Field Evaluation of a Hemozoin-Based Malaria Diagnostic Device in Puerto Lempira, Honduras
Abstract
:1. Introduction
2. Materials and Methods
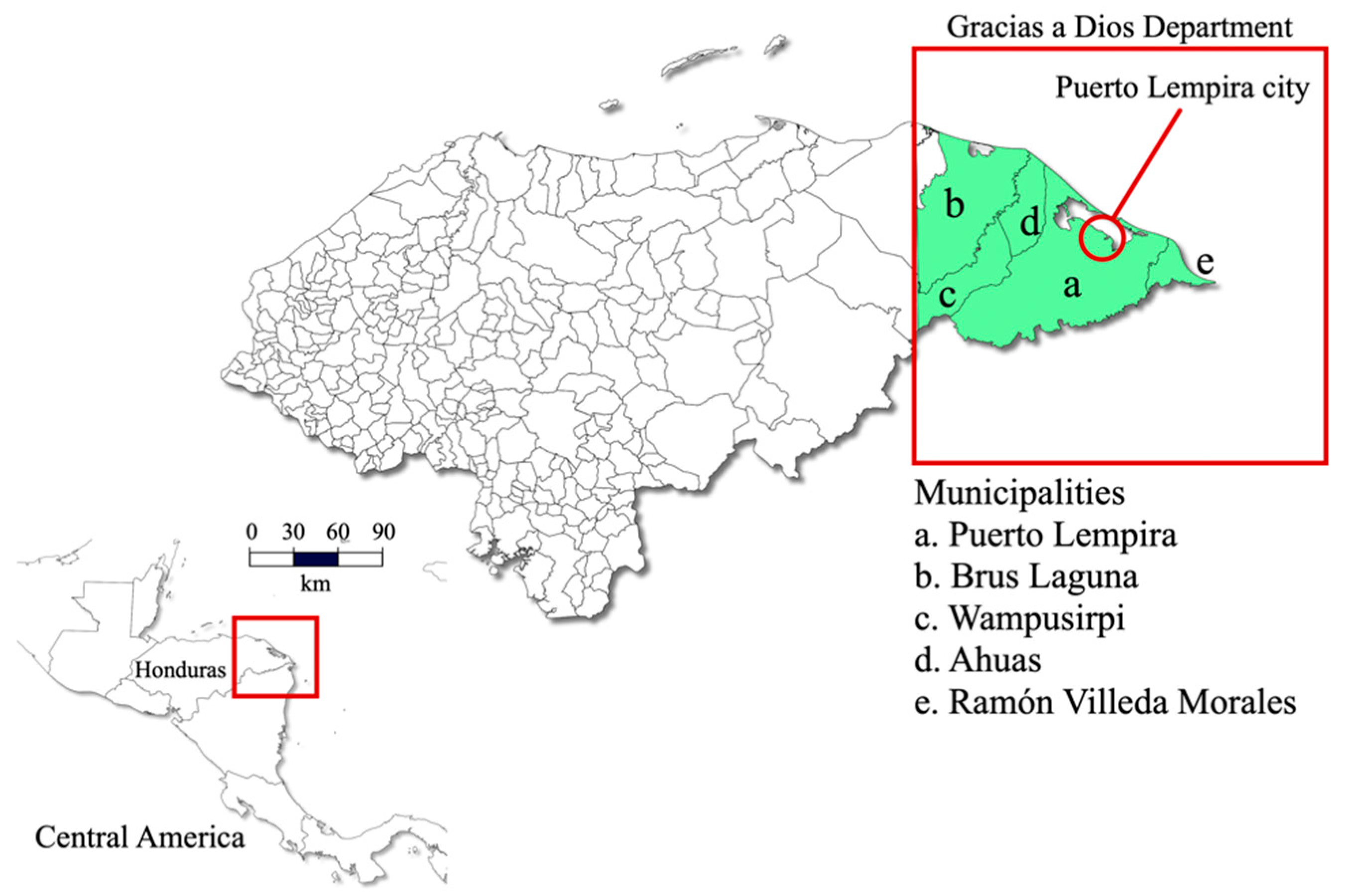
2.1. Study Population
2.2. Study Population and Ethics
2.3. Sample Size
2.4. Microscopy
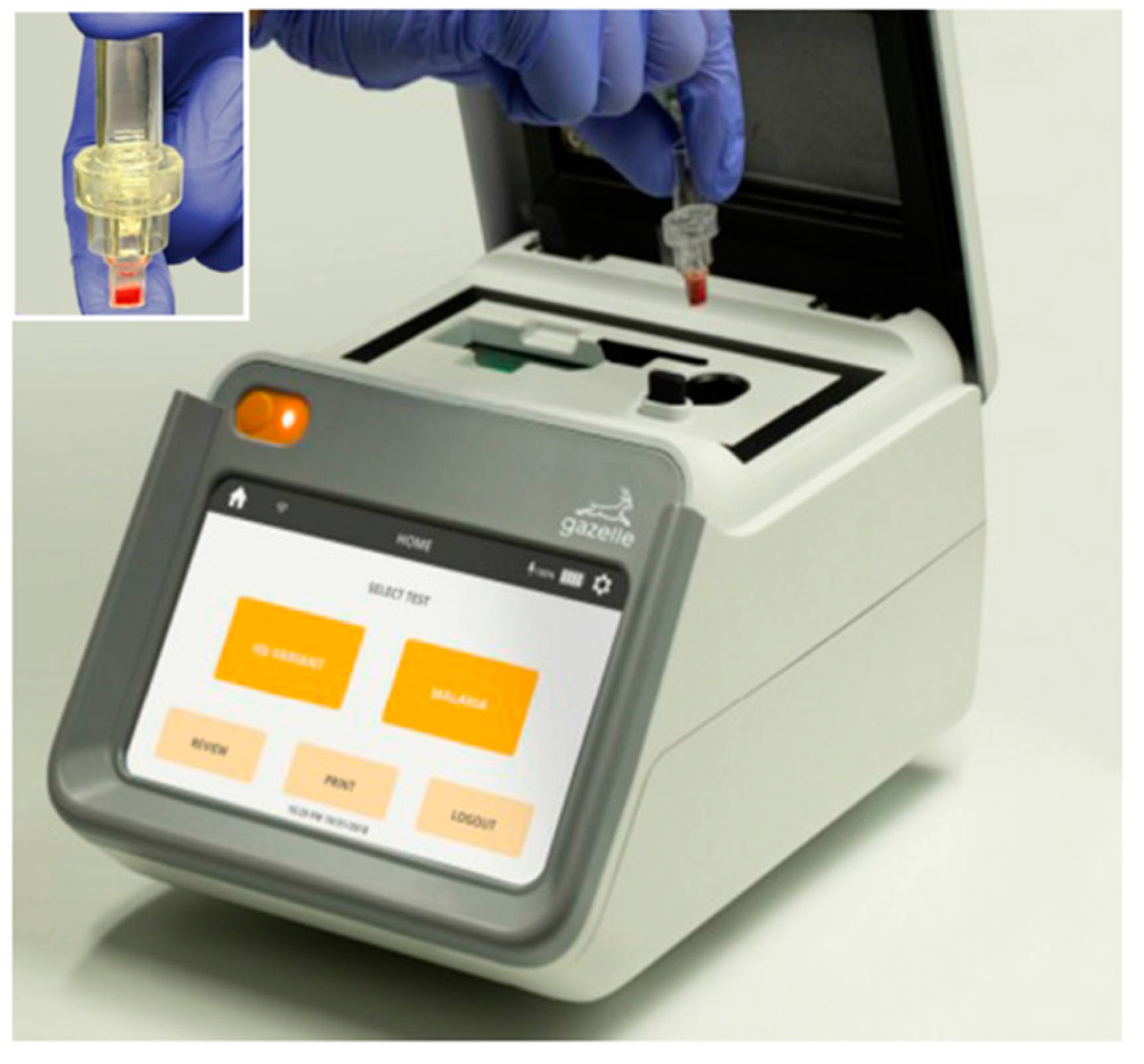
2.5. Gazelle™ Device
2.6. DNA Extraction and Nested PCR
2.7. Turn-Around Time and Cost Analysis
2.8. Statistical Analysis
3. Results
Performance of Microscopy and Gazelle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Technical Strategy for Malaria 2016–2030, 2021 Update; WHO: Geneva, Switzerland, 2021.
- World Health Organization. World Malaria Report; WHO: Geneva, Switzerland, 2021.
- Fitri, L.E.; Widaningrum, T.; Endharti, A.T.; Prabowo, M.H.; Winaris, N.; Nugraha, R.Y.B. Malaria diagnostic update: From conventional to advanced method. J. Clin. Lab. Anal. 2022, 36, e24314. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, D.C.; Williams, J.E. ACP Broadsheet no 148. July 1996. Laboratory diagnosis of malaria. J. Clin. Pathol. 1996, 49, 533–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahama-Maro, J.; D’Acremont, V.; Mtasiwa, D.; Genton, B.; Lengeler, C. Low quality of routine microscopy for malaria at different levels of the health system in Dar es Salaam. Malar. J. 2011, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Shankar, H.; Singh, M.P.; Phookan, S.; Singh, K.; Mishra, N. Diagnostic performance of rapid diagnostic test, light microscopy and polymerase chain reaction during mass survey conducted in low and high malaria-endemic areas from two North-Eastern states of India. Parasitol. Res. 2021, 120, 2251–2261. [Google Scholar] [CrossRef]
- McMorrow, M.L.; Aidoo, M.; Kachur, S.P. Malaria rapid diagnostic tests in elimination settings—Can they find the last parasite? Clin. Microbiol. Infect. 2011, 17, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, F.E.; Sirichaisinthop, J.; Miller, R.S.; Gasser, R.A., Jr.; Wongsrichanalai, C. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am. J. Trop. Med. Hyg. 2003, 69, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Snounou, G.; Viriyakosol, S.; Jarra, W.; Thaithong, S.; Brown, K.N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 1993, 58, 283–292. [Google Scholar] [CrossRef]
- Cunningham, J.; Jones, S.; Gatton, M.L.; Barnwell, J.W.; Cheng, Q.; Chiodini, P.L.; Glenn, J.; Incardona, S.; Kosack, C.; Luchavez, J.; et al. A review of the WHO malaria rapid diagnostic test product testing programme (2008–2018): Performance, procurement and policy. Malar. J. 2019, 18, 387. [Google Scholar] [CrossRef] [Green Version]
- Ogunfowokan, O.; Ogunfowokan, B.A.; Nwajei, A.I. Sensitivity and specificity of malaria rapid diagnostic test (mRDT CareStatTM) compared with microscopy amongst under five children attending a primary care clinic in southern Nigeria. Afr. J. Prim. Health Care Fam. Med. 2020, 12, e1–e8. [Google Scholar] [CrossRef]
- Koita, O.A.; Doumbo, O.K.; Ouattara, A.; Tall, L.K.; Konare, A.; Diakite, M.; Diallo, M.; Sagara, I.; Masinde, G.L.; Doumbo, S.N.; et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am. J. Trop. Med. Hyg. 2012, 86, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontecha, G.; Mejia, R.E.; Banegas, E.; Ade, M.P.; Mendoza, L.; Ortiz, B.; Sabillon, I.; Alvarado, G.; Matamoros, G.; Pinto, A. Deletions of pfhrp2 and pfhrp3 genes of Plasmodium falciparum from Honduras, Guatemala and Nicaragua. Malar. J. 2018, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Sun, Y.; Fan, L.; Wang, D.; Wang, H.; Sun, X.; Zheng, Z. A direct, sensitive and high-throughput genus and species-specific molecular assay for large-scale malaria screening. Infect. Dis. Poverty 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, J.A.; De Paoli, A.; Afara, I.O.; Kochan, K.; Creek, D.J.; Heraud, P.; Wood, B.R. Ultraviolet/Visible and Near-Infrared Dual Spectroscopic Method for Detection and Quantification of Low-Level Malaria Parasitemia in Whole Blood. Anal. Chem. 2021, 93, 13302–13310. [Google Scholar] [CrossRef] [PubMed]
- Sazed, S.A.; Kibria, M.G.; Alam, M.S. An Optimized Real-Time qPCR Method for the Effective Detection of Human Malaria Infections. Diagnostics 2021, 11, 736. [Google Scholar] [CrossRef]
- Orban, A.; Longley, R.J.; Sripoorote, P.; Maneechai, N.; Nguitragool, W.; Butykai, A.; Mueller, I.; Sattabongkot, J.; Karl, S.; Kezsmarki, I. Sensitive detection of Plasmodium vivax malaria by the rotating-crystal magneto-optical method in Thailand. Sci. Rep. 2021, 11, 18547. [Google Scholar] [CrossRef]
- Kumar, B.M.H.; Srikanth, P.C.; Vaibhav, A.M. A novel computation method for detection of Malaria in RBC using Photonic biosensor. Int. J. Inf. Technol. 2021, 13, 2053–2058. [Google Scholar] [CrossRef]
- Kamaliddin, C.; Sutherland, C.J.; Houze, S.; Cottrell, G.; Briand, V.; Castaneda Mogollon, D.; Pillai, D.R. The Role of Ultrasensitive Molecular Methods for Detecting Malaria-The Broader Perspective. Clin. Infect. Dis. 2021, 73, e1387–e1390. [Google Scholar] [CrossRef]
- Yoon, J.; Jang, W.S.; Nam, J.; Mihn, D.C.; Lim, C.S. An Automated Microscopic Malaria Parasite Detection System Using Digital Image Analysis. Diagnostics 2021, 11, 527. [Google Scholar] [CrossRef]
- McGinnis, E.; Chan, G.; Hudoba, M.; Markin, T.; Yakimec, J.; Roland, K. Malaria Screening Using Front-Line Loop-Mediated Isothermal Amplification. Am. J. Clin. Pathol. 2021, 155, 690–697. [Google Scholar] [CrossRef]
- Britton, S.; Cheng, Q.; McCarthy, J.S. Novel molecular diagnostic tools for malaria elimination: A review of options from the point of view of high-throughput and applicability in resource limited settings. Malar. J. 2016, 15, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigala, P.A.; Goldberg, D.E. The peculiarities and paradoxes of Plasmodium heme metabolism. Annu. Rev. Microbiol. 2014, 68, 259–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.E.; Sullivan, D.J., Jr.; Goldberg, D.E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997, 51, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Hanscheid, T.; Langin, M.; Lell, B.; Potschke, M.; Oyakhirome, S.; Kremsner, P.G.; Grobusch, M.P. Full blood count and haemozoin-containing leukocytes in children with malaria: Diagnostic value and association with disease severity. Malar. J. 2008, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inyushin, M.; Kucheryavih, Y.; Kucheryavih, L.; Rojas, L.; Khmelinskii, I.; Makarov, V. Superparamagnetic Properties of Hemozoin. Sci. Rep. 2016, 6, 26212. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, C.; Olson, J.A. Birefringent hemozoin identifies malaria. Am. J. Clin. Pathol. 1986, 86, 360–363. [Google Scholar] [CrossRef]
- Baptista, V.; Costa, M.S.; Calcada, C.; Silva, M.; Gil, J.P.; Veiga, M.I.; Catarino, S.O. The Future in Sensing Technologies for Malaria Surveillance: A Review of Hemozoin-Based Diagnosis. ACS Sens. 2021, 6, 3898–3911. [Google Scholar] [CrossRef]
- Pinto, A.; Archaga, O.; Mejia, A.; Escober, L.; Henriquez, J.; Montoya, A.; Valdivia, H.O.; Fontecha, G. Evidence of a Recent Bottleneck in Plasmodium falciparum Populations on the Honduran-Nicaraguan Border. Pathogens 2021, 10, 1432. [Google Scholar] [CrossRef]
- Escobar, D.; Ascencio, K.; Ortiz, A.; Palma, A.; Fontecha, G. Distribution and phylogenetic diversity of Anopheles species in malaria endemic areas of Honduras in an elimination setting. Parasit. Vectors 2020, 13, 333. [Google Scholar] [CrossRef]
- UICN. La Moskitia Hondureña: Biodiversa, Costera y Entre Espejos de Agua. Available online: https://www.iucn.org/es/news/mexico-america-central-y-el-caribe/202006/la-moskitia-hondurena-biodiversa-costera-y-entre-espejos-de-agua (accessed on 3 May 2022).
- Buderer, N.M. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad. Emerg. Med. 1996, 3, 895–900. [Google Scholar] [CrossRef]
- Secretaría de Salud de Honduras. Norma Nacional de Malaria en Honduras; Secretaría de Salud de Honduras: Tegucigalpa, Honduras, 2017.
- Alger, J. Densidad parasitaria en malaria: Métodos de determinación y su interpretación. Rev. Médica Hondureña 2001, 69, 118–120. [Google Scholar]
- Singh, B.; Bobogare, A.; Cox-Singh, J.; Snounou, G.; Abdullah, M.S.; Rahman, H.A. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 1999, 60, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Fontecha, G.A.; Mendoza, M.; Banegas, E.; Poorak, M.; De Oliveira, A.M.; Mancero, T.; Udhayakumar, V.; Lucchi, N.W.; Mejia, R.E. Comparison of molecular tests for the diagnosis of malaria in Honduras. Malar. J. 2012, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simundic, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb) 2012, 22, 276–282. [Google Scholar] [CrossRef]
- World Health Organization. Malaria Rapid Diagnostic Test. Performance: Results of WHO Product Testing of Malaria RDTs: Round 7 (2015–2016); WHO: Geneva, Switzerland, 2017.
- Valdivia, H.O.; Thota, P.; Braga, G.; Ricopa, L.; Barazorda, K.; Salas, C.; Bishop, D.K.; Joya, C.A. Field validation of a magneto-optical detection device (Gazelle) for portable point-of-care Plasmodium vivax diagnosis. PLoS ONE 2021, 16, e0253232. [Google Scholar] [CrossRef]
- de Melo, G.C.; Netto, R.L.A.; Mwangi, V.I.; Salazar, Y.; de Souza Sampaio, V.; Monteiro, W.M.; de Almeida, E.V.F.F.; Rocheleau, A.; Thota, P.; Lacerda, M.V.G. Performance of a sensitive haemozoin-based malaria diagnostic test validated for vivax malaria diagnosis in Brazilian Amazon. Malar. J. 2021, 20, 146. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.K.; Shrivas, S.; Thota, P.; Singh, M.P.; Rajasubramaniam, S.; Das, A.; Bharti, P.K. First successful field evaluation of new, one-minute haemozoin-based malaria diagnostic device. EClinicalMedicine 2020, 22, 100347. [Google Scholar] [CrossRef]
- Arndt, L.; Koleala, T.; Orban, A.; Ibam, C.; Lufele, E.; Timinao, L.; Lorry, L.; Butykai, A.; Kaman, P.; Molnar, A.P.; et al. Magneto-optical diagnosis of symptomatic malaria in Papua New Guinea. Nat. Commun. 2021, 12, 969. [Google Scholar] [CrossRef]
- Kipanga, P.N.; Omondi, D.; Mireji, P.O.; Sawa, P.; Masiga, D.K.; Villinger, J. High-resolution melting analysis reveals low Plasmodium parasitaemia infections among microscopically negative febrile patients in western Kenya. Malar. J. 2014, 13, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, E.; Zhou, G.; Oo, W.; Afrane, Y.; Githeko, A.; Yan, G. Low parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of Western Kenya. PLoS ONE 2015, 10, e0121763. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.F.; Martinez, N.L.; Gonzalez, I.J.; Arevalo-Herrera, M.; Herrera, S. Evaluation of the loop mediated isothermal DNA amplification (LAMP) kit for malaria diagnosis in P. vivax endemic settings of Colombia. PLoS Negl. Trop. Dis. 2015, 9, e3453. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, C.; Horning, M.P.; Wilson, B.K.; Proctor, J.L.; Hegg, M.C. Limitations of haemozoin-based diagnosis of Plasmodium falciparum using dark-field microscopy. Malar. J. 2014, 13, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebelo, M.; Shapiro, H.M.; Amaral, T.; Melo-Cristino, J.; Hanscheid, T. Haemozoin detection in infected erythrocytes for Plasmodium falciparum malaria diagnosis-prospects and limitations. Acta Trop. 2012, 123, 58–61. [Google Scholar] [CrossRef]

| PCR Reaction | Primer Name | Primer Sequence (5′-3′) | Annealing Temperature | Amplicon Size (bp) |
|---|---|---|---|---|
| Genus Plasmodium first PCR | rPLU1 | TCA AAG ATT AAG CCA TGC AAG TGA | 55 °C | |
| rPLU5 | CCT GTT GTT GCC TTA AAC TYC | |||
| Genus Plasmodium nested PCR | rPLU3 | TTT YTA TAA GGA TAA CTA CGG AAA AGC TGT | 62 °C | 240 |
| rPLU4 | TAC CCG TCA TAG CCA TGT TAG GCC AAT ACC | 107 | ||
| Plasmodium vivax | rVIV1 | CGC TTC TAG CTT AAT CCA CAT AAC TGA TAC | 58 °C | |
| rVIV2 | ACT TCC AAG CCG AAG CAA AGA AAG TCC TTA | 205 | ||
| Plasmodium falciparum | rFAL1 | TTA AAC TGG TTT GGG AAA ACC AAA TAT ATT | 58 °C | |
| rFAL2 | ACA CAA TGA ACT CAA TCA TGA CTA CCC GTC |
| Assay | Positive Samples (%) | Negative Samples (%) | P. vivax (%) | P. falciparum (%) | Mixed Infections (%) |
|---|---|---|---|---|---|
| Microscopy | 50 (22.7%) | 170 (77.3%) | 30 (60%) | 19 (38%) | 1 (2%) |
| Gazelle | 48 (21.8%) | 172 (78.2%) | N.A. | N.A. | N.A. |
| Nested PCR | 77 (35%) | 143 (65%) | 50 (64.9%) | 19 (24.7%) | 8 (10.4%) |
| Microscopy | ||||
|---|---|---|---|---|
| nPCR | P. vivax | P. falciparum | Mixed Infections | Total |
| P. vivax | 27 (54%) | 3 (6%) | 0 | 30 |
| P. falciparum | 0 | 12 (24%) | 0 | 12 |
| Mixed infections | 3 (6%) | 4 (8%) | 1 (2%) | 8 |
| Total | 30 | 19 | 1 | 50 |
| Microscopy | Gazelle | |
|---|---|---|
| Sensitivity | 64.9% | 59.7% |
| Specificity | 100% | 98.6% |
| PPV | 100% | 95.8% |
| NPV | 84.1% | 82.0% |
| Accuracy (effectiveness) | 87.73% | 85% |
| Kappa index | 0.7065 | 0.6389 |
| Assay | Cost (USD) per Sample | Turnaround Time (Minutes) |
|---|---|---|
| Microscopy | <$1 | 15–30 |
| Gazelle | $1.25 | <5 |
| Nested PCR | $15–20 | 1020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontecha, G.; Escobar, D.; Ortiz, B.; Pinto, A.; Serrano, D.; Valdivia, H.O. Field Evaluation of a Hemozoin-Based Malaria Diagnostic Device in Puerto Lempira, Honduras. Diagnostics 2022, 12, 1206. https://doi.org/10.3390/diagnostics12051206
Fontecha G, Escobar D, Ortiz B, Pinto A, Serrano D, Valdivia HO. Field Evaluation of a Hemozoin-Based Malaria Diagnostic Device in Puerto Lempira, Honduras. Diagnostics. 2022; 12(5):1206. https://doi.org/10.3390/diagnostics12051206
Chicago/Turabian StyleFontecha, Gustavo, Denis Escobar, Bryan Ortiz, Alejandra Pinto, Delmy Serrano, and Hugo O. Valdivia. 2022. "Field Evaluation of a Hemozoin-Based Malaria Diagnostic Device in Puerto Lempira, Honduras" Diagnostics 12, no. 5: 1206. https://doi.org/10.3390/diagnostics12051206









