A Systematic Review and Meta-Analysis of the Prevalence of Small Fibre Impairment in Patients with Fibromyalgia
Abstract
:1. Introduction
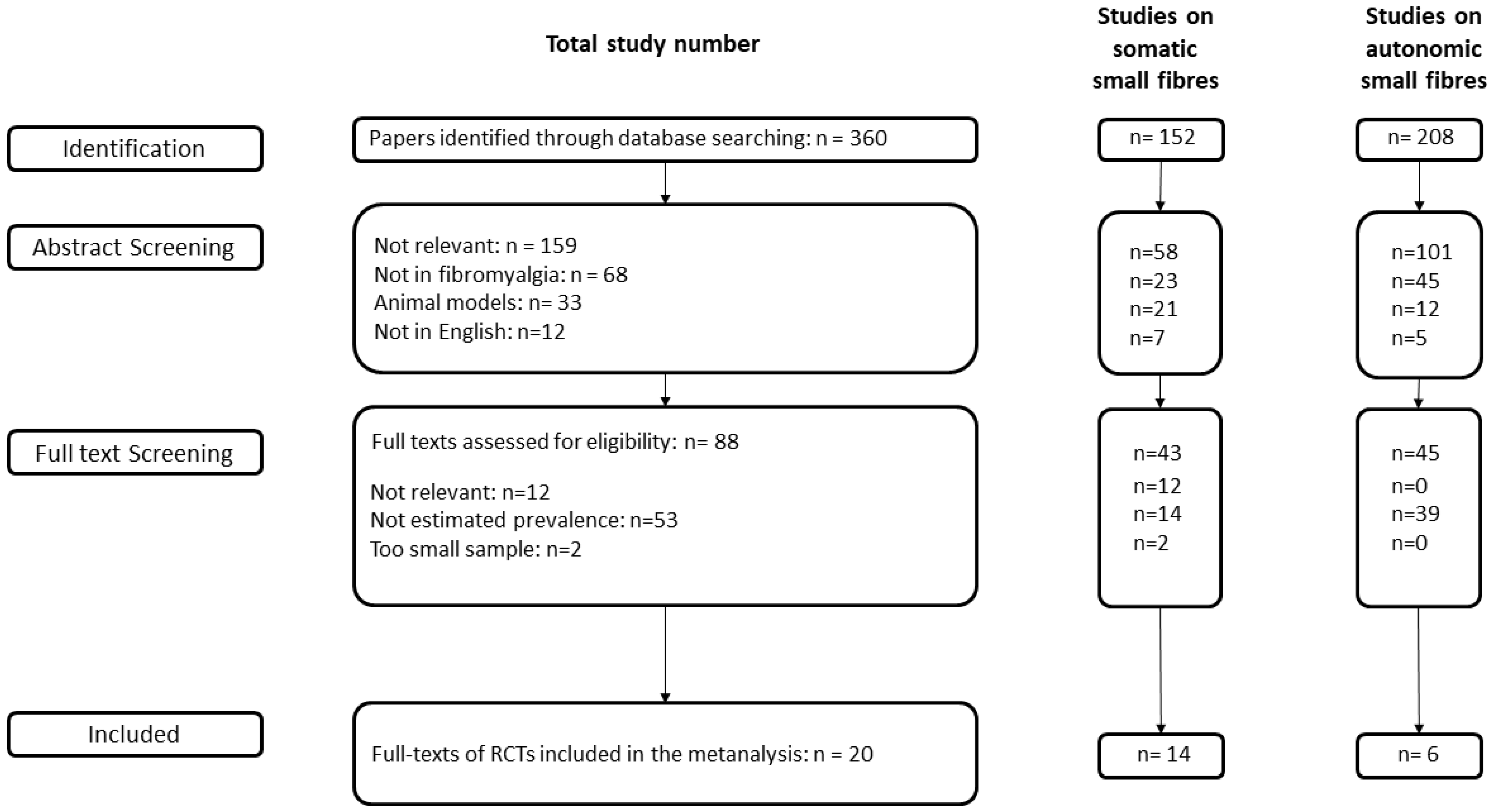
2. Methods
2.1. Search Process
2.2. Meta-Analysis
3. Results
3.1. Somatic Small Fibre Impairment
3.1.1. Subgroup Pooled Analysis—Skin Biopsy
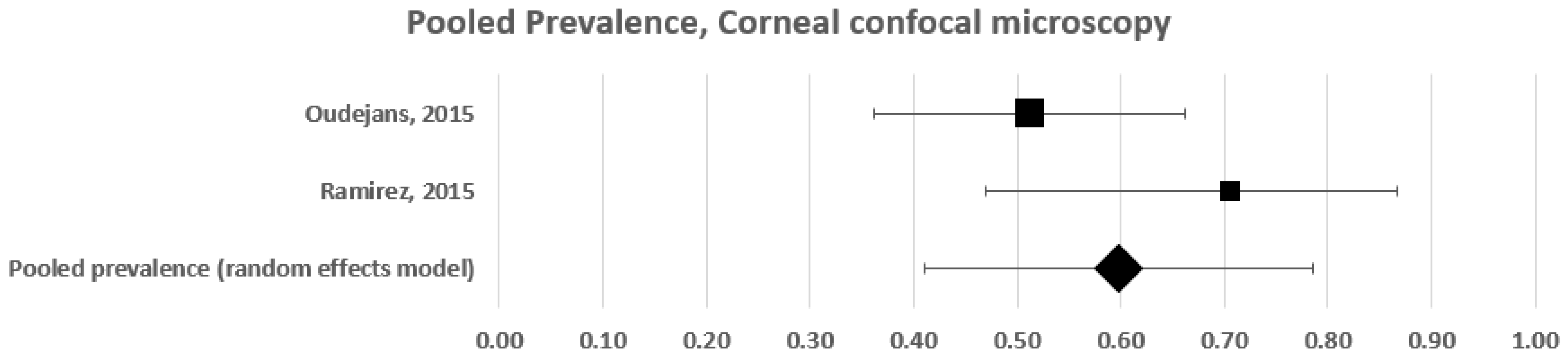
3.1.2. Subgroup Pooled Analysis—Corneal Confocal Microscopy
3.1.3. Subgroup Pooled Analysis—Microneurography
3.2. Autonomic Small Fibre Impairment in Fibromyalgia
Pooled Analysis of Autonomic Small Fibre Impairment Frequency
4. Discussion
4.1. Somatic Small Fibre Impairment
4.2. Autonomic Small Fibre Impairment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Häuser, W.; Fitzcharles, M.A. Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 2018, 20, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheum. 2014, 44, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Tinelli, E.; Gerardi, M.C.; Calistri, V.; Iannuccelli, C.; La Cesa, S.; Tarsitani, L.; Mainero, C.; Sarzi-Puttini, P.; Cruccu, G.; et al. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin. Exp. Rheumatol. 2016, 34, S129–S133. [Google Scholar] [PubMed]
- Truini, A.; Gerardi, M.C.; Di Stefano, G.; La Cesa, S.; Iannuccelli, C.; Pepe, A.; Sarzi-Puttini, P.; Cruccu, G.; Di Franco, M. Hyperexcitability in pain matrices in patients with fibromyalgia. Clin. Exp. Rheumatol. 2015, 33, S68–S72. [Google Scholar]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U.; et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Investig. 2021, 131, e144201. [Google Scholar] [CrossRef]
- Evdokimov, D.; Dinkel, P.; Frank, J.; Sommer, C.; Üçeyler, N. Characterization of dermal skin innervation in fibromyalgia syndrome. PLoS ONE 2020, 15, e0227674. [Google Scholar] [CrossRef] [Green Version]
- Grayston, R.; Czanner, G.; Elhadd, K.; Goebel, A.; Frank, B.; Üçeyler, N.; Malik, R.A.; Alam, U. A systematic review and meta-analysis of the prevalence of small fibre pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin. Arthritis Rheum. 2019, 48, 933–940. [Google Scholar] [CrossRef]
- Ramirez, M.; Martínez-Martínez, L.A.; Hernández-Quintela, E.; Velazco-Casapía, J.; Vargas, A.; Martínez- Lavín, M. Small fibre neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin. Arthritis Rheum. 2015, 45, 214–219. [Google Scholar] [CrossRef]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef]
- Palomo-López, P.; Calvo-Lobo, C.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodriguez-Sanz, D.; Sánchez-Gómez, R.; López-López, D. Quality of life related to foot health status in women with fibromyalgia: A case-control study. Arch. Med. Sci. 2019, 15, 694–699. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health. Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Review Manager (RevMan), version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Boneparth, A.; Chen, S.; Horton, D.B.; Nandini Moorthy, L.; Farquhar, I.; Downs, H.M.; Lee, H.; Oaklander, A.L. Epidermal Neurite Density in Skin Biopsies From Patients With Juvenile Fibromyalgia. J. Rheumatol. 2021, 48, 575–578. [Google Scholar] [CrossRef]
- Fasolino, A.; Di Stefano, G.; Leone, C.; Galosi, E.; Gioia, C.; Lucchino, B.; Terracciano, A.; Di Franco, M.; Cruccu, G.; Truini, A. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. Pain 2020, 161, 2385–2393. [Google Scholar] [CrossRef]
- Vecchio, E.; Lombardi, R.; Paolini, M.; Libro, G.; Delussi, M.; Ricci, K.; Quitadamo, S.G.; Gentile, E.; Girolamo, F.; Iannone, F.; et al. Peripheral and central nervous system correlates in fibromyalgia. Eur. J. Pain 2020, 24, 1537–1547. [Google Scholar] [CrossRef]
- Evdokimov, D.; Frank, J.; Klitsch, A.; Unterecker, S.; Warrings, B.; Serra, J.; Papagianni, A.; Saffer, N.; Altenschildesche, C.M.Z.; Kampik, D.; et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann. Neurol. 2019, 86, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Lawson, V.H.; Grewal, J.; Hackshaw, K.V.; Mongiovi, P.C.; Stino, A.M. Fibromyalgia syndrome and small fibre, early or mild sensory polyneuropathy. Muscle Nerve 2018, 58, 625–630. [Google Scholar] [CrossRef]
- Leinders, M.; Doppler, K.; Klein, T.; Deckart, M.; Rittner, H.; Sommer, C.; Üçeyler, N. Increased cutaneous miR-let-7d expression correlates with small nerve fibre pathology in patients with fibromyalgia syndrome. Pain 2016, 157, 2493–2503. [Google Scholar] [CrossRef]
- De Tommaso, M.; Nolano, M.; Iannone, F.; Vecchio, E.; Ricci, K.; Lorenzo, M.; Delussi, M.; Girolamo, F.; Lavolpe, V.; Provitera, V.; et al. Update on laser-evoked potential findings in fibromyalgia patients in light of clinical and skin biopsy features. J. Neurol. 2014, 261, 461–472. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Donadio, V.; Incensi, A.; Avoni, P.; Liguori, R. Small nerve fibre involvement in patients referred for fibromyalgia. Muscle Nerve 2014, 49, 757–759. [Google Scholar] [CrossRef]
- Kosmidis, M.L.; Koutsogeorgopoulou, L.; Alexopoulos, H.; Mamali, I.; Vlachoyiannopoulos, P.G.; Voulgarelis, M.; Moutsopoulos, H.M.; Tzioufas, A.G.; Dalakas, M.C. Reduction of Intraepidermal Nerve Fibre Density (IENFD) in the skin biopsies of patients with fibromyalgia: A controlled study. J. Neurol. Sci. 2014, 347, 143–147. [Google Scholar] [CrossRef]
- Oaklander, A.L.; Herzog, Z.D.; Downs, H.M.; Klein, M.M. Objective evidence that small fibre polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013, 154, 2310–2316. [Google Scholar] [CrossRef] [Green Version]
- Üçeyler, N.; Zeller, D.; Kahn, A.K.; Kewenig, S.; Kittel-Schneider, S.; Schmid, A.; Casanova-Molla, J.; Reiners, K.; Sommer, C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013, 136, 1857–1867. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Hou, Q.; Argoff, C.E.; Storey, J.S.; Wymer, J.P.; Rice, F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain Med. 2013, 14, 895–915. [Google Scholar] [CrossRef]
- Doppler, K.; Rittner, H.L.; Deckart, M.; Sommer, C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain 2015, 156, 2319–2325. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.H.; Oh, D.H.; Clauw, D.-J. Characteristic electron microscopic findings in the skin of patients with fibromyalgia-preliminary study. Clin. Rheumatol. 2008, 27, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Salemi, S.; Rethage, J.; Wollina, U.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J. Rheumatol. 2003, 30, 146–150. [Google Scholar] [PubMed]
- Enestrom, S.; Bengtsson, A.; Frodin, T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia: Relevant findings or epiphenomena. Scand. J. Rheumatol. 1997, 26, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Kewenig, S.; Kafke, W.; Kittel-Schneider, S.; Sommer, C. Skin cytokine expression in patients with fibromyalgia syndrome is not different from controls. BMC Neurol. 2014, 14, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudejans, L.; He, X.; Niesters, M.; Dahan, A.; Brines, M.; van Velzen, M. Cornea nerve fibre quantification and construction of phenotypes in patients with fibromyalgia. Sci. Rep. 2016, 6, 23573. [Google Scholar] [CrossRef] [Green Version]
- Klitsch, A.; Evdokimov, D.; Frank, J.; Thomas, D.; Saffer, N.; Altenschildesche, C.M.Z.; Sisignano, M.; Kampik, D.; Malik, R.A.; Sommer, C.; et al. Reduced association between dendritic cells and corneal sub-basal nerve fibers in patients with fibromyalgia syndrome. J. Peripher. Nerv. Syst. 2020, 25, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Erkan Turan, K.; Kocabeyoglu, S.; Unal-Cevik, I.; Bezci, F.; Akinci, A.; Irkec, M. Ocular Surface Alterations in the Context of Corneal In Vivo Confocal Microscopic Characteristics in Patients With Fibromyalgia. Cornea 2018, 37, 205–210. [Google Scholar] [CrossRef]
- Van Assche, D.C.S.; Plaghki, L.; Masquelier, E.; Hatem, S.M. Fibromyalgia syndrome-A laser-evoked potentials study unsupportive of small nerve fibre involvement. Eur. J. Pain. 2020, 24, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Gibson, S.J.; Littlejohn, G.O.; Gorman, M.M.; Helme, R.D.; Granges, G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain 1994, 58, 185–193. [Google Scholar] [CrossRef]
- De Tommaso, M.; Federici, A.; Santostasi, R.; Calabrese, R.; Vecchio, E.; Lapadula, G.; Iannone, F.; Lamberti, P.; Livrea, P. Laser-evoked potentials habituation in fibromyalgia. J. Pain 2011, 12, 116–124. [Google Scholar] [CrossRef]
- De Tommaso, M.; Ricci, K.; Libro, G.; Vecchio, E.; Delussi, M.; Montemurno, A.; Lopalco, G.; Iannone, F. Pain Processing and Vegetative Dysfunction in Fibromyalgia: A Study by Sympathetic Skin Response and Laser Evoked Potentials. Pain Res. Treat. 2017, 2017, 9747148. [Google Scholar] [CrossRef] [Green Version]
- Hüllemann, P.; von der Brelie, C.; Manthey, G.; Düsterhöft, J.; Helmers, A.K.; Synowitz, M.; Baron, R. Reduced laser-evoked potential habituation detects abnormal central pain processing in painful radiculopathy patients. Eur. J. Pain 2017, 21, 918–926. [Google Scholar] [CrossRef]
- Hazra, S.; Venkataraman, S.; Handa, G.; Yadav, S.L.; Wadhwa, S.; Singh, U.; Kochhar, K.P.; Deepak, K.K.; Sarkar, K. A Cross-Sectional Study on Central Sensitization and Autonomic Changes in Fibromyalgia. Front. Neurosci. 2020, 14, 788. [Google Scholar] [CrossRef]
- Ozgocmen, S.; Yoldas, T.; Yigiter, R.; Kaya, A.; Ardicoglu, O. R-R interval variation and sympathetic skin response in fibromyalgia. Arch. Med. Res. 2006, 37, 630–634. [Google Scholar] [CrossRef]
- Singh, R.; Rai, N.K.; Rastogi, A.; Endukuru, C.; Joshi, A.; Mishra, S.S. Impact of sleep disturbances and autonomic dysfunction on the quality of life of patients with fibromyalgia. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1021–1029. [Google Scholar] [CrossRef]
- Rost, S.; Crombez, G.; Sütterlin, S.; Vögele, C.; Veirman, E.; Van Ryckeghem, D.M.L. Altered regulation of negative affect in patients with fibromyalgia: A diary study. Eur. J. Pain 2021, 25, 714–724. [Google Scholar] [CrossRef]
- Schamne, J.; Ressetti, C.J.C.; Lima-Silva, A.E.; Okuno, N.M. Impaired Cardiac Autonomic Control in Women With Fibromyalgia Is Independent of Their Physical Fitness. J. Clin. Rheumatol. 2021, 27, S278–S283. [Google Scholar] [CrossRef]
- Reyes-Manzano, C.F.; Lerma, C.; Echeverría, C.J.; Martínez-Lavin, M.; Martínez-Martínez, L.A.; Infante, O.; Guzmán-Vargas, L. Multifractal Analysis Reveals Decreased Non-linearity and Stronger Anticorrelations in Heart Period Fluctuations of Fibromyalgia Patients. Front. Physiol. 2018, 9, 1118. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, A.; Stone, T.M.; Kingsley, J.D. Resistance Exercise Training on Disease Impact, Pain Catastrophizing and Autonomic Modulation in Women with Fibromyalgia. Int. J. Exerc. Sci. 2017, 10, 1184–1195. [Google Scholar]
- Schmidt, J.E.; O’Brien, T.G.; Hooten, W.M.; Joyner, M.J.; Johnson, B.D. The effects of slow-paced versus mechanically assisted breathing on autonomic function in fibromyalgia patients. J. Pain Res. 2017, 10, 2761–2768. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Kim, J.K.; Hong, S.H.; Lee, C.H.; Choi, B.Y. Heart Rate Variability for Quantification of Autonomic Dysfunction in Fibromyalgia. Ann. Rehabil. Med. 2016, 40, 301–309. [Google Scholar] [CrossRef]
- Lee, K.E.; Choi, S.E.; Kang, J.H.; Yim, Y.R.; Kim, J.E.; Lee, J.W.; Wen, L.; Park, D.J.; Kim, T.J.; Park, Y.W.; et al. Comparison of heart rate variability and classic autonomic testing for detection of cardiac autonomic dysfunction in patients with fibromyalgia. Int. J. Rheum. Dis. 2018, 21, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Lerma, C.; Martinez-Martinez, L.A.; Ruiz, N.; Vargas, A.; Infante, O.; Martinez-Lavin, M. Fibromyalgia beyond reductionism. Heart rhythm fractal analysis to assess autonomic nervous system resilience. Scand. J. Rheumatol. 2016, 45, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rosselló, F.; Muñoz, M.A.; Duschek, S.; Montoya, P. Affective Modulation of Brain and Autonomic Responses in Patients With Fibromyalgia. Psychosom. Med. 2015, 77, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Bardal, E.M.; Roeleveld, K.; Mork, P.J. Aerobic and cardiovascular autonomic adaptations to moderate intensity endurance exercise in patients with fibromyalgia. J. Rehabil. Med. 2015, 47, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Zamunér, A.R.; Forti, M.; Andrade, C.P.; Avila, M.A.; da Silva, E. Respiratory Sinus Arrhythmia and its Association with Pain in Women with Fibromyalgia Syndrome. Pain Pract. 2016, 16, 704–711. [Google Scholar] [CrossRef]
- Van Middendorp, H.; Lumley, M.A.; Houtveen, J.H.; Jacobs, J.W.; Bijlsma, J.W.; Geenen, R. The impact of emotion-related autonomic nervous system responsiveness on pain sensitivity in female patients with fibromyalgia. Psychosom. Med. 2013, 75, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Mork, P.J.; Nilsson, J.; Lorås, H.W.; Riva, R.; Lundberg, U.; Westgaard, R.H. Heart rate variability in fibromyalgia patients and healthy controls during non-REM and REM sleep: A case-control study. Scand. J. Rheumatol. 2013, 42, 505–508. [Google Scholar] [CrossRef]
- Lerma, C.; Martinez, A.; Ruiz, N.; Vargas, A.; Infante, O.; Martinez-Lavin, M. Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: Correlation with symptoms severity. Arthritis Res. Ther. 2011, 13, R185. [Google Scholar] [CrossRef] [Green Version]
- Kulshreshtha, P.; Gupta, R.; Yadav, R.K.; Bijlani, R.L.; Deepak, K.K. A comprehensive study of autonomic dysfunction in the fibromyalgia patients. Clin. Auton. Res. 2012, 22, 117–122. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Garrido, S.; Pulgar, A.; Duschek, S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J. Psychosom. Res. 2011, 70, 125–134. [Google Scholar] [CrossRef]
- Reyes Del Paso, G.A.; Garrido, S.; Pulgar, A.; Martín-Vázquez, M.; Duschek, S. Aberrances in autonomic cardiovascular regulation in fibromyalgia syndrome and their relevance for clinical pain reports. Psychosom. Med. 2010, 72, 462–470. [Google Scholar] [CrossRef]
- Di Franco, M.; Iannuccelli, C.; Alessandri, C.; Paradiso, M.; Riccieri, V.; Libri, F.; Valesini, G. Autonomic dysfunction and neuropeptide Y in fibromyalgia. Clin. Exp. Rheumatol. 2009, 27, S75–S78. [Google Scholar]
- Doğru, M.T.; Aydin, G.; Tosun, A.; Keleş, I.; Güneri, M.; Arslan, A.; Ebinç, H.; Orkun, S. Correlations between autonomic dysfunction and circadian changes and arrhythmia prevalence in women with fibromyalgia syndrome. Anadolu Kardiyol Derg. 2009, 9, 110–117. [Google Scholar]
- Wong, A.; Figueroa, A.; Sanchez-Gonzalez, M.A.; Son, W.M.; Chernykh, O.; Park, S.Y. Effectiveness of Tai Chi on Cardiac Autonomic Function and Symptomatology in Women With Fibromyalgia: A Randomized Controlled Trial. J. Aging Phys. Act. 2018, 26, 214–221. [Google Scholar] [CrossRef]
- Zamunér, A.R.; Barbic, F.; Dipaola, F.; Bulgheroni, M.; Diana, A.; Atzeni, F.; Marchi, A.; Sarzi-Puttini, P.; Porta, A.; Furlan, R. Relationship between sympathetic activity and pain intensity in fibromyalgia. Clin. Exp. Rheumatol. 2015, 33, S53–S57. [Google Scholar]
- Furlan, R.; Colombo, S.; Perego, F.; Atzeni, F.; Diana, A.; Barbic, F.; Porta, A.; Pace, F.; Malliani, A.; Sarzi-Puttini, P. Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J. Rheumatol. 2005, 32, 1787–1793. [Google Scholar]
- Naschitz, J.E.; Rozenbaum, M.; Fields, M.C.; Enis, S.; Manor, H.; Dreyfuss, D.; Peck, S.; Peck, E.R.; Babich, J.P.; Mintz, E.P.; et al. Cardiovascular reactivity in fibromyalgia: Evidence for pathogenic heterogeneity. J. Rheumatol. 2005, 32, 335–339. [Google Scholar] [PubMed]
- Cohen, H.; Neumann, L.; Alhosshle, A.; Kotler, M.; Abu-Shakra, M.; Buskila, D. Abnormal sympathovagal balance in men with fibromyalgia. J. Rheumatol. 2001, 28, 581–589. [Google Scholar] [PubMed]
- Raj, S.R.; Brouillard, D.; Simpson, C.S.; Hopman, W.M.; Abdollah, H. Dysautonomia among patients with fibromyalgia: A noninvasive assessment. J. Rheumatol. 2000, 27, 2660–2665. [Google Scholar] [PubMed]
- Cohen, H.; Neumann, L.; Shore, M.; Amir, M.; Cassuto, Y.; Buskila, D. Autonomic dysfunction in patients with fibromyalgia: Application of power spectral analysis of heart rate variability. Semin. Arthritis Rheum. 2000, 29, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lavín, M.; Hermosillo, A.G.; Rosas, M.; Soto, M.E. Circadian studies of autonomic nervous balance in patients with fibromyalgia: A heart rate variability analysis. Arthritis Rheum. 1998, 41, 1966–1971. [Google Scholar] [CrossRef]
- Martínez-Lavín, M.; Hermosillo, A.G.; Mendoza, C.; Ortiz, R.; Cajigas, J.C.; Pineda, C.; Nava, A.; Vallejo, M. Orthostatic sympathetic derangement in subjects with fibromyalgia. J. Rheumatol. 1997, 24, 714–718. [Google Scholar]
- Figueroa, A.; Kingsley, J.D.; McMillan, V.; Panton, L.B. Resistance exercise training improves heart rate variability in women with fibromyalgia. Clin. Physiol. Funct. Imaging 2008, 28, 49–54. [Google Scholar] [CrossRef]
- Reyes Del Paso, G.A.; de la Coba, P. Reduced activity, reactivity and functionality of the sympathetic nervous system in fibromyalgia: An electrodermal study. PLoS ONE 2020, 15, e0241154. [Google Scholar] [CrossRef]
- Pickering, G.; Achard, A.; Corriger, S.; Sickout-Arondo, N.; Macian, V.; Leray, C.; Lucchini, J.; Cardot, M.; Pereira, B. Electrochemical Skin Conductance and Quantitative Sensory Testing on Fibromyalgia. Pain Pract. 2020, 20, 348–356. [Google Scholar] [CrossRef]
- Lush, E.; Salmon, P.; Floyd, A.; Studts, J.L.; Weissbecker, I.; Sephton, S.E. Mindfulness meditation for symptom reduction in fibromyalgia: Psychophysiological correlates. J. Clin. Psychol. Med. Settings 2009, 16, 200–207. [Google Scholar] [CrossRef]
- Thieme, K.; Turk, D.C. Heterogeneity of psychophysiological stress responses in fibromyalgia syndrome patients. Arthritis Res. Ther. 2006, 8, R9. [Google Scholar] [CrossRef] [Green Version]
- Thieme, K.; Rose, U.; Pinkpank, T.; Spies, C.; Turk, D.C.; Flor, H. Psychophysiological responses in patients with fibromyalgia syndrome. J. Psychosom. Res. 2006, 61, 671–679. [Google Scholar] [CrossRef]
- Thieme, K.; Turk, D.C.; Gracely, R.H.; Flor, H. Differential psychophysiological effects of operant and cognitive behavioural treatments in women with fibromyalgia. Eur. J. Pain 2016, 20, 1478–1489. [Google Scholar] [CrossRef]
- Papadopoulou, M.; Papapostolou, A.; Bakola, E.; Masdrakis, V.G.; Moschovos, C.; Chroni, E.; Tsivgoulis, G.; Michopoulos, I. Neurophysiological and ultrasonographic comparative study of autonomous nervous system in patients suffering from fibromyalgia and generalized anxiety disorder. Neurol. Sci. 2021, 43, 2813–2821. [Google Scholar] [CrossRef]
- Ozkan, O.; Yildiz, M.; Köklükaya, E. The correlation of laboratory tests and Sympathetic Skin Response parameters by using artificial neural networks in fibromyalgia patients. J. Med. Syst. 2012, 36, 1841–1848. [Google Scholar] [CrossRef]
- Ozkan, O.; Yildiz, M.; Arslan, E.; Yildiz, S.; Bilgin, S.; Akkus, S.; Koyuncuoglu, H.R.; Koklukaya, E. A Study on the Effects of Sympathetic Skin Response Parameters in Diagnosis of Fibromyalgia Using Artificial Neural Networks. J. Med. Syst. 2016, 40, 54. [Google Scholar] [CrossRef]
- Ulas, U.H.; Unlu, E.; Hamamcioglu, K.; Odabasi, Z.; Cakci, A.; Vural, O. Dysautonomia in fibromyalgia syndrome: Sympathetic skin responses and RR interval analysis. Rheumatol. Int. 2006, 26, 383–387. [Google Scholar] [CrossRef]
- Unlü, E.; Ulaş, U.H.; Gürçay, E.; Tuncay, R.; Berber, S.; Cakçi, A.; Odabaşi, Z. Genital sympathetic skin responses in fibromyalgia syndrome. Rheumatol. Int. 2006, 26, 1025–1030. [Google Scholar] [CrossRef]
- Morf, S.; Amann-Vesti, B.; Forster, A.; Franzeck, U.K.; Koppensteiner, R.; Uebelhart, D.; Sprott, H. Microcirculation abnormalities in patients with fibromyalgia—Measured by capillary microscopy and laser fluxmetry. Arthritis Res. Ther. 2005, 7, R209–R216. [Google Scholar] [CrossRef] [Green Version]
- Jeschonneck, M.; Grohmann, G.; Hein, G.; Sprott, H. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology 2000, 39, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Esen, E.; Çetin, A. Microvascular functions in patients with fibromyalgia syndrome: Effects of physical exercise. Turk. J. Phys. Med. Rehabil. 2017, 63, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K.; Lee, G.S. Clinical Assessment of Autonomic Function in Fibromyalgia by the Refined and Abbreviated Composite Autonomic Symptom Score (COMPASS 31): A Case-Controlled Study. Rev. Recent Clin. Trials 2021. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Atzeni, F.; Diana, A.; Doria, A.; Furlan, R. Increased neural sympathetic activation in fibromyalgia syndrome. Ann. N. Y. Acad. Sci. 2006, 1069, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lavin, M. Fibromyalgia: When Distress Becomes (Un)sympathetic Pain. Pain Res. Treat. 2012, 2012, 981565. [Google Scholar] [CrossRef]
- Martínez-Lavín, M. Fibromyalgia and small fibre neuropathy: The plot thickens! Clin. Rheumatol. 2018, 37, 3167–3171. [Google Scholar] [CrossRef]
- Meeus, M.; Goubert, D.; De Backer, F.; Struyf, F.; Hermans, L.; Coppieters, I.; De Wandele, I.; Da Silva, H.; Calders, P. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: A systematic review. Semin. Arthritis Rheum. 2013, 43, 279–287. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health. 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Bekircan-Kurt, C.E.; Yilmaz, E.; Arslan, D.; Yildiz, F.G.; Dikmetas, Ö.; Ergul-Ulger, Z.; Kocabeyoglu, S.; Irkec, M.; Hekimsoy, V.; Tokgozoglu, L.; et al. The functional and structural evaluation of small fibres in asymptomatic carriers of p.Val50Met (Val30Met) mutation. Neuromuscul. Disord. 2021, 32, 50–56. [Google Scholar] [CrossRef]
- Isak, B.; Oflazoglu, B.; Tanridag, T.; Yitmen, I.; Us, O. Evaluation of peripheral and autonomic neuropathy among patients with newly diagnosed impaired glucose tolerance. Diabetes Metab. Res. Rev. 2008, 24, 563–569. [Google Scholar] [CrossRef]
- Hayano, J.; Yuda, E. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthropol. 2019, 38, 3. [Google Scholar] [CrossRef] [Green Version]
- Kucera, P.; Goldenberg, Z.; Kurca, E. Sympathetic skin response: Review of the method and its clinical use. Bratisl Lek List. 2004, 105, 108–116. [Google Scholar]
- Lefaucheur, J.P.; Zouari, H.G.; Gorram, F.; Nordine, T.; Damy, T.; Planté-Bordeneuve, V. The value of electrochemical skin conductance measurement using Sudoscan® in the assessment of patients with familial amyloid polyneuropathy. Clin. Neurophysiol. 2018, 129, 1565–1569. [Google Scholar] [CrossRef]
- Bradley, M.M.; Codispoti, M.; Cuthbert, B.N.; Lang, P.J. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion 2001, 1, 276–298. [Google Scholar] [CrossRef]




| Reference | Sample Size | Fibromyalgia | Control Group | Prevalence Number | Prevalence Estimate (%) |
|---|---|---|---|---|---|
| Skin biopsy | |||||
| Boneparth 2021 [15] | 38 | 15 | 23 | 8 | 53 |
| Fasolino 2020 [16] | 57 | 57 | - | 18 | 32 |
| Vecchio 2020 [17] | 81 | 81 | - | 69 | 85 |
| Evdokimov 2019 [18] | 128 | 117 | 11 | 74 | 63 |
| Lawson 2018 [19] | 155 | 155 | - | 62 | 40 |
| Leinders 2016 [20] | 116 | 28 | 88 | 14 | 50 |
| De Tommaso 2014 [21] | 81 | 21 | 60 | 16 | 76 |
| Giannoccaro 2014 [22] | 52 | 20 | 32 | 6 | 30 |
| Kosmidis 2014 [23] | 80 | 46 | 34 | 16 | 35 |
| Oaklander 2013 [24] | 57 | 27 | 30 | 11 | 41 |
| Uceyler 2013 [25] | 155 | 24 | 131 | 10 | 42 |
| Corneal confocal microscopy | |||||
| Oudejans 2016 [32] | * | 39 | * | 20 | 51 |
| Ramirez 2015 [8] | 34 | 17 | 17 | 12 | 71 |
| Microneurography | |||||
| Evdokimov 2019 [18] | 41 | 27 | 14 | 11 | 41 |
| Serra 2014 [9] | 56 | 30 | 26 | 9 | 30 |
| Sample Size | Fibromyalgia | Control Group | Prevalence Number | Prevalence Estimate (%) | |
|---|---|---|---|---|---|
| Tilt test | |||||
| Furlan 2005 [64] | 32 | 16 | 16 | 7 | 44 |
| Raj 2000 [67] | 31 | 17 | 14 | 11 | 65 |
| Heart rate variability | |||||
| Singh 2021 [42] | 60 | 30 | 30 | 12 | 40 |
| Lee 2016 [49] | 60 | 35 | 25 | 29 | 83 |
| Skin conductance | |||||
| Pickering 2020 [73] | 100 | 50 | 50 | 14 | 28 |
| Sympathetic skin response | |||||
| De Tommaso 2017 [38] | 80 | 50 | 30 | 9 | 18 |
| Ünlü 2006 [82] | 46 | 28 | 18 | 11 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galosi, E.; Truini, A.; Di Stefano, G. A Systematic Review and Meta-Analysis of the Prevalence of Small Fibre Impairment in Patients with Fibromyalgia. Diagnostics 2022, 12, 1135. https://doi.org/10.3390/diagnostics12051135
Galosi E, Truini A, Di Stefano G. A Systematic Review and Meta-Analysis of the Prevalence of Small Fibre Impairment in Patients with Fibromyalgia. Diagnostics. 2022; 12(5):1135. https://doi.org/10.3390/diagnostics12051135
Chicago/Turabian StyleGalosi, Eleonora, Andrea Truini, and Giulia Di Stefano. 2022. "A Systematic Review and Meta-Analysis of the Prevalence of Small Fibre Impairment in Patients with Fibromyalgia" Diagnostics 12, no. 5: 1135. https://doi.org/10.3390/diagnostics12051135
APA StyleGalosi, E., Truini, A., & Di Stefano, G. (2022). A Systematic Review and Meta-Analysis of the Prevalence of Small Fibre Impairment in Patients with Fibromyalgia. Diagnostics, 12(5), 1135. https://doi.org/10.3390/diagnostics12051135





