequencing of Bladder Mucosal Biopsies. J. Urol. 2019, 202, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Stephens, A.J.; Daisy, C.; Merritt, L.; Newcomb, C.W.; Yang, J.; Dagher, A.; Curatolo, A.; Sachdev, M.; McNeish, B.; et al. Association of Longitudinal Changes in Symptoms and Urinary Biomarkers in Patients with Urological Chronic Pelvic Pain Syndrome: A MAPP Research Network Study. J. Urol. 2021, 205, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Andy, U.; Hassani, D.; Whitmore, K.; Harvie, H.; Malykhina, A.P.; Arya, L. Relationship of Bladder Pain With Clinical and Urinary Markers of Neuroinflammation in Women With Urinary Urgency Without Urinary Incontinence. Female Pelvic Med. Reconstr. Surg. 2020, 27, e418–e422. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Allen, A.; Malykhina, A.P.; Andy, U.; Harvie, H.; Arya, L. Relationship of Pain Catastrophizing With Urinary Biomarkers in Women With Bladder Pain Syndrome. Female Pelvic Med. Reconstr. Surg. 2021, 27, 746–752. [Google Scholar] [CrossRef]
- Landis, J.R.; Williams, D.A.; Lucia, M.S.; Clauw, D.J.; Naliboff, B.D.; Robinson, N.A.; van Bokhoven, A.; Sutcliffe, S.; Schaeffer, A.J.; Rodriguez, L.V.; et al. The MAPP research network: Design, patient characterization and operations. BMC Urol. 2014, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Clemens, J.Q.; Clauw, D.J.; Kreder, K.; Krieger, J.N.; Kusek, J.W.; Lai, H.H.; Rodriguez, L.; Williams, D.A.; Hou, X.; Stephens, A.; et al. Comparison of Baseline Urological Symptoms in Men and Women in the MAPP Research Cohort. J. Urol. 2015, 193, 1554–1558. [Google Scholar] [CrossRef] [Green Version]
- Chidlow, J.H., Jr.; Shukla, D.; Grisham, M.B.; Kevil, C.G. Pathogenic angiogenesis in IBD and experimental colitis: New ideas and therapeutic avenues. Am. J. Physiol. Liver Physiol. 2007, 293, G5–G18. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.H.; Shen, B.; Vijairania, P.; Zhang, X.; Vogt, S.K.; Gereau, I.V.R.W. Anti-vascular endothelial growth factor treatment decreases bladder pain in cyclophosphamide cystitis: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network animal model study. BJU Int. 2017, 120, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Fall, M.; Nordling, J.; Cervigni, M.; Oliveira, P.D.; Fariello, J.; Hanno, P.; Kåbjörn-Gustafsson, C.; Logadottir, Y.; Meijlink, J.; Mishra, N.; et al. Hunner lesion disease differs in diagnosis, treatment and outcome from bladder pain syndrome: An ESSIC working group report. Scand. J. Urol. 2020, 54, 91–98. [Google Scholar] [CrossRef]
- Maeda, D.; Akiyama, Y.; Morikawa, T.; Kunita, A.; Ota, Y.; Katoh, H.; Niimi, A.; Nomiya, A.; Ishikawa, S.; Goto, A.; et al. Hunner-Type (Classic) Interstitial Cystitis: A Distinct Inflammatory Disorder Characterized by Pancystitis, with Frequent Expansion of Clonal B-Cells and Epithelial Denudation. PLoS ONE 2015, 10, e0143316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abreu-Mendes, P.; Costa, A.; Charrua, A.; Pinto, R.A.; Cruz, F. The Role of Urinary VEGF in Observational Studies of BPS/IC Patients: A Systematic Review. Diagnostics 2022, 12, 1037. https://doi.org/10.3390/diagnostics12051037
Abreu-Mendes P, Costa A, Charrua A, Pinto RA, Cruz F. The Role of Urinary VEGF in Observational Studies of BPS/IC Patients: A Systematic Review. Diagnostics. 2022; 12(5):1037. https://doi.org/10.3390/diagnostics12051037
Chicago/Turabian StyleAbreu-Mendes, Pedro, Aurora Costa, Ana Charrua, Rui Almeida Pinto, and Francisco Cruz. 2022. "The Role of Urinary VEGF in Observational Studies of BPS/IC Patients: A Systematic Review" Diagnostics 12, no. 5: 1037. https://doi.org/10.3390/diagnostics12051037






