Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study
Abstract
:1. Introduction
2. Results
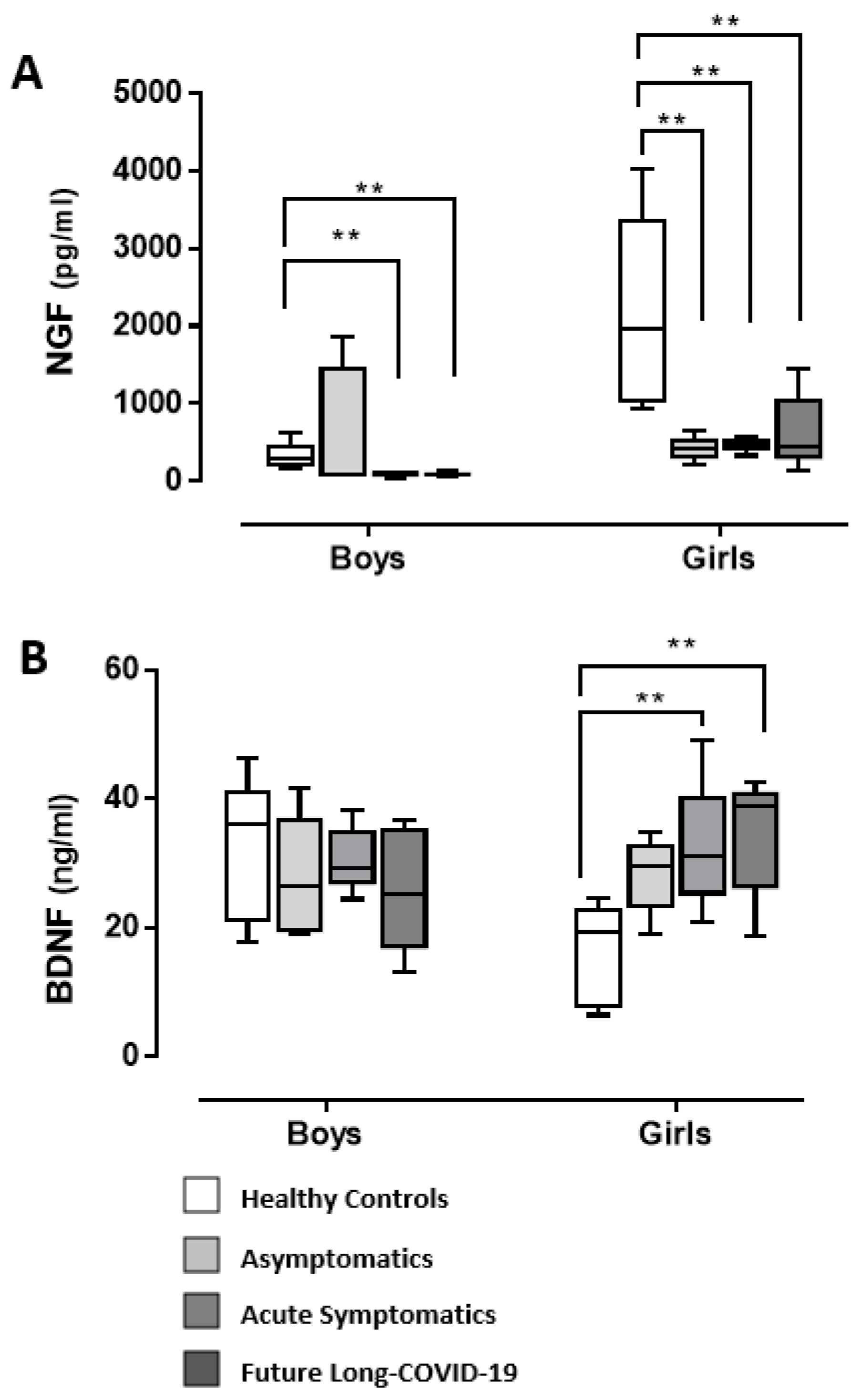
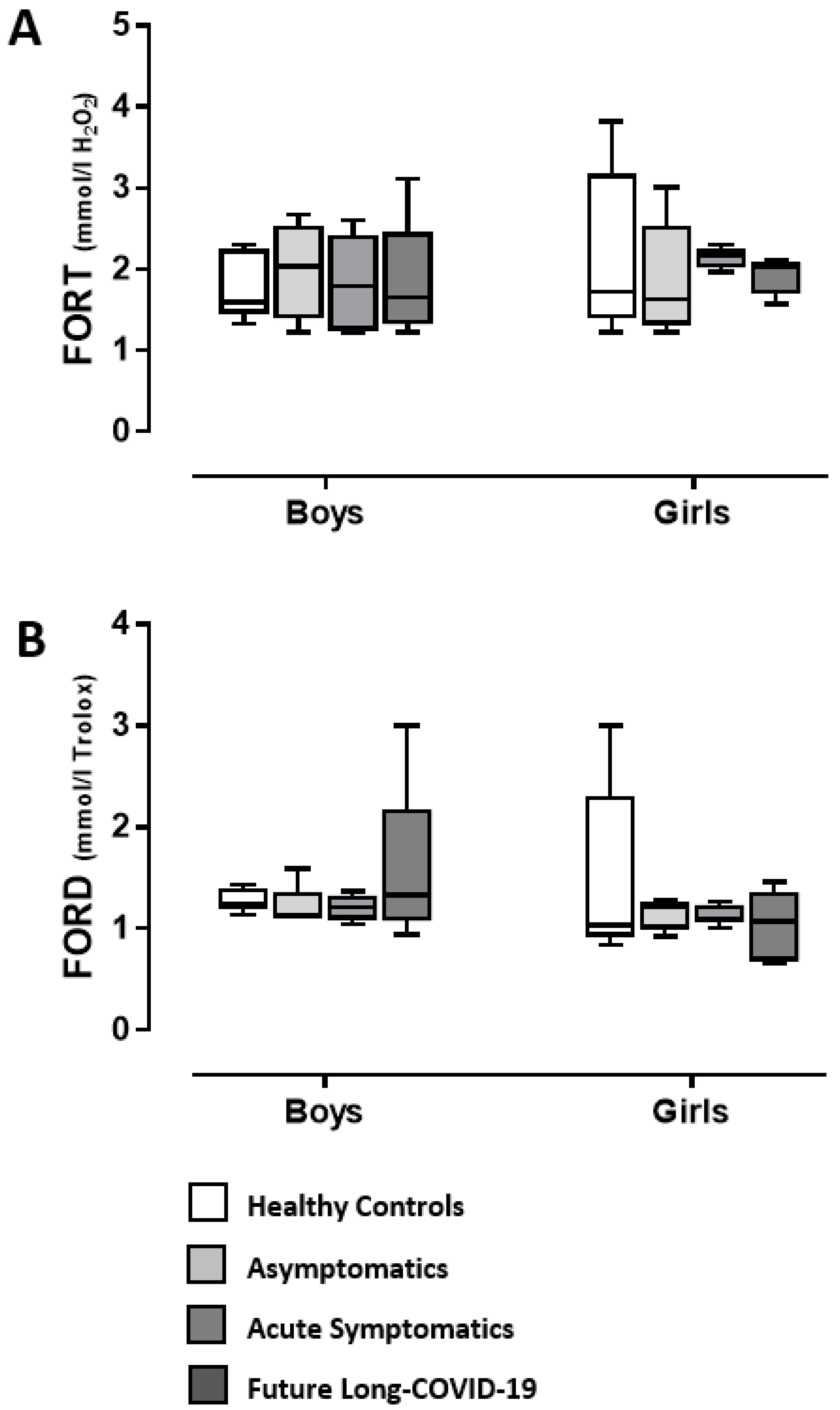
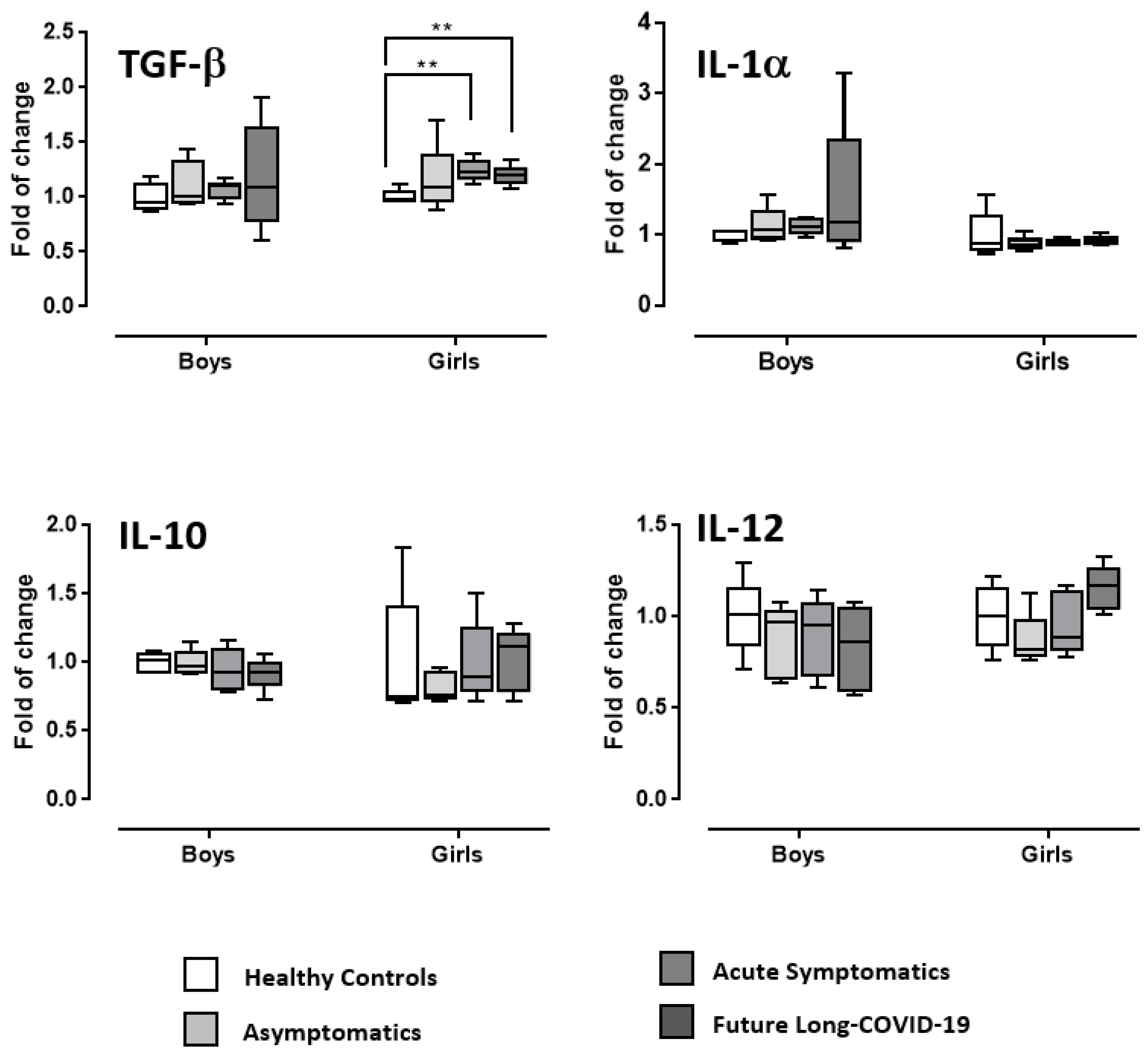
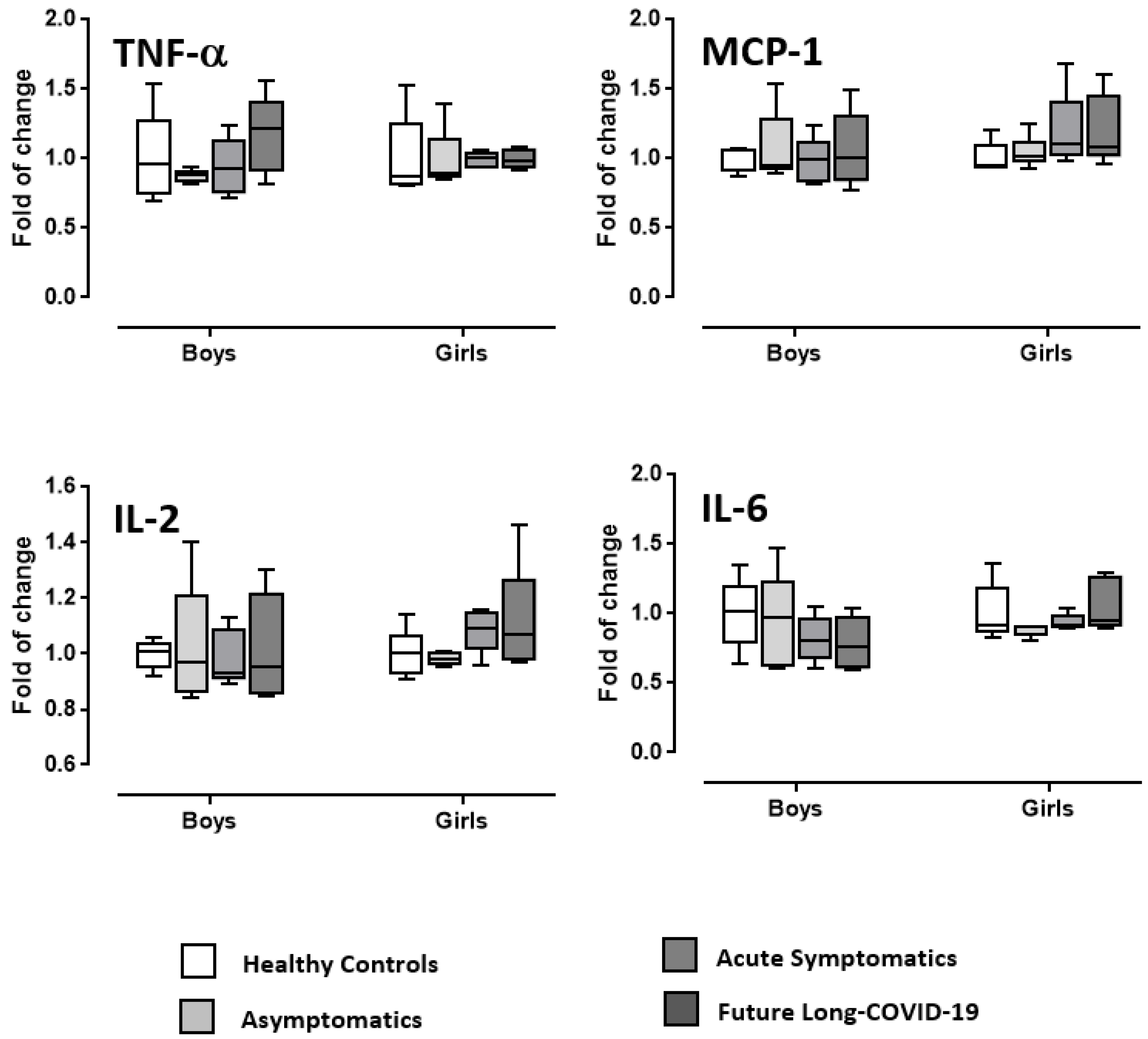
3. Discussion
4. Materials and Methods
4.1. Adolescents’ Recruitment
4.2. Blood Withdrawal
4.3. NGF and BDNF Serum Level Evaluation
4.4. Free Oxygen Radicals Defense (FORD) and Free Oxygen (FORT) Serum Evaluation
4.5. Oxidative Stress ELISA Strip Profiling Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceci, F.M.; Fiore, M.; Gavaruzzi, F.; Angeloni, A.; Lucarelli, M.; Scagnolari, C.; Bonci, E.; Gabanella, F.; Di Certo, M.G.; Barbato, C.; et al. Early Routine Biomarkers of SARS-CoV-2 Morbidity and Mortality: Outcomes from an Emergency Section. Diagnostics 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020, 39, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Pillay, T.S. Gene of the month: The 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. J. Clin. Pathol. 2020, 73, 366–369. [Google Scholar] [CrossRef]
- Sallard, E.; Halloy, J.; Casane, D.; Decroly, E.; van Helden, J. Tracing the origins of SARS-COV-2 in coronavirus phylogenies: A review. Environ. Chem. Lett. 2021, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gabanella, F.; Barbato, C.; Corbi, N.; Fiore, M.; Petrella, C.; de Vincentiis, M.; Greco, A.; Ferraguti, G.; Corsi, A.; Ralli, M.; et al. Exploring Mitochondrial Localization of SARS-CoV-2 RNA by Padlock Assay: A Pilot Study in Human Placenta. Int. J. Mol. Sci. 2022, 23, 2100. [Google Scholar] [CrossRef]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef]
- Doroftei, B.; Ciobica, A.; Ilie, O.-D.; Maftei, R.; Ilea, C. Mini-Review Discussing the Reliability and Efficiency of COVID-19 Vaccines. Diagnostics 2021, 11, 579. [Google Scholar] [CrossRef]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J.; Zhang, J.; Dong, C.; Na, R.; Zheng, L.; et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J. Med. Virol. 2021, 93, 1057–1069. [Google Scholar] [CrossRef]
- Hua, C.-Z.; Miao, Z.-P.; Zheng, J.-S.; Huang, Q.; Sun, Q.-F.; Lu, H.-P.; Su, F.F.; Wang, W.H.; Huang, L.P.; Chen, D.Q.; et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 2804–2812. [Google Scholar] [CrossRef]
- Martins, M.M.; Prata-Barbosa, A.; da Cunha, A.J.L.A. Update on SARS-CoV-2 infection in children. Paediatr. Int. Child Health 2021, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Curatola, A.; Chiaretti, A.; Ferretti, S.; Bersani, G.; Lucchetti, D.; Capossela, L.; Sgambato, A.; Gatto, A. Cytokine Response to SARS-CoV-2 Infection in Children. Viruses 2021, 13, 1868. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Hoang, V.T.; Dao, T.L.; Dudouet, P.; Eldin, C.; Gautret, P. Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef]
- Barbato, C.; Di Certo, M.G.; Gabanella, F.; Petrella, C.; Fiore, M.; Passananti, C.; Colizza, A.; Cavalcanti, L.; Ralli, M.; Greco, A.; et al. Staying tuned for post-COVID-19 syndrome: Looking for new research to sniff out. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5318–5321. [Google Scholar] [CrossRef]
- West, A.E.; Pruunsild, P.; Timmusk, T. Neurotrophins: Transcription and translation. Handb. Exp. Pharmacol. 2014, 220, 67–100. [Google Scholar] [CrossRef]
- Dechant, G.; Neumann, H. Neurotrophins. Adv. Exp. Med. Biol. 2002, 513, 303–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelucci, F.; Piermaria, J.; Gelfo, F.; Shofany, J.; Tramontano, M.; Fiore, M.; Caltagirone, C.; Peppe, A. The effects of motor rehabilitation training on clinical symptoms and serum BDNF levels in Parkinson’s disease subjects. Can. J. Physiol. Pharmacol. 2016, 94, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirassa, P.; Triaca, V.; Amendola, T.; Fiore, M.; Aloe, L. EGF and NGF injected into the brain of old mice enhance BDNF and ChAT in proliferating subventricular zone. J. Neurosci. Res. 2003, 72, 557–564. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; NChaldakov, G.; Fiore, M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Fiore, M.; Tonchev, A.B.; Aloe, L. Neuroadipology: A novel component of neuroendocrinology. Cell. Biol. Int. 2010, 34, 1051–1053. [Google Scholar] [CrossRef]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Auton. Neurosci. 2006, 126, 30–38. [Google Scholar] [CrossRef]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-Derived Neurotrophic Factor and the Development of Structural Neuronal Connectivity. Dev. Neurobiol. 2011, 70, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Herbruggen, O.; Braun, A.; Rochlitzer, S.; Jockers-Scherubl, M.C.; Hellweg, R. Neurotrophic factors—A tool for therapeutic strategies in neurological, neuropsychiatric and neuroimmunological diseases? Curr. Med. Chem. 2007, 14, 2318–2329. [Google Scholar] [CrossRef]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef]
- Liu, P.; Li, S.; Tang, L. Nerve growth factor: A potential therapeutic target for lung diseases. Int. J. Mol. Sci. 2021, 22, 9112. [Google Scholar] [CrossRef] [PubMed]
- Usai, C.; Gibbons, J.M.; Pade, C.; Li, W.; Jacobs, S.R.M.; McKnight, Á.; Kennedy, P.T.; Gill, U.S. The β-NGF/TrkA Signalling Pathway Is Associated with the Production of Anti-Nucleoprotein IgG in Convalescent COVID-19. Front. Immunol. 2022, 12, 813300. [Google Scholar] [CrossRef] [PubMed]
- Minuzzi, L.G.; Seelaender, M.; Silva, B.S.D.A.; Cunha, E.d.B.B.; Deus, M.D.C.; Vasconcellos, F.T.F.; Marqueze, L.F.B.; Gadotti, A.C.; Baena, C.P.; Pereira, T.; et al. COVID-19 Outcome Relates with Circulating BDNF, According to Patient Adiposity and Age. Front. Nutr. 2021, 8, 784429. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi Mehrabani, M.; Karvandi, M.S.; Maafi, P.; Doroudian, M. Neurological complications associated with Covid-19; molecular mechanisms and therapeutic approaches. Rev. Med. Virol. 2022, e2334. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Gholami, M. Possible Neurological and Mental Outcomes of COVID-19 Infection: A Hypothetical Role of ACE-2\Mas\BDNF Signaling Pathway. Int. J. Prev. Med. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.R. New Statistical Procedures for the Social Sciences; Lawrence Erlbaum Associates Inc.: Hillsdale, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 2021, 75, e14357. [Google Scholar] [CrossRef]
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H.; et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Cox, R.J.; Langeland, N. Long COVID: A growing problem in need of intervention. Cell Rep. Med. 2022, 3, 100552. [Google Scholar] [CrossRef] [PubMed]
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; Davis, H.; Deer, R.; Gagnier, J.; Koraishy, F.M.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. MedRxiv preprint Serv. Health Sci.. 2021. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Beltrame, A.; Salguero, P.; Rossi, E.; Conesa, A.; Moro, L.; Bettini, L.R.; Rizzi, E.; D’Angió, M.; Deiana, M.; Piubelli, C.; et al. Association Between Sex Hormone Levels and Clinical Outcomes in Patients With COVID-19 Admitted to Hospital: An Observational, Retrospective, Cohort Study. Front. Immunol. 2022, 13, 834851. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Klein, S.L.; Levin, E.R. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology 2020, 161, bqaa127. [Google Scholar] [CrossRef]
- Chan Sui Ko, A.; Candellier, A.; Mercier, M.; Joseph, C.; Schmit, J.-L.; Lanoix, J.-P.; Andrejak, C. Number of initial symptoms is more related to long COVID-19 than acute severity of infection: A prospective cohort of hospitalized patients. Int. J. Infect. Dis. 2022, 118, 220–223. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Fumagalli, C.; Zocchi, C.; Tassetti, L.; Silverii, M.V.; Amato, C.; Livi, L.; Giovannoni, L.; Verrillo, F.; Bartoloni, A.; Marcucci, R.; et al. Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur. J. Intern. Med. 2021, 97, 36–41. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Hines, D.J.; Hines, R.M.; Lavangnananda, S.; Fels, S.; Medo, E. Influence of Previous COVID-19 and Mastitis Infections on the Secretion of Brain-Derived Neurotrophic Factor and Nerve Growth Factor in Human Milk. Int. J. Mol. Sci. 2021, 22, 3846. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Cirulli, F.; Alleva, E. The NGF saga: From animal models of psychosocial stress to stress-related psychopathology. Front. Neuroendocrinol. 2009, 30, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Bindocci, E.; Alleva, E. NGF, brain and behavioral plasticity. Neural. Plast. 2012, 2012, 784040. [Google Scholar] [CrossRef]
- Badowska-Szalewska, E.; Krawczyk, R.; Ludkiewicz, B.; Moryś, J. The effect of mild stress stimulation on the nerve growth factor (NGF) and tyrosine kinase receptor A (TrkA) immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus and hippocampus in aged vs. adult rats. Neuroscience 2015, 290, 346–356. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; De Gomes, M.G.; Goes, A.T.; Boeira, S.P.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Rocchi, G.; Escelsior, A.; Contini, P.; Colicchio, S.; de Berardis, D.; Amore, M.; Fornaro, P.; Fornaro, M. NGF serum levels variations in major depressed patients receiving duloxetine. Psychoneuroendocrinology 2013, 38, 1824–1828. [Google Scholar] [CrossRef]
- Loades, M.E.; Chatburn, E.; Higson-Sweeney, N.; Reynolds, S.; Shafran, R.; Brigden, A.; Linney, C.; McManus, M.N.; Borwick, C.; Crawley, E. Rapid Systematic Review: The Impact of Social Isolation and Loneliness on the Mental Health of Children and Adolescents in the Context of COVID-19. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1218–1239. [Google Scholar] [CrossRef]
- Morrissette, M. School Closures and Social Anxiety During the COVID-19 Pandemic. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 6–7. [Google Scholar] [CrossRef]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental Health of Children and Adolescents Amidst COVID-19 and Past Pandemics: A Rapid Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschner, Y.; Downey, G.P. Transforming Growth Factor-β: Master Regulator of the Respiratory System in Health and Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lommatzsch, M.; Niewerth, A.; Klotz, J.; Schulte-Herbrüggen, O.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. Platelet and plasma BDNF in lower respiratory tract infections of the adult. Respir. Med. 2007, 101, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Stoll, P.; Wuertemberger, U.; Bratke, K.; Zingler, C.; Virchow, J.C.; Lommatzsch, M. Stage-dependent association of BDNF and TGF-β1 with lung function in stable COPD. Respir. Res. 2012, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Colarusso, C.; Maglio, A.; Terlizzi, M.; Vitale, C.; Molino, A.; Pinto, A.; Vatrella, A.; Sorrentino, R. Post-COVID-19 Patients Who Develop Lung Fibrotic-like Changes Have Lower Circulating Levels of IFN-β but Higher Levels of IL-1α and TGF-β. Biomedicines 2021, 9, 1931. [Google Scholar] [CrossRef]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Chang, C.C.; Fang, W.H.; Chang, H.A.; Chen, T.Y.; Huang, S.Y. Sex-specific association between nerve growth factor polymorphism and cardiac vagal modulation. Psychosom. Med. 2014, 76, 638–643. [Google Scholar] [CrossRef]
- Piccinni, A.; Marazziti, D.; Del Debbio, A.; Bianchi, C.; Roncaglia, I.; Mannari, C.; Origlia, N.; Catena Dell’Osso, M.; Massimetti, G.; Domenici, L.; et al. Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: An analysis of sex differences. Chronobiol. Int. 2008, 25, 819–826. [Google Scholar] [CrossRef]
- Choi, S.W.; Bhang, S.; Ahn, J.H. Diurnal variation and gender differences of plasma brain-derived neurotrophic factor in healthy human subjects. Psychiatry. Res. 2011, 186, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.; Tirassa, P.; Fiore, M.; Pacitti, F.; Quartini, A.; Rosso, P.; Fico, E.; Garavini, A.; Pompili, A.; Vitali, M.; et al. Gender differences in ultradian serum levels of NGF and BDNF correlate with psychophysical traits in healthy humans. Riv. Psichiatr. 2021, 56, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; De Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord. Drug Targets 2021. [Google Scholar] [CrossRef]
- Fiore, M.; Tarani, L.; Radicioni, A.; Spaziani, M.; Ferraguti, G.; Putotto, C.; Gabanella, F.; Maftei, D.; Lattanzi, R.; Minni, A.; et al. Serum Prokineticin-2 in Prepubertal and Adult Klinefelter Individuals. Can. J. Physiol. Pharmacol. 2021, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tarani, L.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Messina, M.P.; Rasio, D.; De Luca, E.; Putotto, C.; et al. Neuroinflammatory Markers in the Serum of Prepubertal Children with down Syndrome. J. Immunol. Res. 2020, 2020, 6937154. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ceccanti, M.; Cestari, V.; Natella, F.; Bello, C.; Coccurello, R.; Mancinelli, R.; Fiore, M. Olive polyphenol effects in a mouse model of chronic ethanol addiction. Nutrition 2017, 33, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlatou, M.G.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Aloe, L.; Fiore, M. Changes in cognition induced by social isolation in the mouse are restored by electro-acupuncture. Physiol. Behav. 2009, 98, 537–542. [Google Scholar] [CrossRef]
- Ceccanti, M.; Coriale, G.; Hamilton, D.A.; Carito, V.; Coccurello, R.; Scalese, B.; Ciafrè, S.; Codazzo, C.; Messina, M.P.; Chaldakov, G.N.; et al. Virtual Morris task responses in individuals in an abstinence phase from alcohol. Can. J. Physiol. Pharmacol. 2018, 96, 128–136. [Google Scholar] [CrossRef]
- Payán-Pernía, S.; Gómez Pérez, L.; Remacha Sevilla, Á.F.; Sierra Gil, J.; Novelli Canales, S. Absolute Lymphocytes, Ferritin, C-Reactive Protein, and Lactate Dehydrogenase Predict Early Invasive Ventilation in Patients with COVID-19. Lab. Med. 2021, 52, 141–145. [Google Scholar] [CrossRef]
| GROUPS | Healthy Controls | Asymptomatics COVID-19 | Acute Symptomatics COVID-19 | Future Long-COVID-19 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Boys (n = 5) | Girls (n = 5) | Boys (n = 5) | Girls (n = 5) | Boys (n = 5) | Girls (n = 5) | Boys (n = 5) | Girls (n = 5) | ||
| SYMPTOMATOLOGY | |||||||||
| Symptoms during acute COVID-19 | Fever | - | - | 0/5 | 0/5 | 2/5 | 4/5 | 1/5 | 3/5 |
| Cough | - | - | 0/5 | 0/5 | 2/5 | 1/5 | 1/5 | 3/5 | |
| Breathing difficulties | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 3/5 | |
| Rhinitis | - | - | 0/5 | 0/5 | 2/5 | 1/5 | 0/5 | 1/5 | |
| Ear infection | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | |
| Pharyngitis | - | - | 0/5 | 0/5 | 1/5 | 2/5 | 3/5 | 1/5 | |
| Respiratory system | - | - | 0/5 | 0/5 | 4/5 | 2/5 | 3/5 | 4/5 | |
| Diarrhea | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | |
| Vomiting | - | - | 0/5 | 0/5 | 0/5 | 1/5 | 2/5 | 0/5 | |
| Nausea | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| Abdominal pain | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/0 | |
| Gastrointestinal pain | - | - | 0/5 | 0/5 | 0/5 | 1/5 | 2/5 | 1/5 | |
| Acute chest pain | - | - | 0/5 | 0/5 | 0/0 | 0/5 | 1/5 | 1/5 | |
| Asthenia | - | - | 0/5 | 0/5 | 2/5 | 2/5 | 4/5 | 4/5 | |
| Ageusia | - | - | 0/5 | 0/5 | 4/5 | 2/5 | 2/5 | 3/5 | |
| Anosmia | - | - | 0/5 | 0/5 | 4/5 | 1/5 | 3/5 | 3/5 | |
| Headache | - | - | 0/5 | 0/5 | 3/5 | 5/5 | 2/5 | 3/5 | |
| Neurological abnormalities | - | - | 0/5 | 0/5 | 5/5 | 5/5 | 4/5 | 5/5 | |
| Skin Rash | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 2/5 | |
| Acute Musculoskeletal Symptoms | - | - | 0/5 | 0/5 | 0/5 | 2/5 | 1/5 | 1/5 | |
| Myalgia | - | - | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 1/5 | |
| GROUPS | Healthy Controls | Asymptomatics COVID-19 | Acute Symptomatics COVID-19 | Future Long-COVID-19 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Boys (n = 5) | Girls (n = 5) | Boys (n = 5) | Girls (n = 5) | Boys (n = 5) | Girls (n = 5) | Boys (n = 5) | Girls (n = 5) | ||
| SYMPTOMATOLOGY | |||||||||
| Symptoms Post-COVID-19 | Fever | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - |
| Cough | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | |
| Breathing difficulties | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| Rhinitis | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Ear infection | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Pharyngitis | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Respiratory system | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | |
| Diarrhea | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Vomiting | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Nausea | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Abdominal pain | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Gastrointestinal pain | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| Acute chest pain | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | ||
| Asthenia | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 3/5 | |
| Ageusia | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | |
| Anosmia | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 1/5 | |
| Headache | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 1/5 | |
| Neurological abnormalities | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 4/5 | 2/5 | |
| Skin Rash | - | - | 0/5 | 0/5 | 0/5 | 0/5 | - | - | |
| Acute Musculoskeletal Symptoms | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| Myalgia | - | - | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrella, C.; Nenna, R.; Petrarca, L.; Tarani, F.; Paparella, R.; Mancino, E.; Di Mattia, G.; Conti, M.G.; Matera, L.; Bonci, E.; et al. Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study. Diagnostics 2022, 12, 1162. https://doi.org/10.3390/diagnostics12051162
Petrella C, Nenna R, Petrarca L, Tarani F, Paparella R, Mancino E, Di Mattia G, Conti MG, Matera L, Bonci E, et al. Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study. Diagnostics. 2022; 12(5):1162. https://doi.org/10.3390/diagnostics12051162
Chicago/Turabian StylePetrella, Carla, Raffaella Nenna, Laura Petrarca, Francesca Tarani, Roberto Paparella, Enrica Mancino, Greta Di Mattia, Maria Giulia Conti, Luigi Matera, Enea Bonci, and et al. 2022. "Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study" Diagnostics 12, no. 5: 1162. https://doi.org/10.3390/diagnostics12051162
APA StylePetrella, C., Nenna, R., Petrarca, L., Tarani, F., Paparella, R., Mancino, E., Di Mattia, G., Conti, M. G., Matera, L., Bonci, E., Ceci, F. M., Ferraguti, G., Gabanella, F., Barbato, C., Di Certo, M. G., Cavalcanti, L., Minni, A., Midulla, F., Tarani, L., & Fiore, M. (2022). Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study. Diagnostics, 12(5), 1162. https://doi.org/10.3390/diagnostics12051162









