Performance of the Abbott Architect Immuno-Chemiluminometric NT-proBNP Assay
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Materials and Methods
2.3. Statistical Analysis
3. Results
3.1. Performance Evaluation
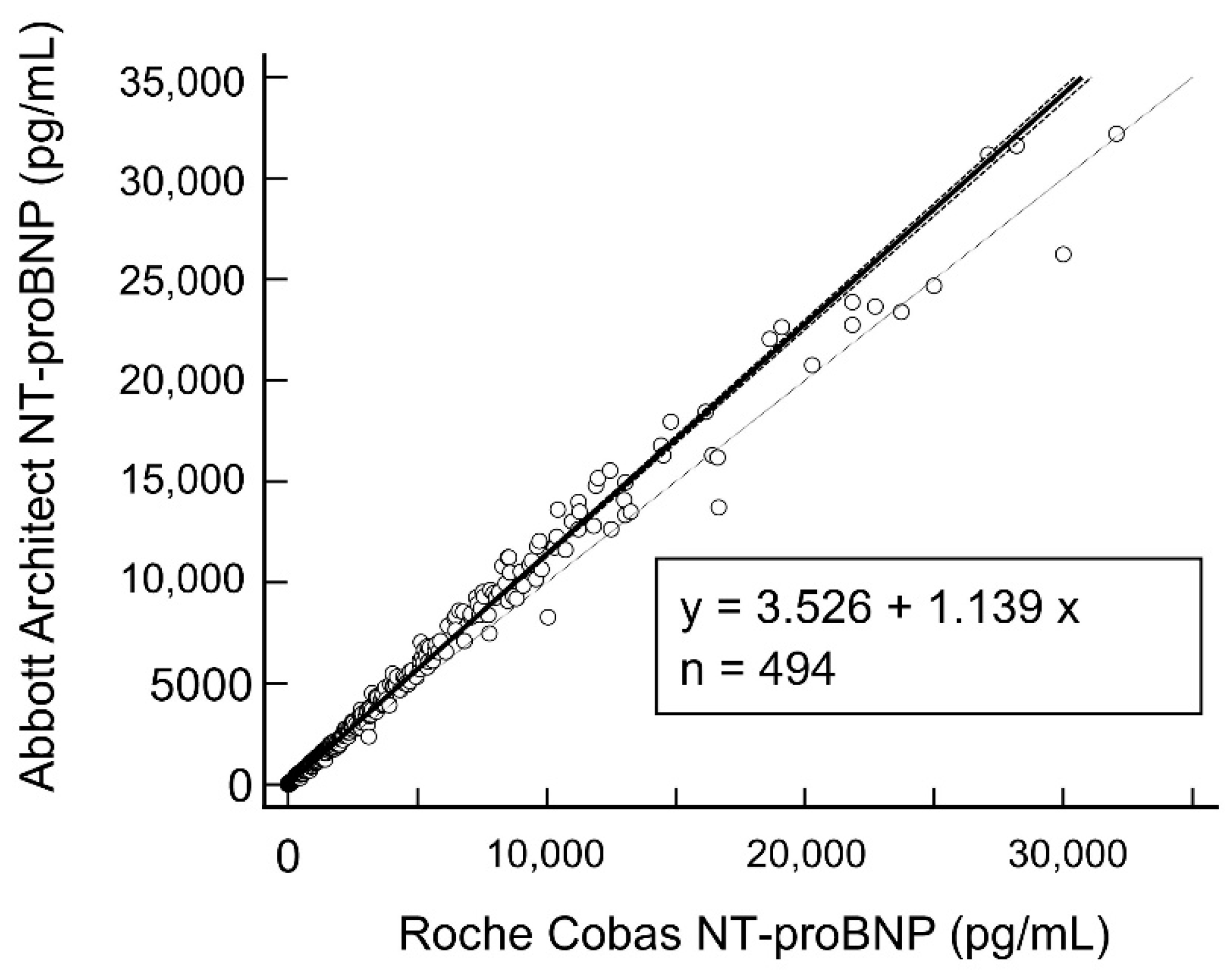
3.2. Combined Regression Analysis and Method Comparison
3.3. Assay Robustness to Biotin Interference
3.4. Influence of Age on NT-proBNP Levels
4. Discussion
- We confirm the assay’s good performance across two sites in two different geographical locations;
- We report the persistent negative bias between the Roche and Abbott assays (Roche < Abbott) across different levels of NT-proBNP;
- The AHA/ESC/ICON age-related NT-proBNP cut-offs to rule-in acute heart failure is also applicable for use on the Abbott NT-proBNP assay.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| HF | Heart failure |
| BNP | B-type natriuretic peptide |
| ARNI | Angiotensin receptor neprilysin inhibitor |
References
- Thygesen, K.; Mair, J.; Mueller, C.; Huber, K.; Weber, M.; Plebani, M.; Hasin, Y.; Biasucci, L.M.; Giannitsis, E.; Lindahl, B.; et al. Recommendations for the use of natriuretic peptides in acute cardiac care: A position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur. Heart J. 2012, 33, 2001–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clerico, A.; Passino, C.; Franzini, M.; Emdin, M. Cardiac biomarker testing in the clinical laboratory: Where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin. Chim. Acta 2015, 443, 17–24. [Google Scholar] [CrossRef]
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: Mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart. J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boar, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart. Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troughton, R.; Felker, G.M.; Januzzi, J.L., Jr. Natriuretic peptide-guided heart failure management. Eur. Heart J. 2014, 35, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huelsmann, M.; Neuhold, S.; Resl, M.; Strunk, G.; Brath, H.; Francesconi, C.; Adlbrecht, C.; Prager, R.; Luger, A.; Pacher, R.; et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): A prospective randomized controlled trial. J. Am. Coll. Cardiol. 2013, 62, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-Type Natriuretic Peptide During Treatment With Sacubitril/Valsartan: The PARADIGM-HF Trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simmonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Lewis, R.A.; Durrington, C.; Condliffe, R.; Kiely, D.G. BNP/NT-proBNP in pulmonary arterial hypertension: Time for point-of-care testing? Eur. Respir. Rev. 2020, 29, 200009. [Google Scholar] [CrossRef] [PubMed]
- Prausmuller, S.; Resl, M.; Arfsten, H.; Spinka, G.; Wurm, R.; Neuhold, S.; Bartko, P.E.; Goliasch, G.; Strunk, G.; Pavo, N.; et al. Performance of the recommended ESC/EASD cardiovascular risk stratification model in comparison to SCORE and NT-proBNP as a single biomarker for risk prediction in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Malachias, M.V.B.; Jhund, P.S.; Claggett, B.L.; Wijkman, M.O.; Bentley-Lewis, R.; Chaturvedi, N.; Desai, A.S.; Haffner, S.M.; Parving, H.H.; Prescott, M.F.; et al. NT-proBNP by Itself Predicts Death and Cardiovascular Events in High-Risk Patients With Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e017462. [Google Scholar] [CrossRef]
- Seegers, J.; Zabel, M.; Gruter, T.; Ammermann, A.; Weber-Kruger, M.; Edelmann, F.; Gelbrich, G.; Binder, L.; Herrmann-Lingen, C.; Groschel, K.; et al. Natriuretic peptides for the detection of paroxysmal atrial fibrillation. Open Heart 2015, 2, e000182. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, W.; Ruhs, H.; Burghaus, R.; Diedrich, C.; Duwal, S.; Eissing, T.; Garmann, D.; Meyer, M.; Ploeger, B.; Lippert, J. NT-proBNP Qualifies as a Surrogate for Clinical End Points in Heart Failure. Clin. Pharmacol. Ther. 2021, 110, 498–507. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Burnett, J.C.B., Jr.; Butler, J.; Camacho, A.; Felker, G.M.; Fiuzat, M.; O’Connor, C.; Solomon, S.D.; Vaduganathan, M.; Zile, M.R.; et al. Natriuretic Peptides as Inclusion Criteria in Clinical Trials: A JACC: Heart Failure Position Paper. JACC Heart Fail. 2020, 8, 347–358. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Cauliez, B.; Guignery, J.; Marinier, S.; Mariau, I.; Lavoinne, A. Two-year stability of NT-proBNP in frozen samples using the Roche Elecsys system. Ann. Clin. Biochem. 2008, 45, 318–319. [Google Scholar] [CrossRef] [Green Version]
- Clerico, A.; Zaninotto, M.; Prontera, C.; Giovannini, S.; Ndreu, R.; Franzini, M.; Zucchelli, G.C.; Plebani, M.; Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical Biochemistry. State of the art of BNP and NT-proBNP immunoassays: The CardioOrmoCheck study. Clin. Chim. Acta 2012, 414, 112–119. [Google Scholar] [CrossRef]
- Ordonez-Llanos, J.; Collinson, P.O.; Christenson, R.H. Amino-terminal pro-B-type natriuretic peptide: Analytic considerations. Am. J. Cardiol. 2008, 101, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Peake, R.W.A.; Turner, H.E.; Leaper, W.; Deans, K.A.; Hannah, A.; Croal, B.L. Comparison of sample types for N-terminal pro-B-type natriuretic peptide measured on the Siemens Immulite 2500 and Dimension Vista LOCI methods. Ann. Clin. Biochem. 2012, 49, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Saenger, A.K.; Jaffe, A.S.; Body, R.; Collinson, P.O.; Kavsak, P.A.; Lam, C.S.P.; Lefevre, G.; Omland, T.; Ordonez-Llanos, J.; Pulkki, K.; et al. Cardiac troponin and natriuretic peptide analytical interferences from hemolysis and biotin: Educational aids from the IFCC Committee on Cardiac Biomarkers (IFCC C-CB). Clin. Chem. Lab. Med. 2019, 57, 633–640. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Kimmenade, R.V.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, I.; Kuan, W.S.; Frampton, C.; Troughton, R.; Liew, O.W.; Chong, J.P.C.; Chan, S.P.; Tan, L.L.; Lin, W.Q.; Pemberton, C.J.; et al. Superior performance of N-terminal pro brain natriuretic peptide for diagnosis of acute decompensated heart failure in an Asian compared with a Western setting. Eur. J. Heart Fail. 2017, 19, 209–217. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Precision of Quantitative Measurement Procedures: Approved Guideline, 3rd ed.; CLSI Document EP05-A3; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline; CLSI Document EP06-A; CLSI: Wayne, PA, USA, 2003. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline, 2nd ed.; CLSI Document EP17-A2; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Collin-Chavagnac, D.; Dehoux, M.; Schellenberg, F.; Cauliez, B.; Maupas-Schwalm, F.; Lefevre, G.; Société Française de Biologie Clinique Cardiac Markers Working Group. Head-to-head comparison of 10 natriuretic peptide assays. Clin. Chem. Lab. Med. 2015, 53, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Masotti, S.; Musetti, V.; Prontera, C.; Storti, S.; Ndreu, R.; Passino, C.; Zucchelli, G.; Clerico, A. Evaluation of analytical performances using standardized analytical protocols and comparison of clinical results of the new ADVIA BNP and NT-proBNP immunoassays for the Centaur XPT platform. Clin. Chem. Lab. Med. 2019, 57, 911–917. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Chen-Tournoux, A.A.; Christenson, R.H.; Doros, G.; Hollander, J.E.; Levy, P.D.; Nagurney, J.T.; Nowak, R.M.; Pang, P.S.; Patel, D.; et al. N-Terminal Pro-B-Type Natriuretic Peptide in the Emergency Department: The ICON-RELOADED Study. J. Am. Coll. Cardiol. 2018, 71, 1191–1200. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, R.; Yang, F.Y.; Xi, L. Natriuretic peptide family as diagnostic/prognostic biomarker and treatment modality in management of adult and geriatric patients with heart failure: Remaining issues and challenges. J. Geriatr. Cardiol. 2018, 15, 540–546. [Google Scholar]
- McCullough, P.A.; Kluger, A.Y. Interpreting the Wide Range of NT-proBNP Concentrations in Clinical Decision Making. J. Am. Coll. Cardiol. 2018, 71, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ferguson, A.; Cervinski, M.A.; Lynch, K.L.; Kyle, P.B. AACC Guidance Document on Biotin Interference in Laboratory Tests. J. Appl. Lab. Med. 2020, 5, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Radulescu, A.; Shrestha, R.T.; Root, M.; Karger, A.B.; Killeen, A.A.; Hodges, J.S.; Fan, S.L.; Ferguson, A.; Garg, U.; et al. Association of Biotin Ingestion With Performance of Hormone and Nonhormone Assays in Healthy Adults. JAMA 2017, 318, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Katzman, B.M.; Lueke, A.J.; Donato, L.J.; Jaffe, A.S.; Baumann, N.A. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin. Biochem. 2018, 60, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Avery, G. Biotin interference in immunoassay: A review for the laboratory scientist. Ann. Clin. Biochem. 2019, 56, 424–430. [Google Scholar] [CrossRef]
- Mrosewski, I.; Urbank, M.; Stauch, T.; Switkowski, R. Interference from High-Dose Biotin Intake in Immunoassays for Potentially Time-Critical Analytes by Roche. Arch. Pathol. Lab. Med. 2020, 144, 1108–1117. [Google Scholar] [CrossRef] [Green Version]


| Roche NT-proBNP Level (pg/mL) | N | Mean NT-proBNP (pg/mL) | Bias (pg/mL), (% of Mean NT-proBNP) |
|---|---|---|---|
| <125 | 75 | 60.9 | 15.5 (25.5%) |
| 125–450 | 116 | 263 | 37.1 (14.1%) |
| 450–900 | 65 | 663 | 57.2 (8.63%) |
| 900–1800 | 68 | 1338 | 155 (11.6%) |
| >1800 | 170 | 7380 | 916 (12.4%) |
| Sample | Biotin (ng/mL) | Average Result (pg/mL) | Average Result (pmol/L) | % Difference vs. Un-Spiked (0) Biotin Sample |
|---|---|---|---|---|
| 1 | 0 | 79.30 | 9.36 | |
| 531.3 | 79.43 | 9.37 | 0.17% | |
| 1062.5 | 78.44 | 9.26 | −1.09% | |
| 1593.8 | 79.89 | 9.43 | 0.74% | |
| 2125.0 | 78.54 | 9027 | −0.96% | |
| 2656.3 | 80.24 | 9.47 | 1.18% | |
| 3187.5 | 78.79 | 9.30 | −0.65% | |
| 3718.8 | 78.80 | 9.30 | −0.64% | |
| 4250.0 | 79.74 | 9.41 | 0.55% | |
| 2 | 0 | 1815.26 | 214.20 | |
| 531.3 | 1760.91 | 207.79 | −2.99% | |
| 1062.5 | 1759.66 | 207.64 | −3.06% | |
| 1593.8 | 1777.98 | 209.80 | −2.05% | |
| 2125.0 | 1797.01 | 212.05 | −1.01% | |
| 2656.3 | 1804.90 | 212.98 | −0.57% | |
| 3187.5 | 1783.38 | 210.44 | −1.76% | |
| 3718.8 | 1765.07 | 208.28 | −2.76% | |
| 4250.0 | 1830.18 | 215.96 | 0.82% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.-S.; Liang, Y.L.; Phua, S.K.; Murtagh, G.; Hoefer, I.E.; Stokwielder, R.H.; Kosevich, M.; Yen, J.; Sickan, J.; Varounis, C.; et al. Performance of the Abbott Architect Immuno-Chemiluminometric NT-proBNP Assay. Diagnostics 2022, 12, 1172. https://doi.org/10.3390/diagnostics12051172
Lau C-S, Liang YL, Phua SK, Murtagh G, Hoefer IE, Stokwielder RH, Kosevich M, Yen J, Sickan J, Varounis C, et al. Performance of the Abbott Architect Immuno-Chemiluminometric NT-proBNP Assay. Diagnostics. 2022; 12(5):1172. https://doi.org/10.3390/diagnostics12051172
Chicago/Turabian StyleLau, Chin-Shern, Ya Li Liang, Soon Kieng Phua, Gillian Murtagh, Imo E. Hoefer, Ron H. Stokwielder, Milica Kosevich, Jennifer Yen, Jaganathan Sickan, Christos Varounis, and et al. 2022. "Performance of the Abbott Architect Immuno-Chemiluminometric NT-proBNP Assay" Diagnostics 12, no. 5: 1172. https://doi.org/10.3390/diagnostics12051172






