Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
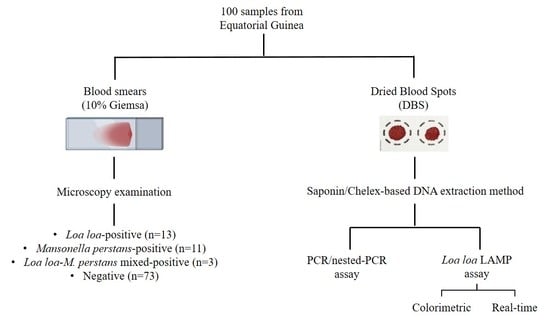
2.2. Samples Obtaining and Selection
2.3. DNA Extraction from Dried Blood Spots
2.4. Molecular Analysis
2.4.1. PCR/Nested-PCR Assay
2.4.2. Colorimetric LAMP Assay
2.4.3. Real-Time LAMP Assay
2.5. Statistics
3. Results
3.1. Application of Molecular Methods on Dried Blood Samples
3.1.1. PCR/Nested-PCR Assay
3.1.2. Colorimetric LAMP Assay
3.1.3. Real-Time LAMP Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boussinesq, M. Loiasis. Ann. Trop. Med. Parasitol. 2006, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Metzger, W.G.; Mordmüller, B. Loa loa-does it deserve to be neglected? Lancet Infect. Dis. 2014, 14, 353–357. [Google Scholar] [CrossRef]
- Gobbi, F.; Bottieau, E.; Bouchaud, O.; Buonfrate, D.; Salvador, F.; Rojo-Marcos, G.; Rodari, P.; Clerinx, J.; Treviño, B.; Herrera-Ávila, J.P.; et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Negl. Trop. Dis. 2018, 12, e0006917. [Google Scholar] [CrossRef] [PubMed]
- Eyebe, S.; Sabbagh, A.; Pion, S.D.; Nana-Djeunga, H.C.; Kamgno, J.; Boussinesq, M.; Chesnais, C.B. Familial aggregation and heritability of loa loa microfilaremia. Clin. Infect. Dis. 2018, 66, 751–757. [Google Scholar] [CrossRef]
- Expanded Special Project for Elimination of NTDs. Available online: https://espen.afro.who.int/ (accessed on 23 November 2021).
- Mathison, B.A.; Couturier, M.R.; Pritt, B.S. Diagnostic identification and differentiation of microfilariae. J. Clin. Microbiol. 2019, 57, e00706-19. [Google Scholar] [CrossRef]
- Ojurongbe, O.; Akindele, A.A.; Adeleke, M.A.; Oyedeji, M.O.; Adedokun, S.A.; Ojo, J.F.; Akinleye, C.A.; Bolaji, O.S.; Adefioye, O.A.; Adeyeba, O.A. Co-endemicity of Loiasis and Onchocerciasis in Rain Forest Communities in Southwestern Nigeria. PLoS Negl. Trop. Dis. 2015, 9, e0003633. [Google Scholar] [CrossRef]
- Simonsen, P.E.; Onapa, A.W.; Asio, S.M. Mansonella perstans filariasis in Africa. Acta Trop. 2011, 120, S109–S120. [Google Scholar] [CrossRef]
- Ta-Tang, T.-H.; Crainey, J.; Post, R.J.; Luz, S.L.; Rubio, J. Mansonellosis: Current perspectives. Res. Rep. Trop. Med. 2018, 9, 9–24. [Google Scholar] [CrossRef]
- Puente, S.; Lago, M.; Subirats, M.; Sanz-Esteban, I.; Arsuaga, M.; Vicente, B.; Alonso-Sardon, M.; Belhassen-Garcia, M.; Muro, A. Imported Mansonella perstans infection in Spain. Infect. Dis. Poverty 2020, 9, 105. [Google Scholar] [CrossRef]
- D’Ambrosio, M.V.; Bakalar, M.; Bennuru, S.; Reber, C.; Skandarajah, A.; Nilsson, L.; Switz, N.; Kamgno, J.; Pion, S.; Boussinesq, M.; et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci. Transl. Med. 2015, 7, 286re4. [Google Scholar] [CrossRef]
- Kamgno, J.; Pion, S.D.; Chesnais, C.B.; Bakalar, M.H.; D’Ambrosio, M.V.; Mackenzie, C.D.; Nana-Djeunga, H.C.; Gounoue-Kamkumo, R.; Njitchouang, G.-R.; Nwane, P.; et al. A Test-and-Not-Treat Strategy for Onchocerciasis in Loa loa–Endemic Areas. N. Engl. J. Med. 2017, 377, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Emukah, E.; Rakers, L.J.; Kahansim, B.; Miri, E.S.; Nwoke, B.E.B.; Griswold, E.; Saka, Y.; Anagbogu, I.; Davies, E.; Ityonzughul, C.; et al. In Southern Nigeria Loa loa Blood Microfilaria Density is Very Low even in Areas with High Prevalence of Loiasis: Results of a Survey Using the New LoaScope Technology. Am. J. Trop. Med. Hyg. 2018, 99, 116–123. [Google Scholar] [CrossRef]
- Pion, S.D.; Nana-Djeunga, H.; Niamsi-Emalio, Y.; Chesnais, C.B.; Deléglise, H.; Mackenzie, C.; Stolk, W.; Fletcher, D.A.; Klion, A.D.; Nutman, T.B.; et al. Implications for annual retesting after a test-and-not-treat strategy for onchocerciasis elimination in areas co-endemic with Loa loa infection: An observational cohort study. Lancet Infect. Dis. 2020, 20, 102–109. [Google Scholar] [CrossRef]
- Johnson, O.; Giorgi, E.; Fronterrè, C.; Amoah, B.; Atsame, J.; Ella, S.N.; Biamonte, M.; Ogoussan, K.; Hundley, L.; Gass, K.; et al. Geostatistical modelling enables efficient safety assessment for mass drug administration with ivermectin in Loa loa endemic areas through a combined antibody and LoaScope testing strategy for elimination of onchocerciasis. PLoS Negl. Trop. Dis. 2022, 16, e0010189. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Ramanathan, R.; Klion, A.D.; Iadarola, M.J.; Nutman, T.B. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J. Clin. Microbiol. 2008, 46, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Amvongo-Adjia, N.; Koudou, B.; Njouendou, A.J.; Chounna Ndongmo, P.W.; Kengne-Ouafo, J.A.; Datchoua-Poutcheu, F.R.; Fovennso, B.A.; Tayong, D.B.; Fombad, F.F.; et al. Cross-Reactivity of Filariais ICT Cards in Areas of Contrasting Endemicity of Loa loa and Mansonella perstans in Cameroon: Implications for Shrinking of the Lymphatic Filariasis Map in the Central African Region. PLoS Negl. Trop. Dis. 2015, 9, e0004184. [Google Scholar] [CrossRef]
- Pedram, B.; Pasquetto, V.; Drame, P.M.; Ji, Y.; Gonzalez-Moa, M.J.; Baldwin, R.K.; Nutman, T.B.; Biamonte, M.A. A novel rapid test for detecting antibody responses to Loa loa infections. PLoS Negl. Trop. Dis. 2017, 11, e0005741. [Google Scholar] [CrossRef]
- Touré, F.S.; Bain, O.; Nerrienet, E.; Millet, P.; Wahl, G.; Toure, Y.; Doumbo, O.; Nicolas, L.; Georges, A.J.; McReynolds, L.A.; et al. Detection of Loa loa-specific DNA in blood from occult-infected individuals. Exp. Parasitol. 1997, 86, 163–170. [Google Scholar] [CrossRef]
- Fink, D.L.; Kamgno, J.; Nutman, T.B. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl. Trop. Dis. 2011, 5, e1299. [Google Scholar] [CrossRef]
- Ta-Tang, T.H.; Moya, L.; Nguema, J.; Aparicio, P.; Miguel-Oteo, M.; Cenzual, G.; Canorea, I.; Lanza, M.; Benito, A.; Crainey, J.L.; et al. Geographical distribution and species identification of human filariasis and onchocerciasis in Bioko Island, Equatorial Guinea. Acta Trop. 2018, 180, 12–17. [Google Scholar] [CrossRef]
- Alhassan, A.; Li, Z.; Poole, C.B.; Carlow, C.K.S. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends Parasitol. 2015, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Mvoulouga, P.O.; Akue, J.P.; Abán, J.L.; Santiago, B.V.; Sánchez, M.C.; Muro, A. Development of a highly sensitive Loop-Mediated Isothermal Amplification (LAMP) method for the detection of Loa loa. PLoS ONE 2014, 9, e94664. [Google Scholar] [CrossRef]
- Drame, P.M.; Fink, D.L.; Kamgno, J.; Herrick, J.A.; Nutman, T.B. Loop-mediated isothermal amplification for rapid and semiquantitative detection of loa loa infection. J. Clin. Microbiol. 2014, 52, 2071–2077. [Google Scholar] [CrossRef]
- Poole, C.B.; Ettwiller, L.; Tanner, N.A.; Evans, T.C.; Wanji, S.; Carlow, C.K.S. Genome filtering for new DNA biomarkers of Loa loa infection suitable for loop-mediated isothermal amplification. PLoS ONE 2015, 10, e0139286. [Google Scholar] [CrossRef]
- Takagi, H.; Itoh, M.; Kasai, S.; Yahathugoda, T.C.; Weerasooriya, M.V.; Kimura, E. Development of loop-mediated isothermal amplification method for detecting Wuchereria bancrofti DNA in human blood and vector mosquitoes. Parasitol. Int. 2011, 60, 493–497. [Google Scholar] [CrossRef]
- Poole, C.B.; Tanner, N.A.; Zhang, Y.; Evans, T.C.; Carlow, C.K.S. Diagnosis of Brugian Filariasis by Loop-Mediated Isothermal Amplification. PLoS Negl. Trop. Dis. 2012, 6, e1948. [Google Scholar] [CrossRef]
- Alhassan, A.; Makepeace, B.L.; Lacourse, E.J.; Osei-Atweneboana, M.Y.; Carlow, C.K.S. A simple isothermal DNA amplification method to screen black flies for Onchocerca volvulus infection. PLoS ONE 2014, 9, 118323. [Google Scholar] [CrossRef]
- Lagatie, O.; Merino, M.; Batsa Debrah, L.; Debrah, A.Y.; Stuyver, L.J. An isothermal DNA amplification method for detection of Onchocerca volvulus infection in skin biopsies. Parasites Vectors 2016, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.B.; Sinha, A.; Ettwiller, L.; Apone, L.; McKay, K.; Panchapakesa, V.; Lima, N.F.; Ferreira, M.U.; Wanji, S.; Carlow, C.K.S. In Silico Identification of Novel Biomarkers and Development of New Rapid Diagnostic Tests for the Filarial Parasites Mansonella perstans and Mansonella ozzardi. Sci. Rep. 2019, 9, 10275. [Google Scholar] [CrossRef] [PubMed]
- Smit, P.W.; Elliott, I.; Peeling, R.W.; Mabey, D.; Newton, P.N. Review article: An overview of the clinical use of filter paper in the diagnosis of tropical diseases. Am. J. Trop. Med. Hyg. 2014, 90, 195–210. [Google Scholar] [CrossRef]
- Simon, N.; Shallat, J.; Williams Wietzikoski, C.; Harrington, W.E. Optimization of Chelex 100 resin-based extraction of genomic DNA from dried blood spots. Biol. Methods Protoc. 2020, 5, bpaa009. [Google Scholar] [CrossRef] [PubMed]
- Bereczky, S.; Mårtensson, A.; Gil, J.P.; Färnert, A. Short report: Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2005, 72, 249–251. [Google Scholar] [CrossRef]
- Schmidt, B.; Xu, W.; González, I.J.; Polley, S.D.; Bell, D.; Shakely, D.; Msellem, M.I.; Björkman, A.; Mårtensson, A. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS ONE 2014, 9, e103905. [Google Scholar] [CrossRef]
- Vincent, J.P.; Komaki-Yasuda, K.; Iwagami, M.; Kawai, S.; Kano, S. Combination of PURE-DNA extraction and LAMP-DNA amplification methods for accurate malaria diagnosis on dried blood spots 11 Medical and Health Sciences 1108 Medical Microbiology. Malar. J. 2018, 17, 373. [Google Scholar] [CrossRef]
- Willard, J.M.; Lee, D.A.; Holland, M.M. Recovery of DNA for PCR amplification from blood and forensic samples using a chelating resin. Methods Mol. Biol. 1998, 98, 9–18. [Google Scholar] [CrossRef]
- Rubio, J.M.; Post, R.J.; Van Leeuwen, W.M.D.; Henry, M.C.; Lindergard, G.; Hommel, M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: The semi-nested multiplex malaria PCR (SnM-PCR). Trans. R. Soc. Trop. Med. Hyg. 2002, 96, S199. [Google Scholar] [CrossRef]
- Hwang, J.; Jaroensuk, J.; Leimanis, M.L.; Russell, B.; McGready, R.; Day, N.; Snounou, G.; Nosten, F.; Imwong, M. Long-term storage limits PCR-based analyses of malaria parasites in archival dried blood spots. Malar. J. 2012, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Baidjoe, A.; Rosenthal, P.J.; Dorsey, G.; Bousema, T.; Greenhouse, B. The effect of storage and extraction methods on amplification of plasmodium falciparum DNA from dried blood spots. Am. J. Trop. Med. Hyg. 2015, 92, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Touré, F.S.; Kassambara, L.; Williams, T.; Millet, P.; Bain, O.; Georges, A.J.; Egwang, T.G. Human occult loiasis: Improvement in diagnostic sensitivity by the use of a nested polymerase chain reaction. Am. J. Trop. Med. Hyg. 1998, 59, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.L.; Lammie, P.J. Laboratory diagnosis of filariasis. Clin. Lab. Med. 1991, 4, 977–1010. [Google Scholar] [CrossRef]
- Plowe, C.V.; Djimde, A.; Bouare, M.; Doumbo, O.; Wellems, T.E. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: Polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 1995, 52, 565–568. [Google Scholar] [CrossRef]
- Blas, I.; Ruíz-Zarzuela, I.; Vallejo, A. WinEpi: Working in Epidemiology. An Online Epidemiological Tool. ISVEE 11: Proceedings of the 11th Symposium of the International Society for Veterinary Epi-Demiology and Economics, Cairns (Australia). Theme 4-Tools & Training for Epidemiologists: Poste. Available online: http://winepi.net/ (accessed on 4 May 2021).
- Walther, M.; Muller, R. Diagnosis of human filariases (except onchocerciasis). Adv. Parasitol. 2003, 53, 149–193. [Google Scholar]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 2013, 54, 506–513. [Google Scholar] [CrossRef]
- Chaorattanakawee, S.; Natalang, O.; Hananantachai, H.; Nacher, M.; Brockman, A.; Krudsood, S.; Looareesuwan, S.; Patarapotikul, J. Storage duration and polymerase chain reaction detection of Plasmodium falciparum from blood spots on filter paper. Am. J. Trop. Med. Hyg. 2003, 69, 42–44. [Google Scholar] [CrossRef]
- Hsiang, M.S.; Lin, M.; Dokomajilar, C.; Kemere, J.; Pilcher, C.D.; Dorsey, G.; Greenhouse, B. PCR-based pooling of dried blood spots for detection of malaria parasites: Optimization and application to a cohort of Ugandan children. J. Clin. Microbiol. 2010, 48, 3539–3543. [Google Scholar] [CrossRef][Green Version]
- Bouyou Akotet, M.K.; Owono-Medang, M.; Mawili-Mboumba, D.P.; Moussavou-Boussougou, M.N.; Nzenze Afène, S.; Kendjo, E.; Kombila, M. The relationship between microfilaraemic and amicrofilaraemic loiasis involving co-infection with Mansonella perstans and clinical symptoms in an exposed population from Gabon. J. Helminthol. 2016, 90, 469–475. [Google Scholar] [CrossRef]
- Whittaker, C.; Walker, M.; Pion, S.D.S.; Chesnais, C.B.; Boussinesq, M.; Basáñez, M.G. The Population Biology and Transmission Dynamics of Loa loa. Trends Parasitol. 2018, 34, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Quyen, T.L.; Hung, T.Q.; Chin, W.H.; Wolff, A.; Bang, D.D. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab Chip 2015, 15, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Eyong, E.E.J.; Tendongfor, N.; Ngwa, C.J.; Esuka, E.N.; Kengne-Ouafo, A.J.; Datchoua-Poutcheu, F.R.; Enyong, P.; Agnew, D.; Eversole, R.R.; et al. Ivermectin treatment of Loa loa hyper-microfilaraemic baboons (Papio anubis): Assessment of microfilarial load reduction, haematological and biochemical parameters and histopathological changes following treatment. PLoS Negl. Trop. Dis. 2017, 11, e0005576. [Google Scholar] [CrossRef] [PubMed]
- Amambo, G.N.; Abong, R.A.; Fombad, F.F.; Njouendou, A.J.; Nietcho, F.; Beng, A.A.; Ritter, M.; Esum, M.E.; Deribe, K.; Cho, J.F.; et al. Validation of Loop Mediated Isothermal Amplification for the Detection of Loa loa Infection in Chrysops sp in Experimental and Natural Field Conditions. Parasit. Vectors 2020, 14, 19. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; Dacal, E.; Rodríguez, E.; Saugar, J.M.; Yepes, E.; Aznar-Ruiz-de-Alegría, M.L.; Espasa, M.; Ninda, A.; Bocanegra, C.; et al. Field and laboratory comparative evaluation of a LAMP assay for the diagnosis of urogenital schistosomiasis in Cubal, Central Angola. Trop. Med. Int. Health 2018, 23, 992–1001. [Google Scholar] [CrossRef]
- Cevallos, W.; Fernández-Soto, P.; Calvopiña, M.; Buendía-Sánchez, M.; López-Abán, J.; Vicente, B.; Muro, A. Diagnosis of amphimeriasis by LAMPhimerus assay in human stool samples long-Term storage onto filter paper. PLoS ONE 2018, 13, e0192637. [Google Scholar] [CrossRef]
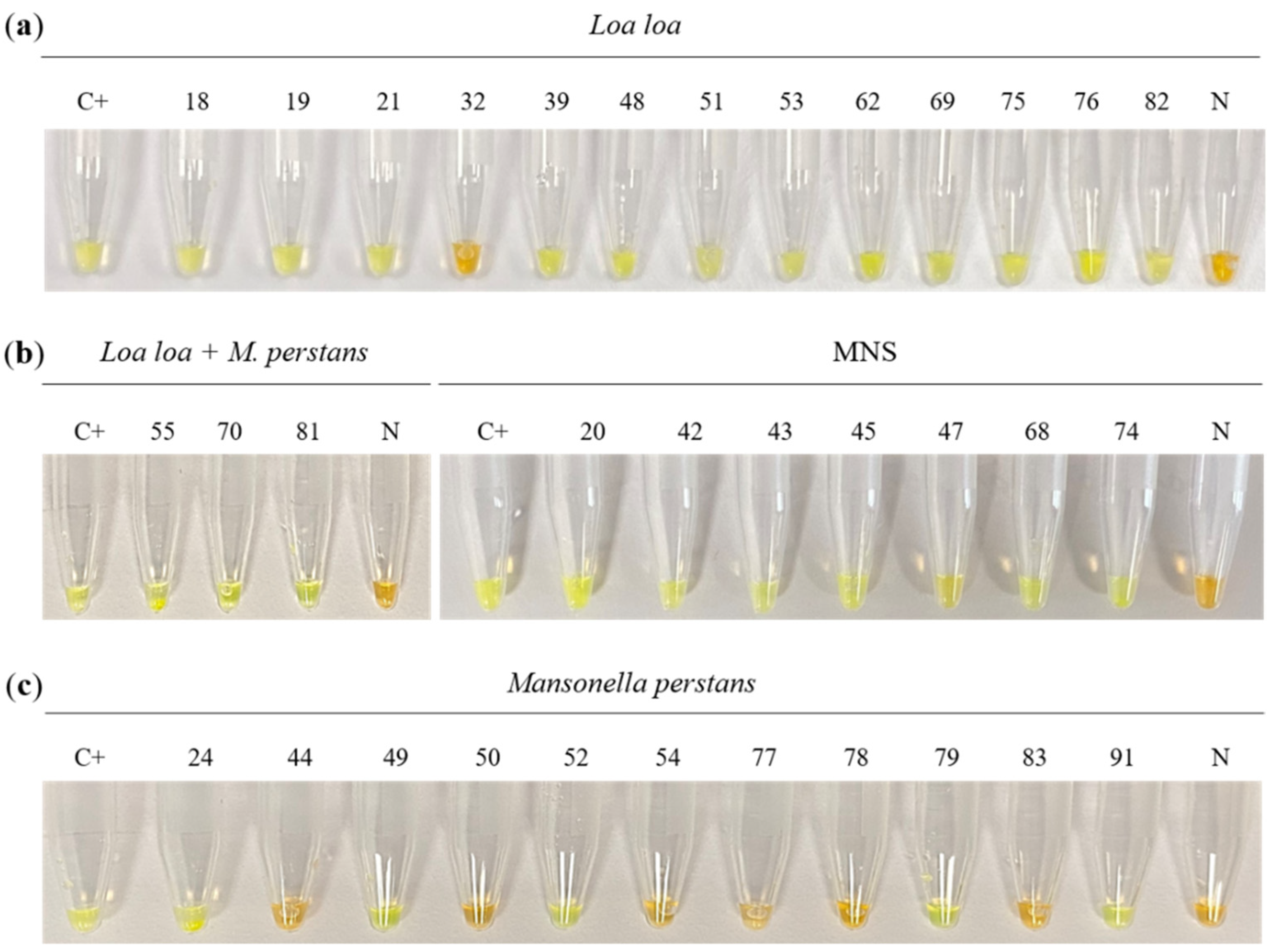



| Sample Groups | Parasitological Finding | Sample Number | mf/mL | PCR/ Nested-PCR | Colorimetric LAMP | Real-Time LAMP |
|---|---|---|---|---|---|---|
| G1 (n = 13) | Loa loa | 18 | 1100 | + | + | + |
| 19 | 300 | + | + | + | ||
| 21 | 500 | + | + | + | ||
| 32 | 2200 | + | - | - | ||
| 39 | 3600 | + | + | + | ||
| 48 | 12,200 | + | + | + | ||
| 51 | 400 | - | + | + | ||
| 53 | 500 | - | + | + | ||
| 62 | 2000 | + | + | + | ||
| 69 | 11,600 | + | + | + | ||
| 75 | 450 | - | + | + | ||
| 76 | 5600 | + | + | + | ||
| 82 | 1900 | + | + | + | ||
| G2 (n = 11) | Mansonella perstans | 24 | 200 | - | + | - |
| 44 | 600 | - | - | - | ||
| 49 | 800 | - | + | - | ||
| 50 | 100 | - | - | - | ||
| 52 | 100 | - | + | + | ||
| 54 | 1300 | - | - | - | ||
| 77 | 3200 | - | - | - | ||
| 78 | 100 | - | - | - | ||
| 79 | 400 | - | + | + | ||
| 83 | 1000 | - | - | - | ||
| 91 | 1000 | - | + | + | ||
| G3 (n = 3) | Loa loa/M. perstans | 55 | 200/200 | - | + | + |
| 70 | 200/200 | + | + | + | ||
| 81 | 6000/1500 | + | + | + | ||
| G4 (n = 73) | No findings | 20 | - | + | - | |
| 42 | - | + | - | |||
| 43 | - | + | + | |||
| 45 | - | + | + | |||
| 47 | - | + | + | |||
| 68 | - | + | - | |||
| 74 | - | + | - | |||
| Remaining nos. up to 100 | - | −66 | −70 |
| Colorimetric LAMP | Real-Time LAMP | PCR/Nested-PCR | |
|---|---|---|---|
| Sensitivity (95% CI) | 94.1% (81.9–105.6%) | 94.1% (82.9–105.3%) | 75.0% (53.8–96.2%) |
| Specificity (95% CI) | 87.5% (78.5–93.3%) | 93.3% (88.2–98.5%) | 100.0% (100.0–100.0%) |
| PPV (95% CI) | 57.1% (36.8–74.3%) | 72.7 % (54.1–91.3%) | 100.0% (100.0–100.0%) |
| NPV (95% CI) | 98.8% (96.0–101.3%) | 98.8% (96.5–101.1%) | 95.5% (91.1–99.8%) |
| Kappa (95% CI) | 62.2% (43.6–80.7%) ** | 76.9% (57.6–96.2%) ** | 83.4% (67.5–99.3%) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Febrer-Sendra, B.; Fernández-Soto, P.; Crego-Vicente, B.; Diego, J.G.-B.; Ta-Tang, T.-H.; Berzosa, P.; Nguema, R.; Ncogo, P.; Romay-Barja, M.; Herrador, Z.; et al. Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples. Diagnostics 2022, 12, 1079. https://doi.org/10.3390/diagnostics12051079
Febrer-Sendra B, Fernández-Soto P, Crego-Vicente B, Diego JG-B, Ta-Tang T-H, Berzosa P, Nguema R, Ncogo P, Romay-Barja M, Herrador Z, et al. Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples. Diagnostics. 2022; 12(5):1079. https://doi.org/10.3390/diagnostics12051079
Chicago/Turabian StyleFebrer-Sendra, Begoña, Pedro Fernández-Soto, Beatriz Crego-Vicente, Juan García-Bernalt Diego, Thuy-Huong Ta-Tang, Pedro Berzosa, Rufino Nguema, Policarpo Ncogo, María Romay-Barja, Zaida Herrador, and et al. 2022. "Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples" Diagnostics 12, no. 5: 1079. https://doi.org/10.3390/diagnostics12051079
APA StyleFebrer-Sendra, B., Fernández-Soto, P., Crego-Vicente, B., Diego, J. G.-B., Ta-Tang, T.-H., Berzosa, P., Nguema, R., Ncogo, P., Romay-Barja, M., Herrador, Z., Benito, A., & Muro, A. (2022). Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples. Diagnostics, 12(5), 1079. https://doi.org/10.3390/diagnostics12051079