The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review
Abstract
:1. Introduction
2. MRI
3. 18F-Fluoroethyl-L-Tyrosine, 18F-FET-PET
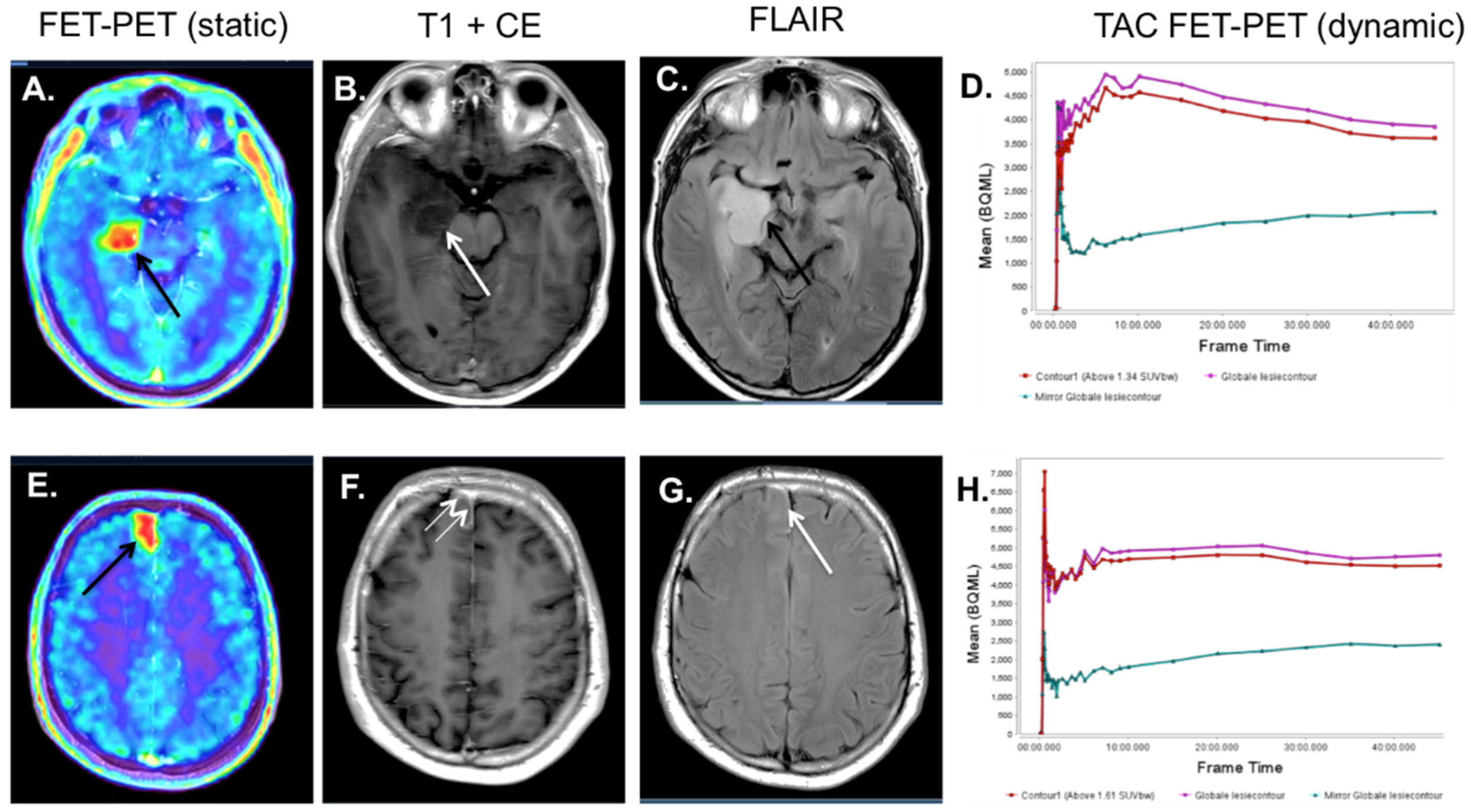
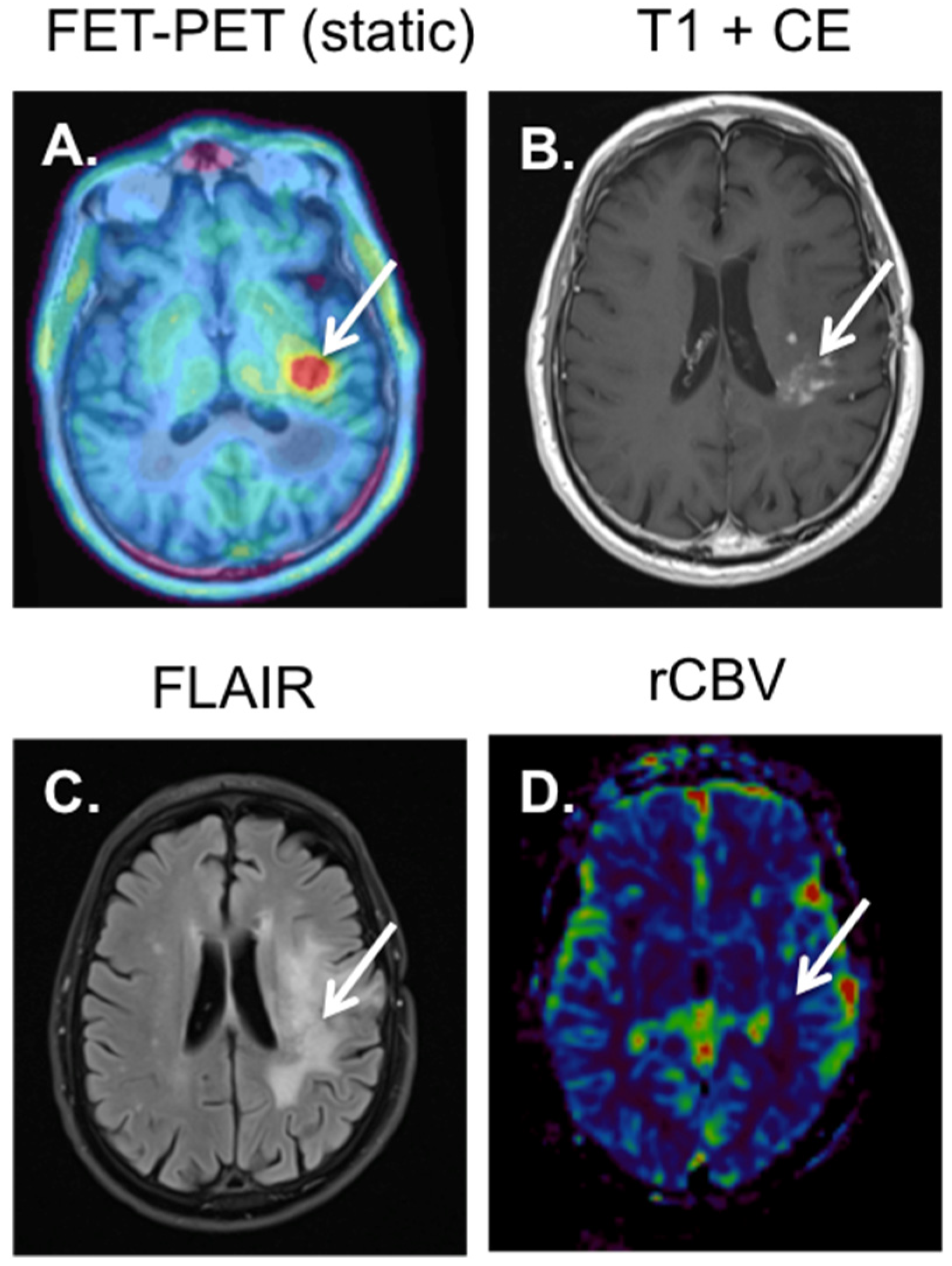
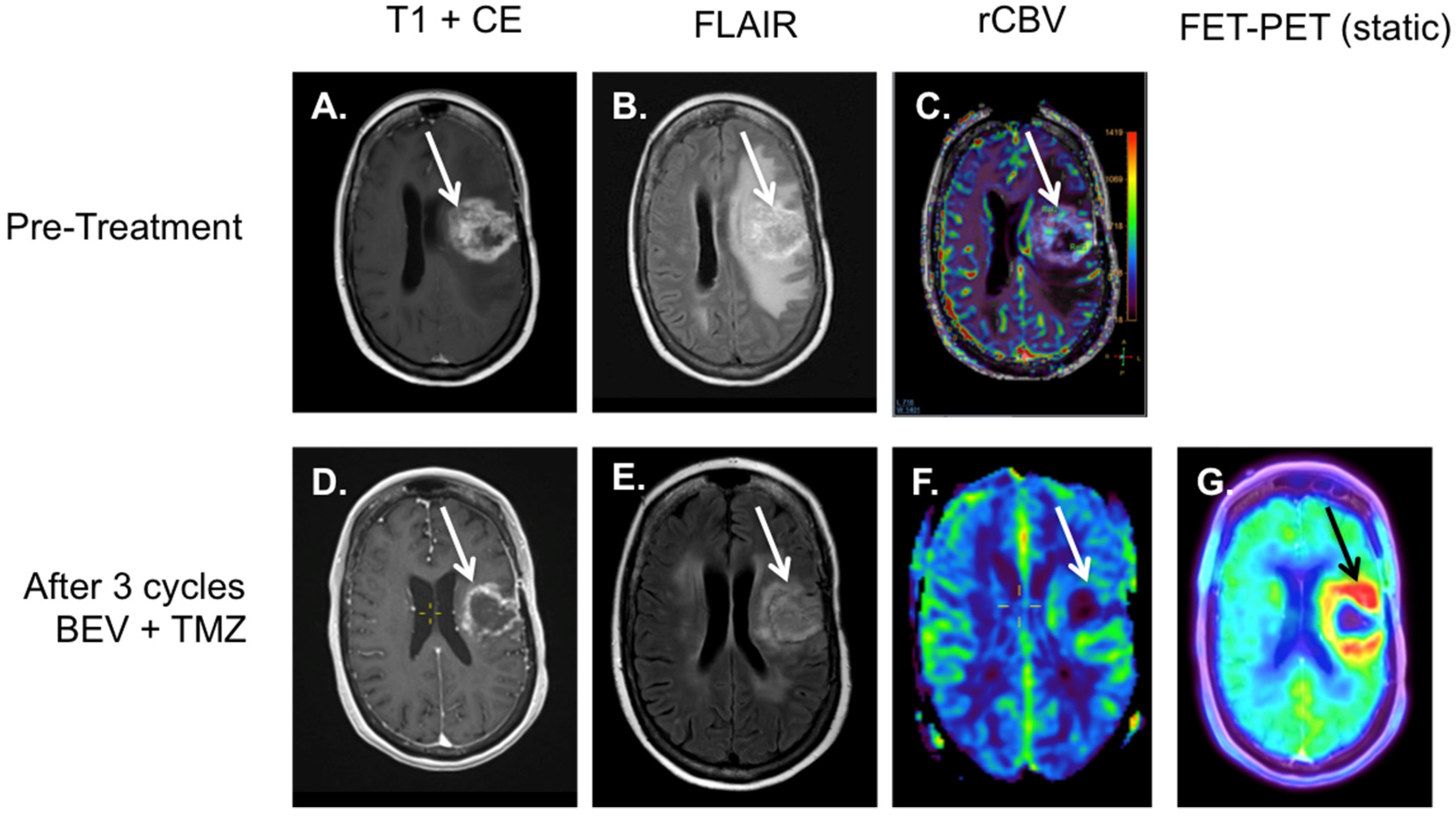
4. 18F-FET-PET-MRI
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [Green Version]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treat-ment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef]
- Chen, W.; Silverman, D.H. Advances in Evaluation of Primary Brain Tumors. Semin. Nucl. Med. 2008, 38, 240–250. [Google Scholar] [CrossRef]
- Thust, S.C.; Heiland, S.; Falini, A.; Jager, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 cen-tres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef] [Green Version]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.-W.; Zilles, K.; Coenen, H.H.; Langen, K.-J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Heiss, W.D. [PET in gliomas. Overview of current studies]. Nuklearmedizin 2014, 53, 163–171. [Google Scholar]
- Dhermain, F. Radiotherapy of high-grade gliomas: Current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin. J. Cancer 2014, 33, 16–24. [Google Scholar] [CrossRef]
- Scott, J.N.; Brasher, P.M.; Sevick, R.J.; Rewcastle, N.B.; Forsyth, P.A. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002, 59, 947–949. [Google Scholar] [CrossRef]
- Muragaki, Y.; Chernov, M.; Maruyama, T.; Ochiai, T.; Taira, T.; Kubo, O.; Nakamura, R.; Iseki, H.; Hori, T.; Takakura, K. Low-Grade Glioma on Stereotactic Biopsy: How Often is the Diagnosis Accurate? Minim. Invasive Neurosurg. 2008, 51, 275–279. [Google Scholar] [CrossRef]
- Hilario, A.; Ramos, A.; Perez-Nunez, A.; Salvador, E.; Millan, J.M.; Lagares, A.; Sepulveda, J.M.; Gonzalez-Leon, P.; Hernandez-Lain, A.; Ricoy, J.R. The added value of apparent diffusion coeffi-cient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am. J. Neuroradiol. 2012, 33, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Kitis, O.; Altay, H.; Calli, C.; Yunten, N.; Akalin, T.; Yurtseven, T. Minimum apparent diffusion coefficients in the evaluation of brain tumors. Eur. J. Radiol. 2005, 55, 393–400. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, X.; She, D.; Lin, Y.; Zhang, Y.; Cao, D. Noninvasive Assessment of IDH Mutational Status in World Health Organi-zation Grade II and III Astrocytomas Using DWI and DSC-PWI Combined with Conventional MR Imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Maynard, J.; Okuchi, S.; Wastling, S.; Busaidi, A.A.; Almossawi, O.; Mbatha, W.; Brandner, S.; Jaunmuktane, Z.; Koc, A.M.; Mancini, L.; et al. World Health Organization Grade II/III Glio-ma Molecular Status: Prediction by MRI Morphologic Features and Apparent Diffusion Coefficient. Radiology 2020, 296, 111–121. [Google Scholar] [CrossRef]
- Thust, S.C.; Hassanein, S.; Bisdas, S.; Rees, J.H.; Hyare, H.; Maynard, J.A.; Brandner, S.; Tur, C.; Jäger, H.R.; Yousry, T.A.; et al. Apparent diffusion coefficient for molecular subtyp-ing of non-gadolinium-enhancing WHO grade II/III glioma: Volumetric segmentation versus two-dimensional region of inter-est analysis. Eur. Radiol. 2018, 28, 3779–3788. [Google Scholar] [CrossRef] [Green Version]
- Le Bihan, D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology 2013, 268, 318–322. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Jelescu, I.O.; Palombo, M.; Bagnato, F.; Schilling, K.G. Challenges for biophysical modeling of microstructure. J. Neurosci. Methods 2020, 344, 108861. [Google Scholar] [CrossRef]
- Haller, S.; Zaharchuk, G.; Thomas, D.; Lovblad, K.-O.; Barkhof, F.; Golay, X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology 2016, 281, 337–356. [Google Scholar] [CrossRef] [Green Version]
- Abrigo, J.M.; Fountain, D.M.; Provenzale, J.M.; Law, E.K.; Kwong, J.S.; Hart, M.G.; Tam, W.W.S. Magnetic resonance perfusion for differentiat-ing low-grade from high-grade gliomas at first presentation. Cochrane Database Syst. Rev. 2018, 1, CD011551. [Google Scholar]
- Yoo, R.-E.; Yun, T.J.; Hwang, I.; Hong, E.K.; Kang, K.M.; Choi, S.H.; Park, C.-K.; Won, J.-K.; Kim, J.-H.; Sohn, C.-H. Arterial spin labeling perfusion-weighted imaging aids in prediction of molecular biomarkers and survival in glioblastomas. Eur. Radiol. 2019, 30, 1202–1211. [Google Scholar] [CrossRef]
- Brendle, C.; Hempel, J.-M.; Schittenhelm, J.; Skardelly, M.; Tabatabai, G.; Bender, B.; Ernemann, U.; Klose, U. Glioma Grading and Determination of IDH Mutation Status and ATRX loss by DCE and ASL Perfusion. Clin. Neuroradiol. 2017, 28, 421–428. [Google Scholar] [CrossRef]
- Welker, K.; Boxerman, J.; Kalnin, A.; Kaufmann, T.; Shiroishi, M.; Wintermark, M.; American Society of Functional Neuroradiology MR Perfusion Standards and Practice Subcommittee of the ASFNR Clinical Practice Committee. ASFNR recommendations for clinical per-formance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am. J. Neuroradiol. 2015, 36, E41–E51. [Google Scholar] [CrossRef] [Green Version]
- Boxerman, J.L.; Quarles, C.C.; Hu, L.S.; Erickson, B.J.; Gerstner, E.R.; Smits, M.; Kaufmann, T.; Barboriak, D.P.; Huang, R.H.; Wick, W.; et al. Consensus recommendations for a dynamic sus-ceptibility contrast MRI protocol for use in high-grade gliomas. Neuro-Oncol. 2020, 22, 1262–1275. [Google Scholar] [CrossRef]
- Thust, S.C.; Bent, M.J.V.D.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.W.; Westerlaan, H.E.; Holtman, G.A.; Aden, K.M.; Van Laar, P.J.; Van Der Hoorn, A. Incidence of Tumour Progression and Pseudoprogression in High-Grade Gliomas: A Systematic Review and Meta-Analysis. Clin. Neuroradiol. 2018, 28, 401–411. [Google Scholar] [CrossRef]
- van Dijken, B.R.J.; van Laar, P.J.; Holtman, G.A.; van der Hoorn, A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef] [Green Version]
- Essock-Burns, E.; Lupo, J.M.; Cha, S.; Polley, M.Y.; Butowski, N.A.; Chang, S.M.; Nelson, S.J. Assessment of perfusion MRI-derived parame-ters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma. Neuro-Oncol. 2011, 13, 119–131. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Pichler, P.; Karl, M.; Brandner, S.; Lerch, C.; Renner, B.; Heinz, G. Quantification of serial changes in cerebral blood vol-ume and metabolism in patients with recurrent glioblastoma undergoing antiangiogenic therapy. Eur. J. Radiol. 2015, 84, 1128–1136. [Google Scholar] [CrossRef]
- Leao, D.; Craig, P.; Godoy, L.; Da La Leite, C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef]
- Krukowski, P.; Podgorski, P.; Guzinski, M.; Szewczyk, P.; Sasiadek, M. Analysis of the brain proton magnetic resonance spectros-copy—Differences between normal grey and white matter. Pol. J. Radiol. 2010, 75, 22–26. [Google Scholar]
- Usinskiene, J.; Ulyte, A.; Bjornerud, A.; Venius, J.; Katsaros, V.K.; Rynkeviciene, R.; Letautiene, S.; Norkus, D.; Suziedelis, K.; Rocka, S.; et al. Optimal differentiation of high- and low-grade glioma and metastasis: A meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 2016, 58, 339–350. [Google Scholar] [CrossRef]
- Lee, E.; Lee, S.; Agid, R.; Bae, J.; Keller, A.; Terbrugge, K. Preoperative Grading of Presumptive Low-Grade Astrocytomas on MR Imaging: Diagnostic Value of Minimum Apparent Diffusion Coefficient. Am. J. Neuroradiol. 2008, 29, 1872–1877. [Google Scholar] [CrossRef] [Green Version]
- Murakami, R.; Hirai, T.; Sugahara, T.; Fukuoka, H.; Toya, R.; Nishimura, S.; Kitajima, M.; Okuda, T.; Nakamura, H.; Oya, N.; et al. Grading astrocytic tumors by using apparent dif-fusion coefficient parameters: Superiority of a one- versus two-parameter pilot method. Radiology 2009, 251, 838–845. [Google Scholar] [CrossRef]
- Hakyemez, B.; Erdogan, C.; Ercan, I.; Ergin, N.; Uysal, S.; Atahan, S. High-grade and low-grade gliomas: Differentiation by using perfusion MR imaging. Clin. Radiol. 2005, 60, 493–502. [Google Scholar] [CrossRef]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, M.; et al. 2-hydroxyglutarate detection by magnetic reso-nance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Suh, C.H.; Kim, H.S.; Jung, S.C.; Choi, C.G.; Kim, S.J. 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: A systemic review and meta-analysis using individual patient data. Neuro-Oncol. 2018, 20, 1573–1583. [Google Scholar] [CrossRef] [Green Version]
- Andronesi, O.C.; Arrillaga-Romany, I.C.; Ly, K.I.; Bogner, W.; Ratai, E.M.; Reitz, K.; Lafrate, A.J.; Dietrich, J.; Gerstner, E.R.; Chi, A.S.; et al. Pharmacodynamics of mutant-IDH1 inhibi-tors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat. Commun. 2018, 9, 1474. [Google Scholar] [CrossRef]
- Choi, C.; Raisanen, J.M.; Ganji, S.K.; Zhang, S.; McNeil, S.S.; An, Z.; Madan, A.; Hatanpaa, K.J.; Vemireddy, V.; Sheppard, C.A.; et al. Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients With IDH-Mutant Glioma. J. Clin. Oncol. 2016, 34, 4030–4039. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, M.I.; Young, R.J.; Rubel, J.; Rosenblum, M.; Tisnado, J.; Briggs, S.; Arevalo-Perez, J.; Cross, J.R.; Campos, C.; Straley, K.; et al. Integration of 2-hydroxyglutarate-proton mag-netic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro-Oncol. 2016, 18, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, C.; Stoffels, G.; Filß, C.; Heinzel, A.; Lohmann, P.; Willuweit, A.; Ermert, J.; Coenen, H.H.; Mottaghy, F.M.; Galldiks, N.; et al. Current trends in the use of O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) in neurooncology. Nucl. Med. Biol. 2021, 92, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guide-lines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: Version 1. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [Green Version]
- Vander Borght, T.; Asenbaum, S.; Bartenstein, P.; Halldin, C.; Kapucu, O.; Van Laere, K.; Varrone, A.; Tatsch, K. EANM procedure guidelines for brain tumour imaging using labelled amino acid analogues. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1374–1380. [Google Scholar] [CrossRef] [Green Version]
- Rapp, M.; Heinzel, A.; Galldiks, N.; Stoffels, G.; Felsberg, J.; Ewelt, C.; Sabel, M.; Steiger, H.J.; Reifenberger, G.; Beez, T.; et al. Diagnostic performance of 18F-FET PET in newly diag-nosed cerebral lesions suggestive of glioma. J. Nucl. Med. 2013, 54, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Pichler, R.; Dunzinger, A.; Wurm, G.; Pichler, J.; Weis, S.; Nußbaumer, K.; Topakian, R.; Aigner, R.M. Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur. J. Pediatr. 2010, 37, 1521–1528. [Google Scholar] [CrossRef]
- Jansen, N.L.; Graute, V.; Armbruster, L.; Suchorska, B.; Lutz, J.; Eigenbrod, S.; Cumming, P.; Bartenstein, P.; Tonn, J.-C.; Kreth, F.W.; et al. MRI-suspected low-grade glioma: Is there a need to perform dynamic FET PET? Eur. J. Pediatr. 2012, 39, 1021–1029. [Google Scholar] [CrossRef]
- Verger, A.; Stoffels, G.; Bauer, E.K.; Lohmann, P.; Blau, T.; Fink, G.R.; Neumaier, B.; Shah, N.J.; Langen, K.-J.; Galldiks, N. Static and dynamic 18F–FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur. J. Pediatr. 2018, 45, 443–451. [Google Scholar] [CrossRef]
- Vettermann, F.; Suchorska, B.; Unterrainer, M.; Nelwan, D.; Forbrig, R.; Ruf, V.; Wenter, V.; Kreth, F.-W.; Herms, J.; Bartenstein, P.; et al. Non-invasive prediction of IDH-wildtype genotype in gliomas using dynamic 18F-FET PET. Eur. J. Pediatr. 2019, 46, 2581–2589. [Google Scholar] [CrossRef]
- Jansen, N.L.; Schwartz, C.; Graute, V.; Eigenbrod, S.; Lutz, J.; Egensperger, R.; Pöpperl, G.; Kretzschmar, H.A.; Cumming, P.; Bartenstein, P.; et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [18F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro-Oncol. 2012, 14, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.F.; Delgado, A.F. Discrimination between Glioma Grades II and III Using Dynamic Susceptibility Perfusion MRI: A Meta-Analysis. AJNR Am. J. Neuroradiol. 2017, 38, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piroth, M.D.; Prasath, J.; Willuweit, A.; Stoffels, G.; Sellhaus, B.; van Osterhout, A.; Geisler, S.; Shah, N.J.; Eble, M.J.; Coenen, H.H.; et al. Uptake of O-(2-[18F]fluoroethyl)-L-tyrosine in reactive astrocytosis in the vicinity of cerebral gliomas. Nucl. Med. Biol. 2013, 40, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Floeth, F.W.; Sabel, M.; Stoffels, G.; Pauleit, D.; Hamacher, K.; Steiger, H.-J.; Langen, K.-J. Prognostic Value of 18F-Fluoroethyl-l-Tyrosine PET and MRI in Small Nonspecific Incidental Brain Lesions. J. Nucl. Med. 2008, 49, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Hutterer, M.; Bumes, E.; Riemenschneider, M.J.; Grosse, J.; Hellwig, D.; Galldiks, N.; Langen, K.-J.; Hau, P. AIDS-Related Central Nervous System Toxoplasmosis with Increased 18F-Fluoroethyl-L-Tyrosine Amino Acid PET Uptake Due to LAT1/2 Expression of Inflamma-tory Cells. Clin. Nucl. Med. 2017, 42, e506–e508. [Google Scholar] [CrossRef]
- Galldiks, N.; Law, I.; Pope, W.; Arbizu, J.; Langen, K.-J. The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. NeuroImage Clin. 2017, 13, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.-J. 18F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef]
- Kertels, O.; Mihovilovic, M.I.; Linsenmann, T.; Kessler, A.F.; Tran-Gia, J.; Kircher, M.; Brumberg, J.; Monoranu, C.M.; Samnick, S.; Ernestus, R.-I.; et al. Clinical Utility of Different Approaches for Detection of Late Pseudoprogression in Glioblastoma With O-(2-[18F]Fluoroethyl)-l-Tyrosine PET. Clin. Nucl. Med. 2019, 44, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblas-toma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Muoio, B.; Giovanella, L.; Treglia, G. Recent Developments of 18F-FET PET in Neuro-oncology. Curr. Med. Chem. 2018, 25, 3061–3073. [Google Scholar] [CrossRef]
- Galldiks, N.; Dunkl, V.; Ceccon, G.; Tscherpel, C.; Stoffels, G.; Law, I.; Henriksen, O.M.; Muhic, A.; Poulsen, H.S.; Steger, J.; et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur. J. Pediatr. 2018, 45, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Rapp, M.; Stoffels, G.; Fink, G.R.; Shah, N.J.; Coenen, H.H.; Sabel, M.; Langen, K.-J. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-l-tyrosine PET in comparison to MRI. Eur. J. Nucl. Med. Mol. Imaging 2012, 40, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, M.; Nowosielski, M.; Putzer, D.; Waitz, D.; Tinkhauser, G.; Kostron, H.; Muigg, A.; Virgolini, I.J.; Staffen, W.; Trinka, E.; et al. O-(2-18F-Fluoroethyl)-L-Tyrosine PET Predicts Failure of Antiangiogenic Treatment in Patients with Recurrent High-Grade Glioma. J. Nucl. Med. 2011, 52, 856–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verger, A.; Filss, C.P.; Lohmann, P.; Stoffels, G.; Sabel, M.; Wittsack, H.J.; Kops, E.R.; Galldiks, N.; Fink, G.R.; Shah, N.J.; et al. Comparison of 18F-FET PET and perfusion-weighted MRI for glioma grading: A hybrid PET/MR study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2257–2265. [Google Scholar] [CrossRef]
- Filss, C.P.; Galldiks, N.; Stoffels, G.; Sabel, M.; Wittsack, H.J.; Turowski, B.; Antoch, G.; Zhang, K.; Fink, G.R.; Coenen, H.H.; et al. Comparison of 18F-FET PET and Perfusion-Weighted MR Imaging: A PET/MR Imaging Hybrid Study in Patients with Brain Tumors. J. Nucl. Med. 2014, 55, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Verger, A.; Filss, C.P.; Lohmann, P.; Stoffels, G.; Sabel, M.; Wittsack, H.J.; Kops, E.R.; Galldiks, N.; Fink, G.; Shah, N.J.; et al. Comparison of O-(2-(18)F-Fluoroethyl)-L-Tyrosine Positron Emission Tomography and Perfusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Patients with Progressive and Recurrent Glioma: A Hybrid Positron Emission Tomography/Magnetic Resonance Study. World Neurosurg. 2018, 113, e727–e737. [Google Scholar]
- Song, S.; Cheng, Y.; Ma, J.; Wang, L.; Dong, C.; Wei, Y.; Xu, G.; An, Y.; Qi, Z.; Lin, Q.; et al. Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: A biopsy validation study. Eur. J. Pediatr. 2020, 47, 1458–1467. [Google Scholar] [CrossRef] [Green Version]
- Jena, A.; Taneja, S.; Singh, A.; Negi, P.; Sarin, R.; Das, P.K.; Singhal, M. Reliability of 18F-FDG PET Metabolic Parameters Derived Using Simultaneous PET/MRI and Correlation with Prognostic Factors of Invasive Ductal Carcinoma: A Feasibility Study. Am. J. Roentgenol. 2017, 209, 662–670. [Google Scholar] [CrossRef]
- Heinzel, A.; Stock, S.; Langen, K.-J.; Müller, D. Cost-effectiveness analysis of FET PET-guided target selection for the diagnosis of gliomas. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1089–1096. [Google Scholar] [CrossRef]
- van de Weijer, T.; Broen, M.P.G.; Moonen, R.P.M.; Hoeben, A.; Anten, M.; Hovinga, K.; Compter, I.; van der Pol, J.A.J.; Jacquerie, A.; Mottaghy, F.; et al. 18F-FET-PET/MRI in de neuro-oncologie: Het beste van 2 werelden. Tijdschr. Neurol. Neurochir. 2022, 123, 77–85. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Weijer, T.; Broen, M.P.G.; Moonen, R.P.M.; Hoeben, A.; Anten, M.; Hovinga, K.; Compter, I.; van der Pol, J.A.J.; Mitea, C.; Lodewick, T.M.; et al. The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review. Diagnostics 2022, 12, 1202. https://doi.org/10.3390/diagnostics12051202
van de Weijer T, Broen MPG, Moonen RPM, Hoeben A, Anten M, Hovinga K, Compter I, van der Pol JAJ, Mitea C, Lodewick TM, et al. The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review. Diagnostics. 2022; 12(5):1202. https://doi.org/10.3390/diagnostics12051202
Chicago/Turabian Stylevan de Weijer, Tineke, Martijn P. G. Broen, Rik P. M. Moonen, Ann Hoeben, Monique Anten, Koos Hovinga, Inge Compter, Jochem A. J. van der Pol, Cristina Mitea, Toine M. Lodewick, and et al. 2022. "The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review" Diagnostics 12, no. 5: 1202. https://doi.org/10.3390/diagnostics12051202
APA Stylevan de Weijer, T., Broen, M. P. G., Moonen, R. P. M., Hoeben, A., Anten, M., Hovinga, K., Compter, I., van der Pol, J. A. J., Mitea, C., Lodewick, T. M., Jacquerie, A., Mottaghy, F. M., Wildberger, J. E., & Postma, A. A. (2022). The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review. Diagnostics, 12(5), 1202. https://doi.org/10.3390/diagnostics12051202





