A Predictive Nomogram of Early Recurrence for Patients with AFP-Negative Hepatocellular Carcinoma Underwent Curative Resection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Follow-Up
2.3. Clinicopathologic Characteristics
2.4. MRI Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Independent Prognostic Factors of AFP-NHCC Patients
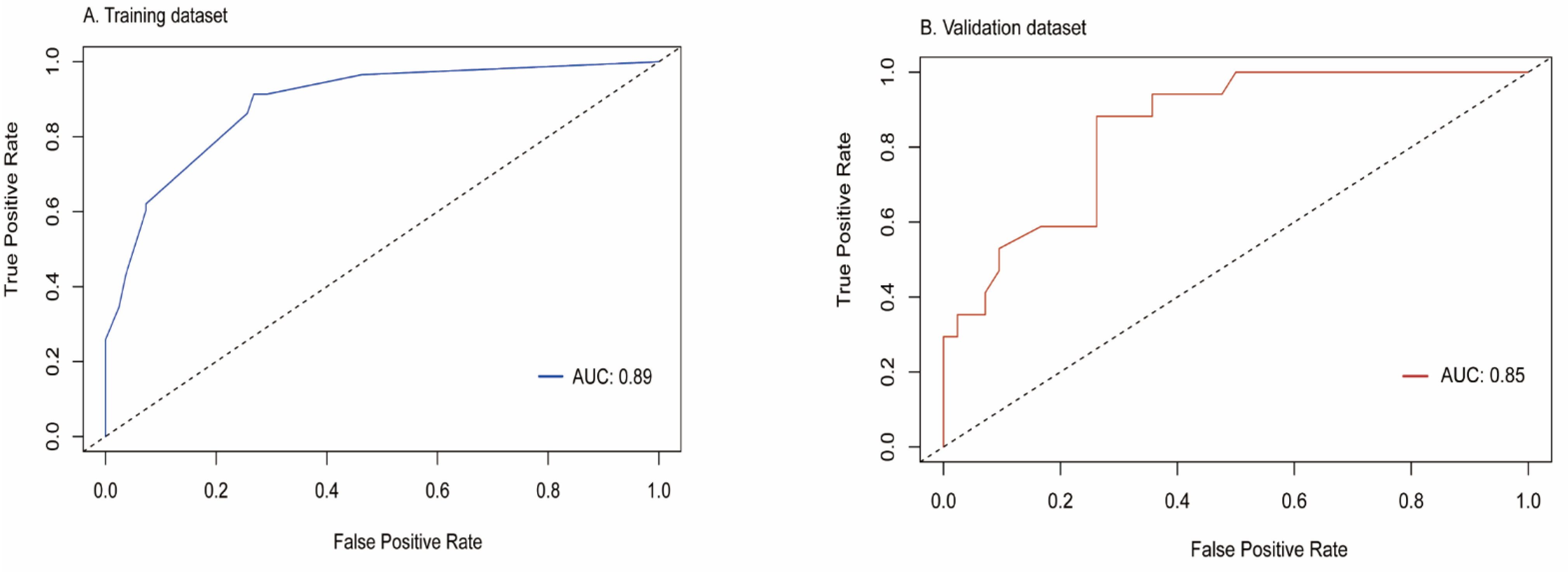
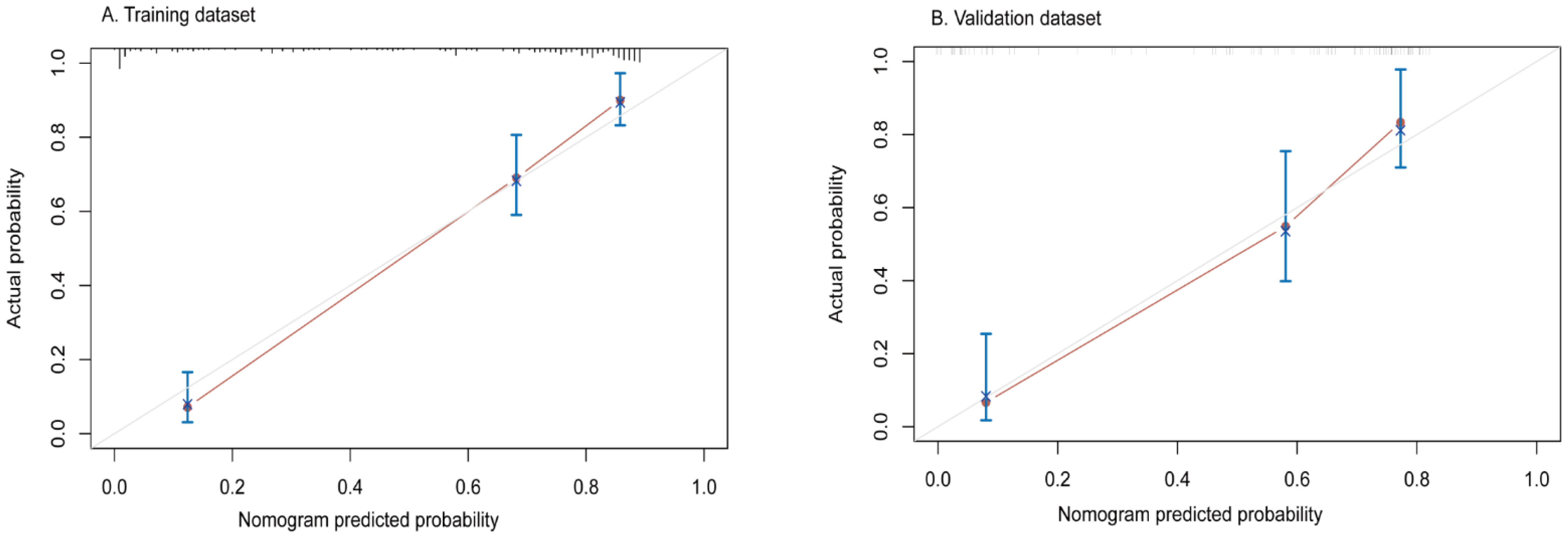
3.3. Construction and Evaluation of Nomogram
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gao, T.; Zhi, J.; Mu, C.; Gu, S.; Xiao, J.; Yang, J.; Wang, Z.; Xiang, Y. One-step detection for two serological biomarker species to improve the diagnostic accuracy of hepatocellular carcinoma. Talanta 2018, 178, 89–93. [Google Scholar] [CrossRef]
- Guo, S.; Chen, W.; Luo, Y.; Ren, F.; Zhong, T.; Rong, M.; Dang, Y.; Feng, Z.; Chen, G. Clinical implication of long non-coding RNA NEAT1 expression in hepatocellular carcinoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 5395–5402. [Google Scholar] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Augusto, V.; Singal, A.G.; Eli, P.; Sasan, R.; Riccardo, L.; Kazuhiko, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef]
- Chan, A.W.; Chan, S.L.; Wong, G.L.; Wong, V.W.; Chong, C.C.; Lai, P.; Chan, H.L.; To, K.F. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann. Surg. Oncol. 2015, 22, 4138–4148. [Google Scholar] [CrossRef]
- Tang, A.; Hallouch, O.; Chernyak, V.; Kamaya, A.; Sirlin, C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2018, 43, 13–25. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [Green Version]
- Cucchetti, A.; Piscaglia, F.; Grigioni, A.D.E.; Ravaioli, M.; Cescon, M.; Zanello, M.; Grazi, G.L.; Golfieri, R.; Grigioni, W.F.; Pinna, A.D. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: A pilot study. J. Hepatol. 2010, 52, 880–888. [Google Scholar] [CrossRef]
- Chan, A.W.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Tada, T.; Chong, C.C.; Xiang, B.D.; Li, L.Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- She, S.; Xiang, Y.; Yang, M.; Ding, X.; Liu, X.; Ma, L.; Liu, Q.; Liu, B.; Lu, Z.; Li, S.; et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int. J. Oncol. 2015, 47, 543–554. [Google Scholar] [CrossRef]
- Wang, M.; Devarajan, K.; Singal, A.G.; Marrero, J.A.; Dai, J.; Feng, Z.; Rinaudo, J.A.; Srivastava, S.; Evans, A.; Hann, H.W.; et al. The doylestown algorithm: A test to improve the performance of AFP in the detection of hepatocellular carcinoma. Cancer Prev. Res. 2016, 9, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Bai, D.S.; Zhang, C.; Chen, P.; Jin, S.J.; Jiang, G.Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017, 7, 12870. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Rodríguez, R.; Romero-Gutiérrez, M.; Artaza-Varasa, T.; González-Frutos, C.; Ciampi-Dopazo, J.J.; de-la-Cruz-Pérez, G.; Sánchez-Ruano, J.J. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev. Esp. Enferm. Dig. 2012, 104, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, S.W.; Liu, L.L.; Yang, X.; Cai, S.H.; Yun, J.P. A model combining TNM stage and tumor size shows utility in predicting recurrence among patients with hepatocellular carcinoma after resection. Cancer Manag. Res. 2018, 10, 3707–3715. [Google Scholar] [CrossRef] [Green Version]
- Necchi, A.; Sonpavde, G.; Vullo, S.L.; Giardiello, D.; Bamias, A.; Crabb, S.J.; Harshman, L.C.; Bellmunt, J.; De Giorgi, U.; Sternberg, C.N.; et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: Retrospective international study of invasive/advanced cancer of the urothelium (RISC). Eur. Urol. 2017, 71, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desseroit, M.C.; Visvikis, D.; Tixier, F.; Majdoub, M.; Perdrisot, R.; Guillevin, R.; Cheze Le Rest, C.; Hatt, M. Erratum to: Development of a nomogram combining clinical staging with 18F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Miao, L.F.; Ye, X.H.; Cui, M.S.; He, X.F. Development of prognostic signature and nomogram for patients with breast cancer. Medicine 2019, 98, e14617. [Google Scholar] [CrossRef]
- Ferrer-Fàbrega, J.; Forner, A.; Liccioni, A.; Miquel, R.; Molina, V.; Navasa, M.; Fondevila, C.; García-Valdecasas, J.C.; Bruix, J.; Fuster, J. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 2016, 63, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Tsilimigras, D.I.; Sahara, K.; Moris, D.; Hyer, J.; Paredes, A.Z.; Bagante, F.; Merath, K.; Farooq, A.S.; Ratti, F.; Marques, H.P.; et al. Effect of Surgical Margin Width on Patterns of Recurrence among Patients Undergoing R0 Hepatectomy for T1 Hepatocellular Carcinoma: An International Multi-Institutional Analysis. J. Gastrointest. Surg. 2020, 24, 1552–1560. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, H.; Lee, D.H.; Hur, B.Y.; Hwang, Y.J.; Suh, K.S.; Han, J.K. Gadoxetate-enhanced MRI Features of Proliferative Hepatocellular Carcinoma Are Prognostic after Surgery. Radiology 2021, 300, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.I.; Jang, K.M.; Kim, S.H.; Kim, Y.K.; Kim, H.; Ahn, S.H. Preoperative Prediction for Early Recurrence Can Be as Accurate as Postoperative Assessment in Single Hepatocellular Carcinoma Patients. Korean J. Radiol. 2020, 21, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lim, H.K.; Lee, W.J.; Choi, D.; Park, C.K. Scirrhous hepatocellular carcinoma: Comparison with usual hepatocellular carcinoma based on CT-pathologic features and long-term results after curative resection. Eur. J. Radiol. 2009, 69, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Ikeda, K.; Seko, Y.; Hosaka, T.; Kobayashi, M.; Saitoh, S.; Kumada, H. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR Am. J. Roentgenol. 2011, 197, W665–W673. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Ikeda, K.; Hirakawa, M.; Yatsuji, H.; Sezaki, H.; Hosaka, T.; Akuta, N.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; et al. New classification of dynamic computed tomography images predictive of malignant characteristics of hepatocellular carcinoma. Hepatol. Res. 2010, 40, 1006–1014. [Google Scholar] [CrossRef]
- Ng, I.O.; Lai, E.C.; Fan, S.T.; Ng, M.M. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer 1992, 70, 45–49. [Google Scholar] [CrossRef]
- Lewis, R.H.; Glazer, E.S.; Bittenbinder, D.M.; O’Brien, T.; Deneve, J.L.; Shibata, D.; Behrman, S.W.; Vanatta, J.M.; Satapathy, S.K.; Dickson, P.V. Outcomes following resection of hepatocellular carcinoma in the absence of cirrhosis. J. Gastrointest. Cancer 2019, 50, 808–815. [Google Scholar] [CrossRef]
- Segal, E.; Sirlin, C.B.; Ooi, C.; Adler, A.S.; Gollub, J.; Chen, X.; Chan, B.K.; Matcuk, G.R.; Barry, C.T.; Chang, H.Y.; et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 2007, 25, 675–680. [Google Scholar] [CrossRef]
- Renzulli, M.; Brocchi, S.; Cucchetti, A.; Mazzotti, F.; Mosconi, C.; Sportoletti, C.; Brandi, G.; Pinna, A.D.; Golfieri, R. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology 2016, 279, 432–442. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Li, Z.; Xie, C.; Qin, S.; Yan, M.; Ke, Q.; Jin, X.; Lin, T.; Zhou, M.; et al. Two-trait predictor of venous invasion on contrast-enhanced CT as a preoperative predictor of outcomes for early-stage hepatocellular carcinoma after hepatectomy. Front. Oncol. 2021, 11, 688087. [Google Scholar] [CrossRef] [PubMed]
- Tarao, K.; Takemiya, S.; Tamai, S.; Sugimasa, Y.; Ohkawa, S.; Akaike, M.; Tanabe, H.; Shimizu, A.; Yoshida, M.; Kakita, A. Relationship between the recurrence of hepatocellular carcinoma (HCC) and serum alanine aminotransferase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC. Cancer 1997, 79, 688–694. [Google Scholar] [CrossRef]
- Obulhasim, G.; Yasen, M.; Kajino, K.; Mogushi, K.; Tanaka, S.; Mizushima, H.; Tanaka, H.; Arii, S.; Hino, O. Up-regulation of dbpA mRNA in hepatocellular carcinoma associated with metabolic syndrome. Hepatol. Int. 2013, 7, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Zhang, H.; Wu, X.F.; Han, S.; Jiao, C.Y.; Wang, D.; Wang, K.; Li, X.C. Clinical efficacy and prognostic factors analysis following curative hepatectomy for hepatocellular carcinoma patients with different China Liver Cancer Staging. Chin. J. Surg. 2021, 59, 134–143. [Google Scholar] [PubMed]
- Ng, K.K.; Cheng, N.M.; Huang, J.; Liao, M.; Chong, C.C.; Lee, K.F.; Wong, J.; Cheung, S.Y.; Lok, H.T.; Fung, A.K.; et al. Development and validation of a novel nomogram predicting 10-year actual survival after curative hepatectomy for hepatocellular carcinoma. Surgeon 2021, 19, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.T.; Zhao, C.; Peng, N.F.; Yang, Y.; Zhong, J.H.; Yang, T.; Zheng, M.H.; Wang, Y.Y.; Gong, W.F.; Xiang, B.D.; et al. Association between age and overall survival of patients with hepatocellular carcinoma after hepatic resection. J. Surg. Oncol. 2016, 114, 966–970. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | AFP− (<20 ug/mL) (n = 199) | AFP+ (≥20 ug/mL) (n = 231) | p-Value |
|---|---|---|---|
| Patient demographic | |||
| Age | 60.00 (54.00–66.00) | 56.00 (49.00–62.00) | <0.001 |
| Gender | 0.058 | ||
| Male | 173 | 185 | |
| Female | 26 | 46 | |
| Liver disease | 0.262 | ||
| HBV/HCV | 125 | 157 | |
| Other | 74 | 74 | |
| Liver cirrhosis | 0.018 | ||
| Present | 69 | 56 | |
| Absent | 130 | 175 | |
| Ascites | 0.309 | ||
| Present | 25 | 37 | |
| Absent | 174 | 194 | |
| Laboratory parameters | |||
| ALT (IU/L) | 32.00 (21.00–45.00) | 34.00 (24.00–53.00) | 0.114 |
| AST (IU/L) | 33.00 (25.00–48.00) | 38.00 (27.00–55.00) | 0.027 |
| ALB | 42.50 (40.10–45.20) | 42.20 (39.30–44.80) | 0.227 |
| TBIL | 16.40 (11.90–20.80) | 16.30 (13.20–20.80) | 0.459 |
| DBIL | 3.20 (2.30–4.10) | 3.30 (2.50–4.20) | 0.239 |
| CR | 65.00 (58.00–73.00) | 65.0 (57.00–75.00) | 0.708 |
| ALP (IU/L) | 98.00 (78.00–127.25) | 109.00 (89.00–133.00) | 0.002 |
| GGT (IU/L) | 49.00 (32.00–96.00) | 66.00 (39.00–120.00) | 0.002 |
| PLT | 165.00 (126.00–214.00) | 168.00 (122.00–217.00) | 0.966 |
| CA199 | 16.86 (8.70–29.34) | 20.91 (11.62–36.32) | 0.005 |
| CEA | 2.57 (1.58–3.74) | 2.67 (1.78–3.79) | 0.995 |
| Child-Pugh grade | 0.059 | ||
| A | 196 | 221 | |
| B | 3 | 11 | |
| MRI features | |||
| Multifocality | 0.100 | ||
| Solitary | 166 | 178 | |
| Multiple | 33 | 53 | |
| L-max | 0.007 | ||
| ≤5 cm | 118 | 110 | |
| >5 cm | 81 | 121 | |
| Tumor margin | 0.006 | ||
| Smooth | 45 | 29 | |
| Non-smooth | 154 | 202 | |
| Tumor-capsule | 0.183 | ||
| Present | 179 | 198 | |
| Absent | 20 | 33 | |
| Peritumoral enhancement | <0.001 | ||
| Present | 18 | 50 | |
| Absent | 181 | 181 | |
| Rim enhancement | <0.001 | ||
| Present | 35 | 79 | |
| Absent | 164 | 152 | |
| TTPVI | 0.001 | ||
| Present | 99 | 151 | |
| Absent | 100 | 80 | |
| Intra-hemorrhage | 0.152 | ||
| Present | 33 | 51 | |
| Absent | 166 | 180 | |
| Intra-necrosis | 0.401 | ||
| Present | 63 | 82 | |
| Absent | 136 | 149 | |
| Histologic characteristics | |||
| Histologic grade | <0.001 | ||
| Poor | 42 | 92 | |
| Mediate | 149 | 137 | |
| Well | 8 | 2 | |
| Satellite nodules | 0.002 | ||
| Present | 27 | 59 | |
| Absent | 172 | 172 | |
| MVI | <0.001 | ||
| Present | 79 | 131 | |
| Absent | 120 | 100 | |
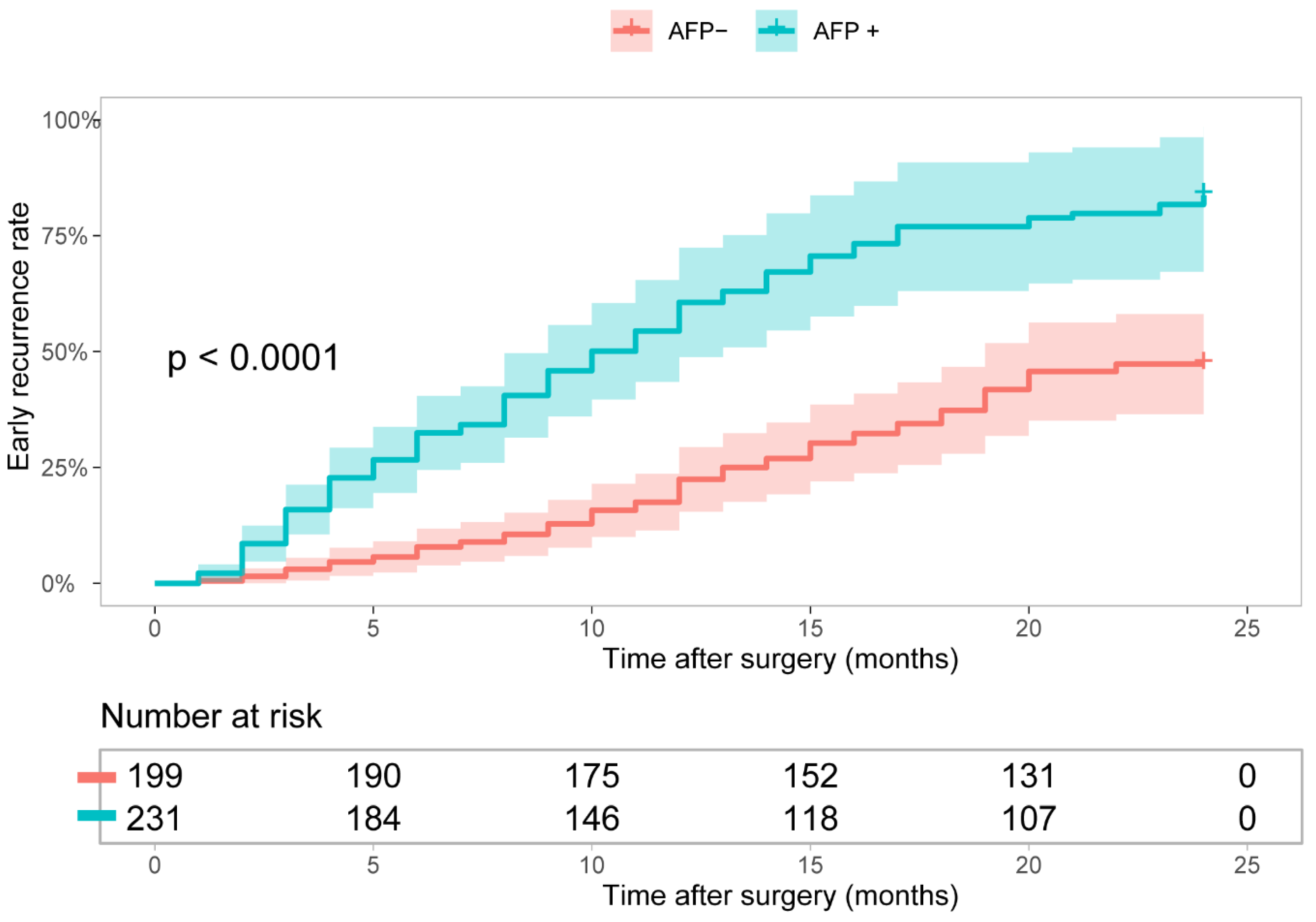
| Early recurrence | <0.001 | ||
| Present | 75 | 131 | |
| Absent | 124 | 100 |
| Characteristic | Training Dataset | Validation Dataset | p-Value |
|---|---|---|---|
| n = 139 | n = 60 | ||
| Patient demographics | |||
| Age (y) | 59.647 (9.263) | 58.617 (8.857) | 0.466 |
| Gender | 1.000 | ||
| Female | 18 | 8 | |
| Male | 121 | 52 | |
| Liver disease | 1.000 | ||
| Hepatitis B/C virus | 87 | 38 | |
| Absent | 52 | 22 | |
| Liver cirrhosis | 0.821 | ||
| Present | 47 | 22 | |
| Absent | 92 | 38 | |
| Ascites | 0.654 | ||
| Present | 16 | 9 | |
| Absent | 123 | 51 | |
| Surgical treatment | |||
| Major hepatectomy | 33 | 20 | 0.160 |
| Minor hepatectomy | 106 | 40 | |
| Laboratory factors | |||
| ALB (g/L) > 40, ≤40 | 103, 36 | 49, 11 | 0.331 |
| ALT (IU/L) > 50, ≤50 | 30, 109 | 14, 46 | 0.930 |
| AST (IU/L) > 40, ≤40 | 49, 90 | 18, 42 | 0.578 |
| TBIL (μmol/L) > 19, ≤19 | 50, 89 | 16, 44 | 0.265 |
| DBIL (μmol/L) > 3.4, ≤3.4 | 64, 75 | 25, 35 | 0.678 |
| GGT (IU/L) > 40, ≤40 | 52, 87 | 32, 28 | 0.287 |
| ALP (IU/L) > 125, ≤125 | 38, 101 | 13, 47 | 0.507 |
| CR (μmol/L) > 110, ≤110 | 2, 137 | 0, 60 | 0.873 |
| CEA (ng/mL) > 3.4, ≤3.4 | 42, 97 | 14, 46 | 0.412 |
| CA199(U/mL) > 37, ≤37 | 22, 117 | 8, 52 | 0.14 |
| PT (s) > 13, ≤13 | 4, 135 | 2, 58 | 1.000 |
| PLT (109/L) > 300, ≤300 | 5, 134 | 2, 58 | 1.000 |
| Child-Pugh grade A, B | 137,2 | 59, 1 | 1.000 |
| MRI features | |||
| Multifocality | 0.851 | ||
| Solitary | 115 | 51 | |
| Multiple | 24 | 9 | |
| L-max > 5, ≤5 | 53, 86 | 28, 32 | 0.333 |
| Tumor margin | 1.000 | ||
| Smooth | 31 | 14 | |
| Non-smooth | 108 | 46 | |
| Tumor-capsule | 0.785 | ||
| Present | 124 | 55 | |
| Absent | 15 | 5 | |
| Peritumoral enhancement | 1.000 | ||
| Present | 13 | 5 | |
| Absent | 126 | 55 | |
| Rim enhancement | 0.700 | ||
| Present | 23 | 12 | |
| Absent | 116 | 48 | |
| Intra-hemorrhage | 25, 114 | 8, 52 | 0.547 |
| Intra-necrosis | 41, 98 | 22, 38 | 0.4055 |
| TTPVI | 1.000 | ||
| Present | 70 | 30 | |
| Absent | 69 | 30 | |
| Histologic features | |||
| Histologic grade | 0.758 | ||
| Poorly | 31 | 11 | |
| Moderately | 103 | 46 | |
| Well | 5 | 3 | |
| Satellite nodules | 0.693 | ||
| Present | 115 | 51 | |
| Absent | 24 | 9 | |
| MVI | 0.477 | ||
| Present | 132 | 51 | |
| Absent | 17 | 9 | |
| Early recurrence | 0.547 | ||
| Present | 50 | 25 | |
| Absent | 89 | 35 |
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Patient demographics | ||||
| Age (y) | 1.016 (0.986–1.047) | 0.286 | ||
| Gender | 1.940 (0.698–5.390) | 0.204 | ||
| Female | ||||
| Male | ||||
| Liver disease | 0.930 (0.525–1.646) | 0.803 | ||
| Hepatitis B/C virus | ||||
| Absent | ||||
| Liver cirrhosis | 1.275 (0.696–2.335) | 0.431 | ||
| Present | ||||
| Absent | ||||
| Ascites | 1.904 (0.925–3.919) | 0.081 | ||
| Present | ||||
| Absent | ||||
| Surgical treatment | 2.139 (1.190–3.843) | 0.011 | 1.047 (0.486–1.876) | 0.894 |
| Major hepatectomy | ||||
| Minor hepatectomy | ||||
| Laboratory factors | ||||
| ALB (g/L) > 40, ≤40 | 0.596 (0.329–1.080) | 0.088 | ||
| ALT (IU/L) > 50, ≤50 | 1.440 (0.765–2.711) | 0.258 | ||
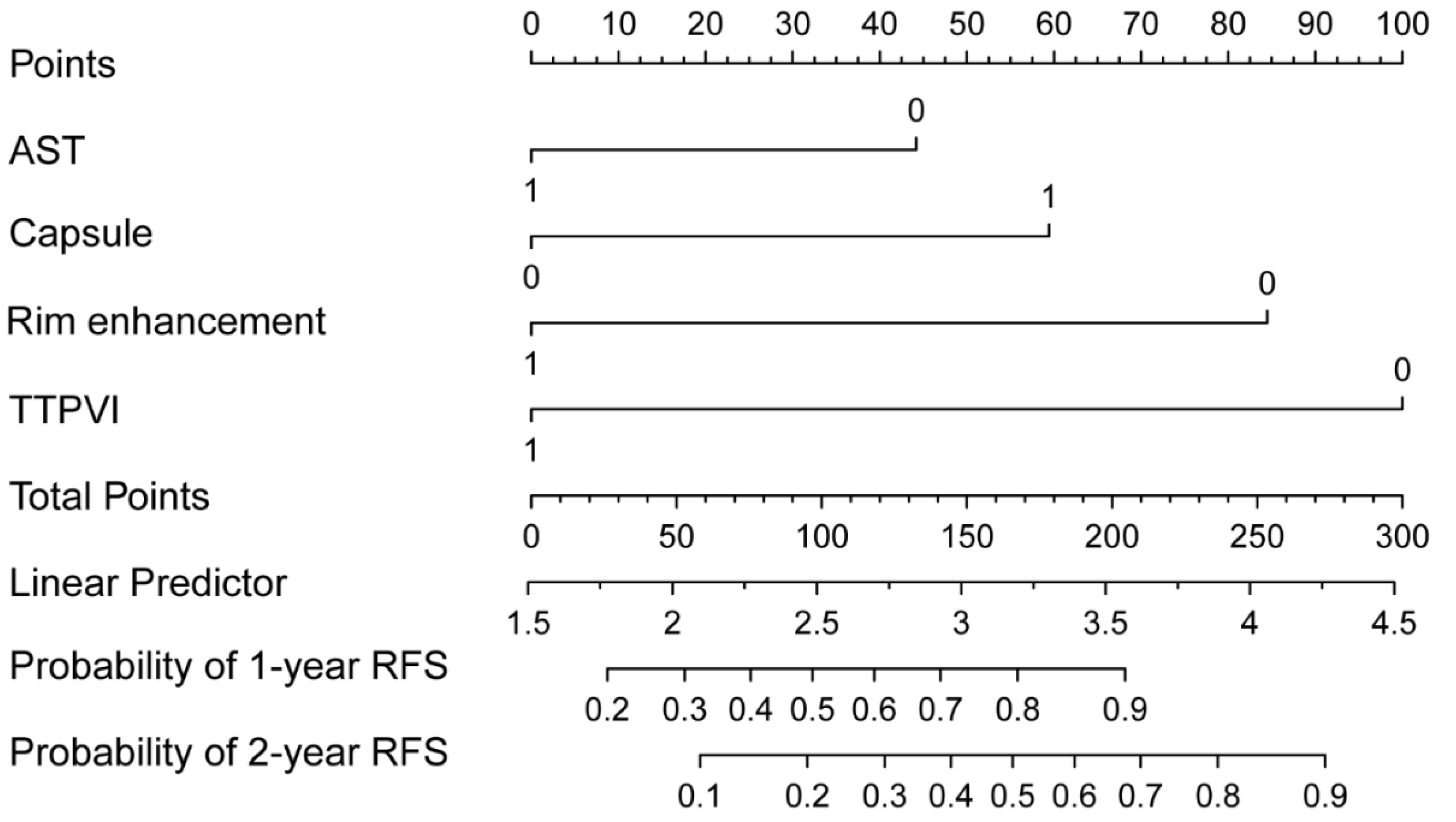
| AST (IU/L) > 40, ≤40 | 2.324 (1.333–4.050) | 0.003 | 1.975 (1.045–3.732) | 0.036 |
| TBIL (μmol/L) > 19, ≤19 | 1.368 (0.780–2.399) | 0.275 | ||
| DBIL (μmol/L) > 3.4, ≤3.4 | 1.688 (0.965–2.952) | 0.066 | ||
| GGT (IU/L) > 40, ≤40 | 0.608 (0.348–1.060) | 0.079 | ||
| ALP (IU/L) > 125, ≤125 | 1.002 (1.000–1.005) | 0.069 | ||
| CR (μmol/L) > 110, ≤110 | 0.994 (0.974–1.014) | 0.529 | ||
| CEA (ng/mL) > 3.4, ≤3.4 | 1.209 (0.667–2.190) | 0.532 | ||
| CA199 (U/mL) > 37, ≤37 | 2.237 (1.168–4.284) | 0.015 | 1.023 (0.498–2.101) | 0.950 |
| PT (s) > 13, ≤13 | 1.433 (0.993–2.068) | 0.055 | ||
| PLT (109/L) | 0.998 (0.993–1.002) | 0.337 | ||
| Child-Pugh grade A, B | 2.089 (0.288–15.136) | 0.466 | ||
| MRI features | ||||
| Multifocality | 2.728 (1.503–4.949) | 0.001 | 1.424 (0.726–2.794) | 0.303 |
| Solitary | ||||
| Multiple | ||||
| L-max > 5, ≤5 | 1.228 (0.697–2.163) | 0.477 | ||
| Tumor margin | 3.056 (1.213–7.703) | 0.018 | 1.488 (0.563–3.929) | 0.422 |
| Smooth | ||||
| Non-smooth | ||||
| Tumor-capsule | 0.205 (0.105–0.398) | <0.001 | 0.422 (0.198–0.900) | 0.026 |
| Present | ||||
| Absent | ||||
| Peritumoral enhancement | 3.215 (1.604–6.448) | 0.001 | 1.183 (0.518–2.704) | 0.689 |
| Present | ||||
| Absent | ||||
| Rim enhancement | 6.173(3.438–11.084) | <0.001 | 2.819 (1.267–6.273) | 0.011 |
| Present | ||||
| Absent | ||||
| Intra-hemorrhage | 1.632 (0.853–3.124) | 0.139 | ||
| Intra-necrosis | 1.616 (0.907–2.880) | 0.104 | ||
| TTPVI | 18.061 (6.481–50.333) | <0.001 | 11.665 (3.978–34.203) | <0.001 |
| Present | ||||
| Absent | ||||
| Histologic features | ||||
| Histologic grade | 1.164 (0.756–1.791) | 0.491 | ||
| Poorly | ||||
| Moderately | ||||
| Well | ||||
| Satellite nodules | 1.067 (0.843–1.351) | 0.589 | ||
| Present | ||||
| Absent | ||||
| MVI | 1.139 (0.844–1.535) | 0.395 | ||
| Present | ||||
| Absent | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Han, L.; Xiao, B.; Li, X.; Ye, Z. A Predictive Nomogram of Early Recurrence for Patients with AFP-Negative Hepatocellular Carcinoma Underwent Curative Resection. Diagnostics 2022, 12, 1073. https://doi.org/10.3390/diagnostics12051073
Li W, Han L, Xiao B, Li X, Ye Z. A Predictive Nomogram of Early Recurrence for Patients with AFP-Negative Hepatocellular Carcinoma Underwent Curative Resection. Diagnostics. 2022; 12(5):1073. https://doi.org/10.3390/diagnostics12051073
Chicago/Turabian StyleLi, Wencui, Lizhu Han, Bohan Xiao, Xubin Li, and Zhaoxiang Ye. 2022. "A Predictive Nomogram of Early Recurrence for Patients with AFP-Negative Hepatocellular Carcinoma Underwent Curative Resection" Diagnostics 12, no. 5: 1073. https://doi.org/10.3390/diagnostics12051073





