A Survey on the Role of Artificial Intelligence in Biobanking Studies: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality Evaluation
3. Results
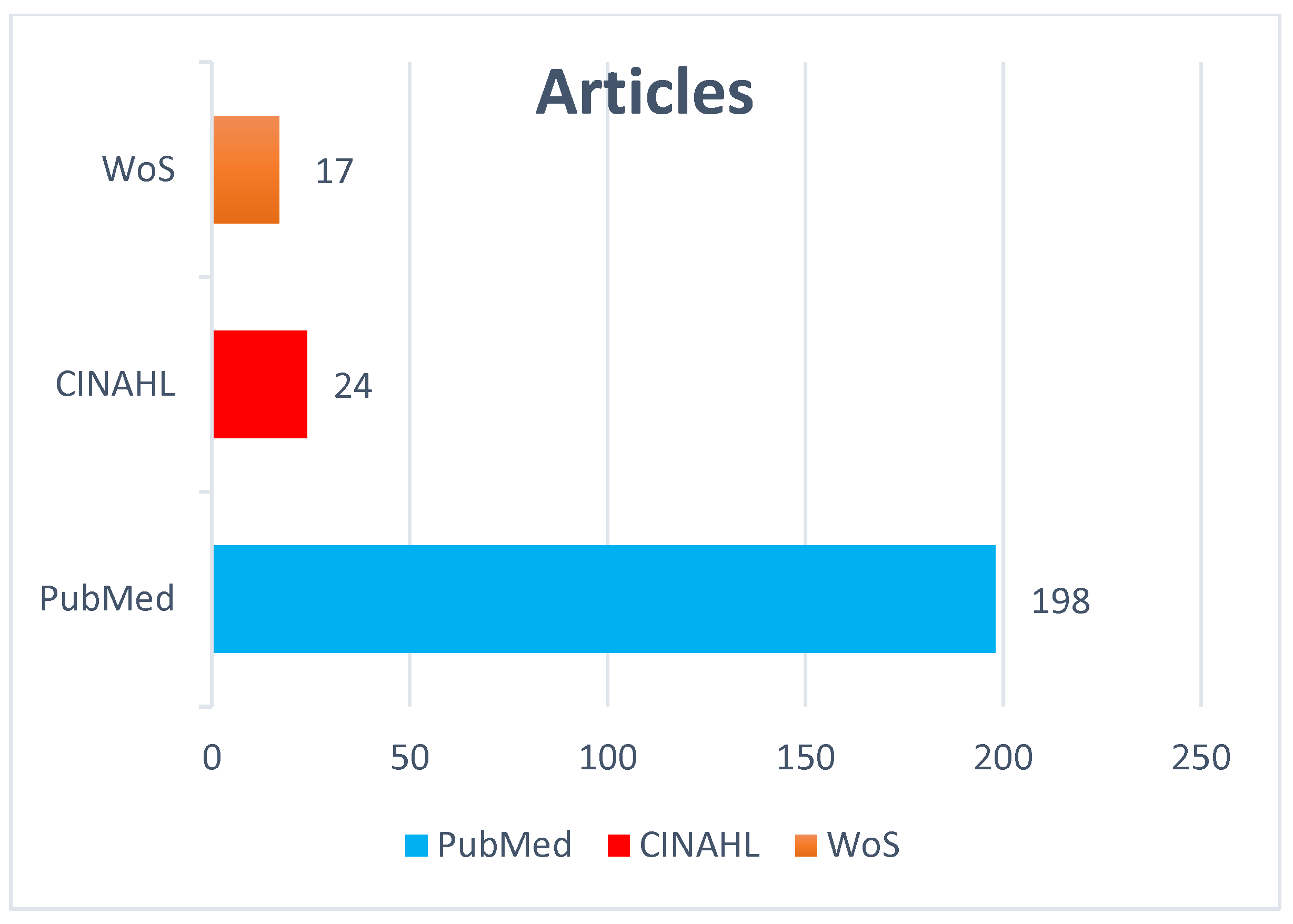
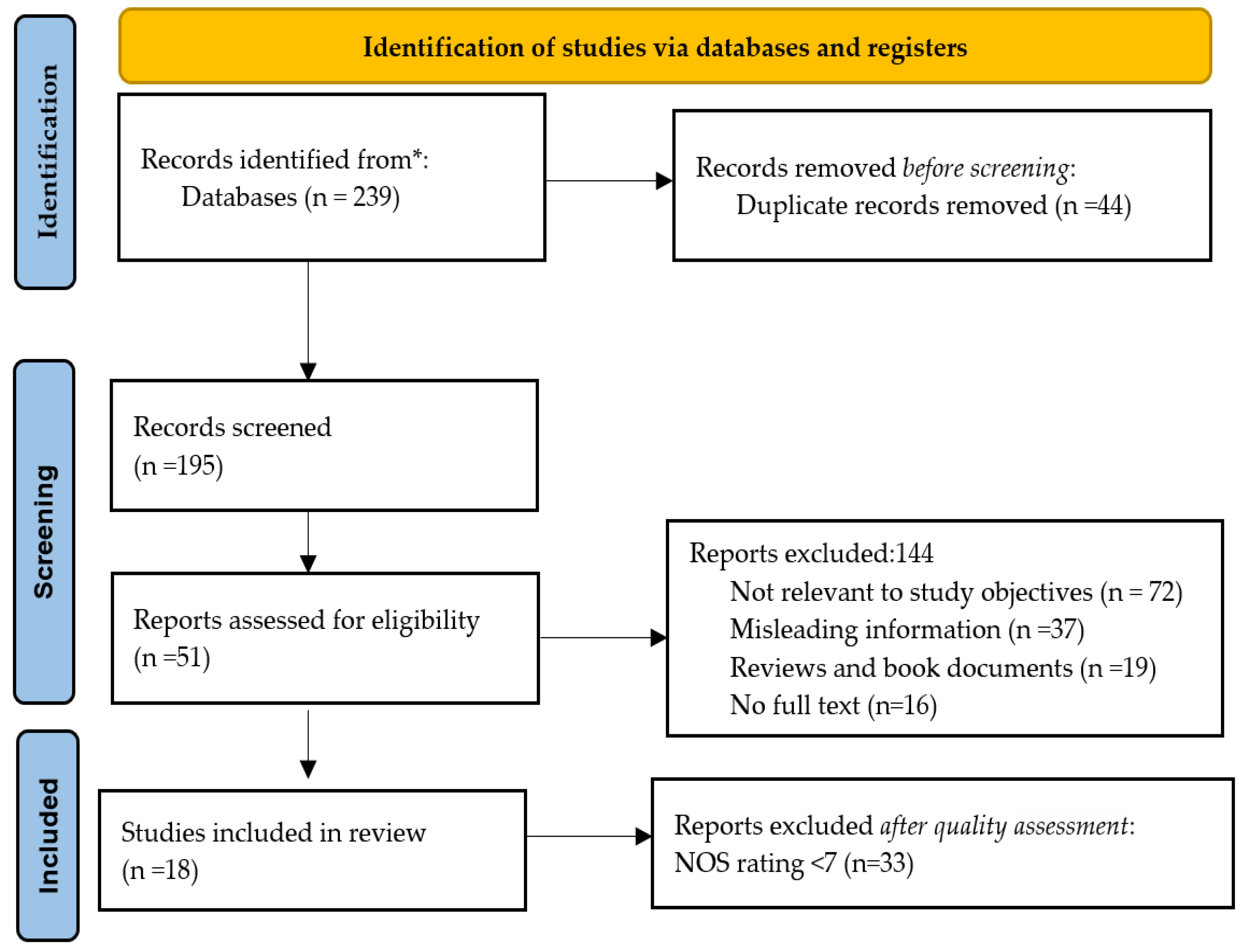
3.1. Search Outcomes
3.2. Study Characteristics
Biobanking Studies Associated with Image Datasets
3.3. Applications of AI in Disease Detection with Biobanking Datasets
3.3.1. Alzheimer’s Disease Detection
3.3.2. Cardiovascular Diseases
3.3.3. Chronic Diseases
3.3.4. Disease Subtype Classification
3.3.5. Pandemics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Project Acronym
References
- Villa, G.; Romagnoli, S. Registers and biobanks in ICU and anesthesia. Minerva Anestesiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.; Bernardini, S.; Ferrari, M.; Fortina, P.; Gouget, B.; Gruson, D.; Lang, T.; Loh, T.P.; Morris, H.A.; Park, J.Y.; et al. Key questions about the future of laboratory medicine in the next decade of the 21st century: A report from the IFCC-Emerging Technologies Division. Clin. Chim. Acta 2019, 495, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Kinkorová, J.; Topolčan, O. Biobanks in Horizon 2020: Sustainability and attractive perspectives. EPMA J. 2018, 9, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, K.; Klein, K.O.; Shao, X.; Fritzler, M.J.; Hudson, M.; Colmegna, I.; Pastinen, T.; Bernatsky, S.; Greenwood, C.M.T. Thousands of CpGs Show DNA Methylation Differences in ACPA-Positive Individuals. Genes 2021, 12, 1349. [Google Scholar] [CrossRef]
- Benjamins, J.W.; Yeung, M.W.; van de Vegte, Y.J.; Said, M.A.; van der Linden, T.; Ties, D.; Juarez-Orozco, L.E.; Verweij, N.; van der Harst, P. Genomic insights in ascending aortic size and distensibility. EBioMedicine 2022, 75, 103783. [Google Scholar] [CrossRef]
- Leming, M.; Suckling, J. Deep learning for sex classification in resting-state and task functional brain networks from the UK Biobank. NeuroImage 2021, 241, 118409. [Google Scholar] [CrossRef]
- Viertler, C.; Zatloukal, K. Biobanken und Biomolekulare Ressourcen Forschungsinfrastruktur (BBMRI). Der Pathologe 2008, 29 (Suppl. 2), 210–213. [Google Scholar] [CrossRef][Green Version]
- Kulkarni, S.; Jha, S. Artificial Intelligence, Radiology, and Tuberculosis: A Review. Acad. Radiol. 2019, 27, 71–75. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhao, L.; Liu, Z.; Wu, X.; Chen, J.; Long, E.; Lin, D.; Zhu, Y.; Chen, C.; Lin, Z.; et al. Implementation of artificial intelligence in medicine: Status analysis and development suggestions. Artif. Intell. Med. 2020, 102, 101780. [Google Scholar] [CrossRef]
- Holmes, J.H.; Sacchi, L.; Bellazzi, R.; Peek, N. Artificial Intelligence in Medicine AIME 2015. Artif. Intell. Med. 2017, 81, 1–2. [Google Scholar] [CrossRef]
- Tack, C. Artificial intelligence and machine learning | applications in musculoskeletal physiotherapy. Musculoskelet. Sci. Pract. 2018, 39, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, O.; Aromolaran, D.; Isewon, I.; Oyelade, J. Machine learning approach to gene essentiality prediction: A review. Brief. Bioinform. 2021, 22, bbab128. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yoon, J.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning–Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; E Goodman, K.; Kaminsky, J.; Lessler, J. What is Machine Learning? A Primer for the Epidemiologist. Am. J. Epidemiol. 2019, 188, 2222–2239. [Google Scholar] [CrossRef]
- Vogeley, K.; Bente, G. Artificial humans: Psychology and neuroscience perspectives on embodiment and nonverbal communication. Neural Netw. 2010, 23, 1077–1090. [Google Scholar] [CrossRef]
- Huang, H.; Hsu, B.W.; Lee, C.; Tseng, V.S. Development of a light-weight deep learning model for cloud applications and remote diagnosis of skin cancers. J. Dermatol. 2020, 48, 310–316. [Google Scholar] [CrossRef]
- Noor, M.B.T.; Zenia, N.Z.; Kaiser, M.S.; Mahmud, M.; Al Mamun, S. Detecting Neurodegenerative Disease from MRI: A Brief Review on a Deep Learning Perspective. Lect. Notes Comput. Sci. 2019, 11976, 115–125. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Liu, M.; Zhang, D. A Survey on Deep Learning for Neuroimaging-Based Brain Disorder Analysis. Front. Neurosci. 2020, 14, 779. [Google Scholar] [CrossRef]
- Padilla, L.M.; Creem-Regehr, S.H.; Hegarty, M.; Stefanucci, J.K. Decision making with visualizations: A cognitive framework across disciplines. Cogn. Res. Princ. Implic. 2018, 3, 29. [Google Scholar] [CrossRef]
- Chen, M.; Decary, M. Artificial intelligence in healthcare: An essential guide for health leaders. Health Manag. Forum 2019, 33, 10–18. [Google Scholar] [CrossRef]
- Baskaran, L.; Ying, X.; Xu, Z.; Al’Aref, S.J.; Lee, B.C.; Lee, S.-E.; Danad, I.; Park, H.-B.; Bathina, R.; Baggiano, A.; et al. Machine learning insight into the role of imaging and clinical variables for the prediction of obstructive coronary artery disease and revascularization: An exploratory analysis of the CONSERVE study. PLoS ONE 2020, 15, e0233791. [Google Scholar] [CrossRef] [PubMed]
- Vodencarevic, A.; Tascilar, K.; Hartmann, F.; Reiser, M.; Hueber, A.J.; Haschka, J.; Bayat, S.; Meinderink, T.; Knitza, J.; on behalf of the RETRO study group; et al. Advanced machine learning for predicting individual risk of flares in rheumatoid arthritis patients tapering biologic drugs. Arthritis Res. Ther. 2021, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- WCross, W.F.; West, J.C.; Pisani, A.R.; Crean, H.F.; Nielsen, J.L.; Kay, A.H.; Caine, E.D. A randomized controlled trial of suicide prevention training for primary care providers: A study protocol. BMC Med. Educ. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.A.; Zallman, L.; Pagán, J.A.; Ortega, A.N.; Wang, Y.; Tatar, M.; Stimpson, J.P. Comparison of Use of Health Care Services and Spending for Unauthorized Immigrants vs Authorized Immigrants or US Citizens Using a Machine Learning Model. JAMA Netw. Open 2020, 3, e2029230. [Google Scholar] [CrossRef] [PubMed]
- Strang, K.D.; Sun, Z. Hidden big data analytics issues in the healthcare industry. Health Inform. J. 2019, 26, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Narita, A.; Ueki, M.; Tamiya, G. Artificial intelligence powered statistical genetics in biobanks. J. Hum. Genet. 2020, 66, 61–65. [Google Scholar] [CrossRef]
- Marmor, R.A.; Clay, B.; Millen, M.; Savides, T.J.; Longhurst, C.A. The Impact of Physician EHR Usage on Patient Satisfaction. Appl. Clin. Inform. 2018, 09, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Alemayehu, D.; Zou, K.H. Applications of ROC Analysis in Medical Research. Acad. Radiol. 2012, 19, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Jiang, Y.; Huang, H.; Swaroop, A.; Chew, E.Y.; Weeks, D.E.; Chen, W.; Ding, Y. Genome-Wide Association Studies-Based Machine Learning for Prediction of Age-Related Macular Degeneration Risk. Transl. Vis. Sci. Technol. 2021, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Solem, E.; Petersen, T.S.; Hansen, C.; Hansen, C.; Lioma, C.; Igel, C.; Boomsma, W.; Krause, O.; Lorenzen, S.; Selvan, R.; et al. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national European cohort of 5594 patients. Sci. Rep. 2021, 11, 3246. [Google Scholar] [CrossRef]
- Rim, T.H.; Lee, C.J.; Tham, Y.-C.; Cheung, N.; Yu, M.; Lee, G.; Kim, Y.; Ting, D.S.W.; Chong, C.C.Y.; Choi, Y.S.; et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit. Health 2021, 3, e306–e316. [Google Scholar] [CrossRef]
- MDabbah, M.A.; Reed, A.B.; Booth, A.T.C.; Yassaee, A.; Despotovic, A.; Klasmer, B.; Binning, E.; Aral, M.; Plans, D.; Morelli, D.; et al. Machine learning approach to dynamic risk modeling of mortality in COVID-19: A UK Biobank study. Sci. Rep. 2021, 11, 16936. [Google Scholar] [CrossRef]
- Alaa, A.M.; Bolton, T.; Di Angelantonio, E.; Rudd, J.H.F.; Van Der Schaar, M. Cardiovascular disease risk prediction using automated machine learning: A prospective study of 423,604 UK Biobank participants. PLoS ONE 2019, 14, e0213653. [Google Scholar] [CrossRef]
- Tian, J.; Smith, G.; Guo, H.; Liu, B.; Pan, Z.; Wang, Z.; Xiong, S.; Fang, R. Modular machine learning for Alzheimer’s disease classification from retinal vasculature. Sci. Rep. 2021, 11, 238. [Google Scholar] [CrossRef]
- Alfaro-Almagro, F.; Jenkinson, M.; Bangerter, N.K.; Andersson, J.L.; Griffanti, L.; Douaud, G.; Sotiropoulos, S.N.; Jbabdi, S.; Hernandez-Fernandez, M.; Vallee, E.; et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 2018, 166, 400–424. [Google Scholar] [CrossRef]
- Peng, H.; Gong, W.; Beckmann, C.F.; Vedaldi, A.; Smith, S.M. Accurate brain age prediction with lightweight deep neural networks. Med. Image Anal. 2021, 68, 101871. [Google Scholar] [CrossRef]
- Alkaabi, L.A.; Ahmed, L.S.; Al Attiyah, M.F.; Abdel-Rahman, M.E. Predicting hypertension using machine learning: Findings from Qatar Biobank Study. PLoS ONE 2020, 15, e0240370. [Google Scholar] [CrossRef]
- Langner, T.; Strand, R.; Ahlström, H.; Kullberg, J. Large-scale biometry with interpretable neural network regression on UK Biobank body MRI. Sci. Rep. 2020, 10, 17752. [Google Scholar] [CrossRef]
- Attar, R.; Pereañez, M.; Gooya, A.; Albà, X.; Zhang, L.; de Vila, M.H.; Lee, A.M.; Aung, N.; Lukaschuk, E.; Sanghvi, M.M.; et al. Quantitative CMR population imaging on 20,000 subjects of the UK Biobank imaging study: LV/RV quantification pipeline and its evaluation. Med. Image Anal. 2019, 56, 26–42. [Google Scholar] [CrossRef]
- Willetts, M.; Hollowell, S.; Aslett, L.; Holmes, C.; Doherty, A. Statistical machine learning of sleep and physical activity phenotypes from sensor data in 96,220 UK Biobank participants. Sci. Rep. 2018, 8, 7961. [Google Scholar] [CrossRef]
- Reinbolt, R.E.; Sonis, S.; Timmers, C.D.; Fernandez-Martinez, J.L.; Cernea, A.; Deandrés-Galiana, E.J.; Hashemi, S.; Miller, K.; Pilarski, R.; Lustberg, M.B. Genomic risk prediction of aromatase inhibitor-related arthralgia in patients with breast cancer using a novel machine-learning algorithm. Cancer Med. 2017, 7, 240–253. [Google Scholar] [CrossRef]
- Naito, T.; Suzuki, K.; Hirata, J.; Kamatani, Y.; Matsuda, K.; Toda, T.; Okada, Y. A deep learning method for HLA imputation and trans-ethnic MHC fine-mapping of type 1 diabetes. Nat. Commun. 2021, 12, 1639. [Google Scholar] [CrossRef]
- Dinsdale, N.K.; Bluemke, E.; Smith, S.M.; Arya, Z.; Vidaurre, D.; Jenkinson, M.; Namburete, A.I. Learning patterns of the ageing brain in MRI using deep convolutional networks. NeuroImage 2020, 224, 117401. [Google Scholar] [CrossRef]
- Alipanahi, B.; Hormozdiari, F.; Behsaz, B.; Cosentino, J.; McCaw, Z.R.; Schorsch, E.; Sculley, D.; Dorfman, E.H.; Foster, P.J.; Peng, L.H.; et al. Large-scale machine-learning-based phenotyping significantly improves genomic discovery for optic nerve head morphology. Am. J. Hum. Genet. 2021, 108, 1217–1230. [Google Scholar] [CrossRef]
- Schulz, M.-A.; Chapman-Rounds, M.; Verma, M.; Bzdok, D.; Georgatzis, K. Inferring disease subtypes from clusters in explanation space. Sci. Rep. 2020, 10, 12900. [Google Scholar] [CrossRef]
- Ullah, E.; Mall, R.; Rawi, R.; Moustaid-Moussa, N.; Butt, A.A.; Bensmail, H. Harnessing Qatar Biobank to understand type 2 diabetes and obesity in adult Qataris from the First Qatar Biobank Project. J. Transl. Med. 2018, 16, 99. [Google Scholar] [CrossRef]
- Vergani, V.; Razavi, R.; Puyol-Antón, E.; Ruijsink, B. Deep Learning for Classification and Selection of Cine CMR Images to Achieve Fully Automated Quality-Controlled CMR Analysis From Scanner to Report. Front. Cardiovasc. Med. 2021, 8, 1260. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J. Presymptomatic studies in ALS: Rationale, challenges, and approach. Neurology 2012, 79, 1732–1739. [Google Scholar] [CrossRef]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLOS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef]
- Subramanian, R.; He, Q.; Pascual, M. Quantifying asymptomatic infection and transmission of COVID-19 in New York City using observed cases, serology, and testing capacity. Proc. Natl. Acad. Sci. USA 2021, 118, e2019716118. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-”how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Budd, S.; Robinson, E.C.; Kainz, B. A survey on active learning and human-in-the-loop deep learning for medical image analysis. Med. Image Anal. 2021, 71, 102062. [Google Scholar] [CrossRef]
- Kiesow, H.; Spreng, R.N.; Holmes, A.J.; Chakravarty, M.M.; Marquand, A.F.; Yeo, B.T.T.; Bzdok, D. Deep learning identifies partially overlapping subnetworks in the human social brain. Commun. Biol. 2021, 4, 65. [Google Scholar] [CrossRef]
- Liss, D.T.; Finch, E.A.; Gregory, D.L.; Cooper, A.; Ackermann, R.T. Design and participant characteristics for a randomized effectiveness trial of an intensive lifestyle intervention to reduce cardiovascular risk in adults with type 2 diabetes: The I-D-HEALTH study. Contemp. Clin. Trials. 2016, 46, 114–121. [Google Scholar] [CrossRef]
- Kim, H.; Lim, D.; Kim, Y. Classification and Prediction on the Effects of Nutritional Intake on Overweight/Obesity, Dyslipidemia, Hypertension and Type 2 Diabetes Mellitus Using Deep Learning Model: 4–7th Korea National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2021, 18, 5597. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Han, X.; Zhang, Y.; Li, F.; Li, H. Single-Nucleotide Polymorphisms Promote Dysregulation Activation by Essential Gene Mediated Bio-Molecular Interaction in Breast Cancer. Front. Oncol. 2021, 11, 791943. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Juárez, M.A.; Torres-Gómez, A.; Hoyo-Ulloa, I.; León-Montiel, R.D.J.; U’Ren, A.B. Identification of high-risk COVID-19 patients using machine learning. PLoS ONE 2021, 16, e0257234. [Google Scholar] [CrossRef] [PubMed]
- Weissler, E.H.; Naumann, T.; Andersson, T.; Ranganath, R.; Elemento, O.; Luo, Y.; Ghassemi, M. The role of machine learning in clinical research: Transforming the future of evidence generation. Trials 2021, 22, 537. [Google Scholar] [CrossRef] [PubMed]
- Riegman, P.H.; Morente, M.M.; Betsou, F.; De Blasio, P.; Geary, P. Biobanking for better healthcare. Mol. Oncol. 2008, 2, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Cianflone, A.; Grimaldi, A.M.; Incoronato, M.; Bevilacqua, P.; Messina, F.; Baselice, S.; Soricelli, A.; Mirabelli, P.; Salvatore, M. Biobanking in health care: Evolution and future directions. J. Transl. Med. 2019, 17, 172. [Google Scholar] [CrossRef]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Sinitsyna, A.; Izotov, A.; Kaysheva, A. Biobanks—A Platform for Scientific and Biomedical Research. Diagnostics 2020, 10, 485. [Google Scholar] [CrossRef]
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Oschwald, J.; Guye, S.; Liem, F.; Rast, P.; Willis, S.; Röcke, C.; Jäncke, L.; Martin, M.; Mérillat, S. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev. Neurosci. 2019, 31, 1–57. [Google Scholar] [CrossRef]
- Mu, S.H.; Xu, M.; Duan, J.X.; Zhang, J.; Tan, L.H. Localizing Age-Related Changes in Brain Structure Using Voxel-Based Morphometry. Neural Plast. 2017, 2017, 6303512. [Google Scholar] [CrossRef]
- Ramanoël, S.; York, E.; Le Petit, M.; Lagrené, K.; Habas, C.; Arleo, A. Age-Related Differences in Functional and Structural Connectivity in the Spatial Navigation Brain Network. Front. Neural Circuits 2019, 13, 69. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.W.; Karthikesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. NPJ Digit. Med. 2021, 4, 65. [Google Scholar] [CrossRef]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit. Health 2019, 1, e271–e297. [Google Scholar] [CrossRef]
- Lundervold, A.; Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys. 2018, 29, 102–127. [Google Scholar] [CrossRef]
- Rodrigue, A.L.; Mastrovito, D.; Esteban, O.; Durnez, J.; Koenis, M.M.; Janssen, R.; Alexander-Bloch, A.; Knowles, E.M.; Mathias, S.R.; Mollon, J.; et al. Searching for Imaging Biomarkers of Psychotic Dysconnectivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 1135–1144. [Google Scholar] [CrossRef]
- Cuellar, L.K.; Friedrich, A.; Gabernet, G.; de la Garza, L.; Fillinger, S.; Seyboldt, A.; Koch, T.; Oven-Krockhaus, S.Z.; Wanke, F.; Richter, S.; et al. A data management infrastructure for the integration of imaging and omics data in life sciences. BMC Bioinform. 2022, 23, 61. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Choi, E.; Kaplin, S.; Venner, E.; Murugan, M.; Wang, Z.; Glicksberg, B.S.; Amos, C.I.; Schatz, M.C.; et al. Artificial Intelligence and Cardiovascular Genetics. Life 2022, 12, 279. [Google Scholar] [CrossRef]
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic principles of biobanking: From biological samples to precision medicine for patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef]
- Bard, A.; Raisi-Estabragh, Z.; Ardissino, M.; Lee, A.M.; Pugliese, F.; Dey, D.; Sarkar, S.; Munroe, P.B.; Neubauer, S.; Harvey, N.C.; et al. Automated Quality-Controlled Cardiovascular Magnetic Resonance Pericardial Fat Quantification Using a Convolutional Neural Network in the UK Biobank. Front. Cardiovasc. Med. 2021, 8, 677574. [Google Scholar] [CrossRef]
- Samuel, G.; Lucivero, F.; Lucassen, A.M. Sustainable biobanks: A case study for a green global bioethics. Glob. Bioeth. 2022, 33, 50. [Google Scholar] [CrossRef]
- Kinkorová, J.; Topolčan, O. Biobanks in the era of big data: Objectives, challenges, perspectives, and innovations for predictive, preventive, and personalised medicine. EPMA J. 2020, 11, 333. [Google Scholar] [CrossRef]
- Budimir, D.; Polašek, O.; Marušić, A.; Kolčić, I.; Zemunik, T.; Boraska, V.; Jerončić, A.; Boban, M.; Campbell, H.; Rudan, I. Ethical aspects of human biobanks: A systematic review. Croat. Med. J. 2011, 52, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.W.; Saria, S.; Ohno-Machado, L.; Shah, A.; Escobar, G. Big Data In Health Care: Using Analytics To Identify And Manage High-Risk And High-Cost Patients. Health Aff. 2014, 33, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Reina-Romo, E.; Mandal, S.; Amorim, P.; Bloemen, V.; Ferraris, E.; Geris, L. Towards the Experimentally-Informed In Silico Nozzle Design Optimization for Extrusion-Based Bioprinting of Shear-Thinning Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- The Diagnostic Process-Improving Diagnosis in Health Care-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK338593/ (accessed on 28 March 2022).
| N | Study Type | Country | Sample | Experimental Setup | Findings | Performance Metric | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Experimental | UK | 19000 T1-weighted MRI (data spilt was done for training 12,802 and testing 6885) | A three-dimensional CNN model was developed for the prediction of chronological age. | Predicted age against true age of both male and female groups from linear and nonlinear registered images. | - | [46] |
| 2 | Experimental | UK | 81,830 fundus images with random seed for training and testing | An ML model was employed for predicting optic nerve head features. | Vertical cup-to-disc ratio (VCDR), a diagnostic parameter and cardinal endophenotype for glaucoma are predicted. | - | [47] |
| 3 | Experimental | UK | 35,358 subjects with 32,215 Caucasians | ML models were developed for age-related macular degeneration (AMD) risk prediction. | ML models were more satisfactory than normal controls. | ROC: 0.81 | [32] |
| 4 | Experimental | Japan, UK | 416,846 subjects (62,387 subjects from Japan, 354,459 from the UK) | Developed DEEP*HLA deep learning models for human leukocyte antigen (HLA). | DEEP*HLA applied to subjects and succeed in linked class I and II HLA variation shared risk from those populations. | Sensitivity: 98.7% | [45] |
| 5 | Experimental | UK | Around 500,000 individuals in the age range 40 to 69 | Proposed pipeline to classify Alzheimer’s disease accurately. | Modular ML models had high accuracy to detect and classify Alzheimer’s disease | Accuracy: 82.44% | [37] |
| 6 | Experimental | UK, Denmark | 5594 patients | Developed and validated ML model and predicted risk of COVID-19. | ML models can predict hospital and ICU admissions risk for COVID-19 patients by using age, gender, and BMI demographic variables. | ROC: 0.80 | [33] |
| 7 | Experimental | South Korea, Singapore, UK | 216,152 retinal images | Five datasets from three different biobanks were used to train and validate deep learning models for coronary artery calcium (CAC) scores. | In South Korea, 6.3% of participants had cardiovascular events, and in Singapore and the UK 3.6%, and 0.7% of participants had fatal cardiovascular events, respectively. | ROC: 0.74 | [34] |
| 8 | Experimental | UK | 11,245 participants | Designed and validated ML model to predict mortality risk of COVID-19. | ML models are highly accurate with patient characteristics, brief medical history, symptoms, and vital signs. | ROC: 0.91 | [35] |
| 9 | RCT | UK | 14,503 T1-weighted structural MRI data | Data spilt was done for training 12,949 and testing 6885. Simple Fully Convolutional Network (FCN) to predict brain age. | 99.5% accuracy for sex classification and brain age prediction. | Accuracy: 99.5% | [39] |
| 10 | Cross-sectional | Qatar | 987 Qatar residents | Machine learning models used to predict Hypertension. | ML models are a rapid productive model to predict Hypertension. | Accuracy: 82.1% | [40] |
| 11 | Experimental | UK, ATLAS | Madelon dataset (16 classes, 50 features), fashion-MINST dataset (dimensionality: 782, sample size: 70,000), T1 brain MRI data of 10,000 participants (UKBB), 60,498 gene expressions of 8500 participants (TCGA) | Introduced an approach to discover disease subtypes: Classifier trained as healthy vs diseased to extract instance information instead of analyzing raw data. | Clustering is helpful in understanding and identification of disease subtypes. | - | [48] |
| 12 | Experimental | UK | MRI data of 32,000 participants | A neural network trained to understand various biological metrics from MRI images. | The neural network showed sturdy results to infer body measurement with MRI data. | Accuracy: 99.97% | [41] |
| 13 | Experimental | UK | 20,000 subjects’ cardiac magnetic resonance (CMR) image | Fully automatic image analysis pipeline. | Experimental setup provided better significance among automation indexes and manual reference indexes. It produced similar accuracy in segmentation for humans. | Accuracy: 93% | [42] |
| 14 | Experimental | UK | 423,604 | ML model developed to predict cardiovascular diseases (CVD) using auto prognosis. | Auto prognosis predicted 268 more cases than the Framingham score, and also consider more predictors. | ROC: 0.77, sensitivity: 69.9% | [36] |
| 15 | Statistical | Qatar | 1000 | ML models and Panorama state of the art statistics methods are used to understand type 2 diabetics and obesity. | Expose the risk factor and association between diabetics and obesity to subjects. | - | [49] |
| 16 | Experimental | UK | 96,220 participants | ML models to detect human sleep and activity from wrist-worn accelerometer data. | To evaluate human lifestyle and health behaviors with machine learning. | Accuracy: 87% | [43] |
| 17 | Experimental | UK | 700 patients with cancer | Systematic chart review on patients with AI treatment with stage I-III BC. | This study is the primary link to a cluster of specific single nucleotide polymorphisms (SNP/gene) to aromatase inhibitor-related arthralgia (AIA) risk independent of candidate gene bias. | Accuracy: 75.93% | [44] |
| 18 | Epidemiological | UK | 10,000 MRI Images | An automated processing and quality control (QC) pipeline was established. | Raw images data is converted to useful information to further research. | Accuracy: 99.1% | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battineni, G.; Hossain, M.A.; Chintalapudi, N.; Amenta, F. A Survey on the Role of Artificial Intelligence in Biobanking Studies: A Systematic Review. Diagnostics 2022, 12, 1179. https://doi.org/10.3390/diagnostics12051179
Battineni G, Hossain MA, Chintalapudi N, Amenta F. A Survey on the Role of Artificial Intelligence in Biobanking Studies: A Systematic Review. Diagnostics. 2022; 12(5):1179. https://doi.org/10.3390/diagnostics12051179
Chicago/Turabian StyleBattineni, Gopi, Mohmmad Amran Hossain, Nalini Chintalapudi, and Francesco Amenta. 2022. "A Survey on the Role of Artificial Intelligence in Biobanking Studies: A Systematic Review" Diagnostics 12, no. 5: 1179. https://doi.org/10.3390/diagnostics12051179
APA StyleBattineni, G., Hossain, M. A., Chintalapudi, N., & Amenta, F. (2022). A Survey on the Role of Artificial Intelligence in Biobanking Studies: A Systematic Review. Diagnostics, 12(5), 1179. https://doi.org/10.3390/diagnostics12051179








