Quantitative Sodium (23Na) MRI in Pediatric Gliomas: Initial Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sodium MR Imaging Acquisition
2.3. Sodium MR Imaging Acquisition
2.4. Post-Processing and Quantitative Sodium MR Imaging
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
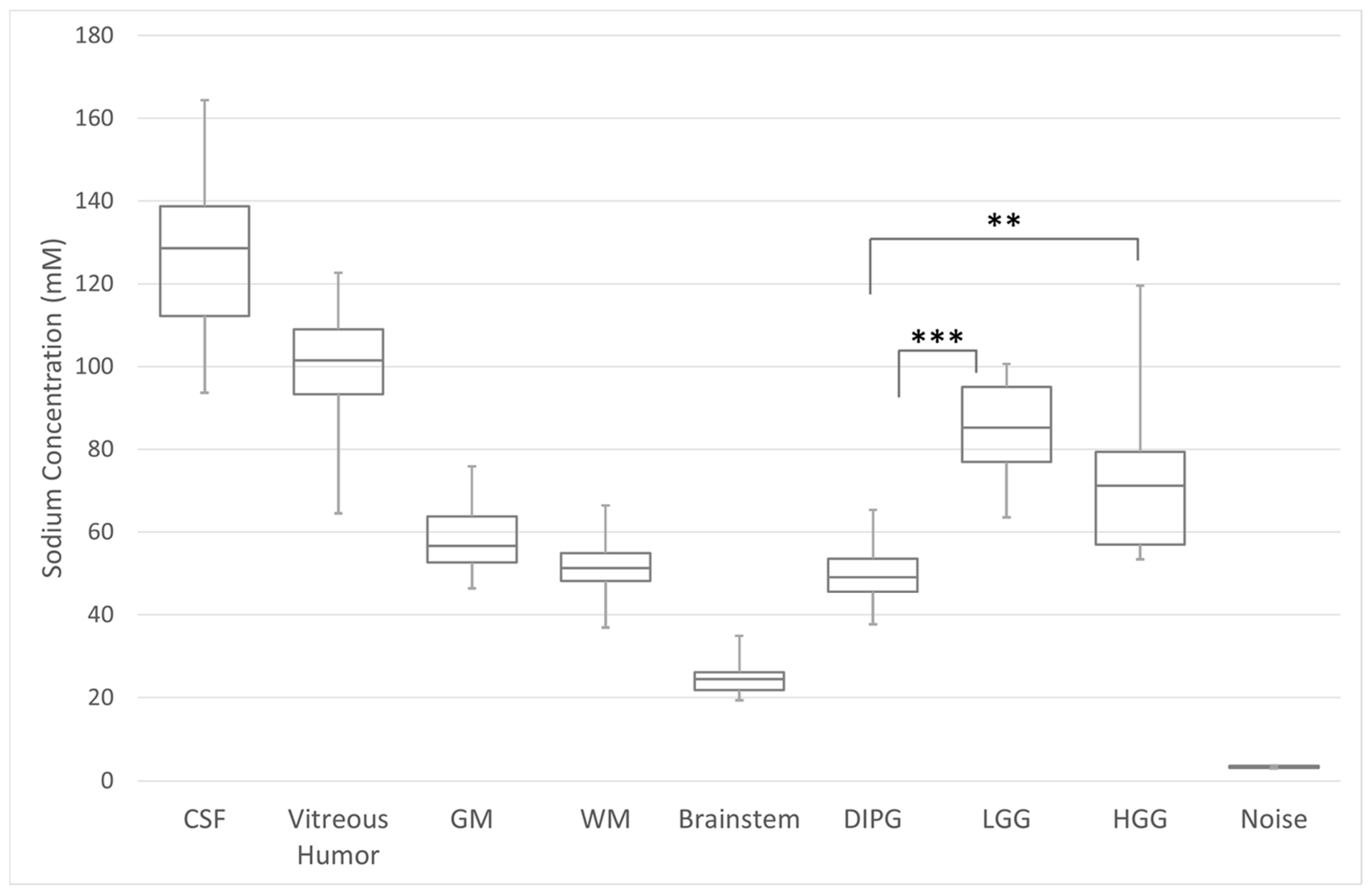
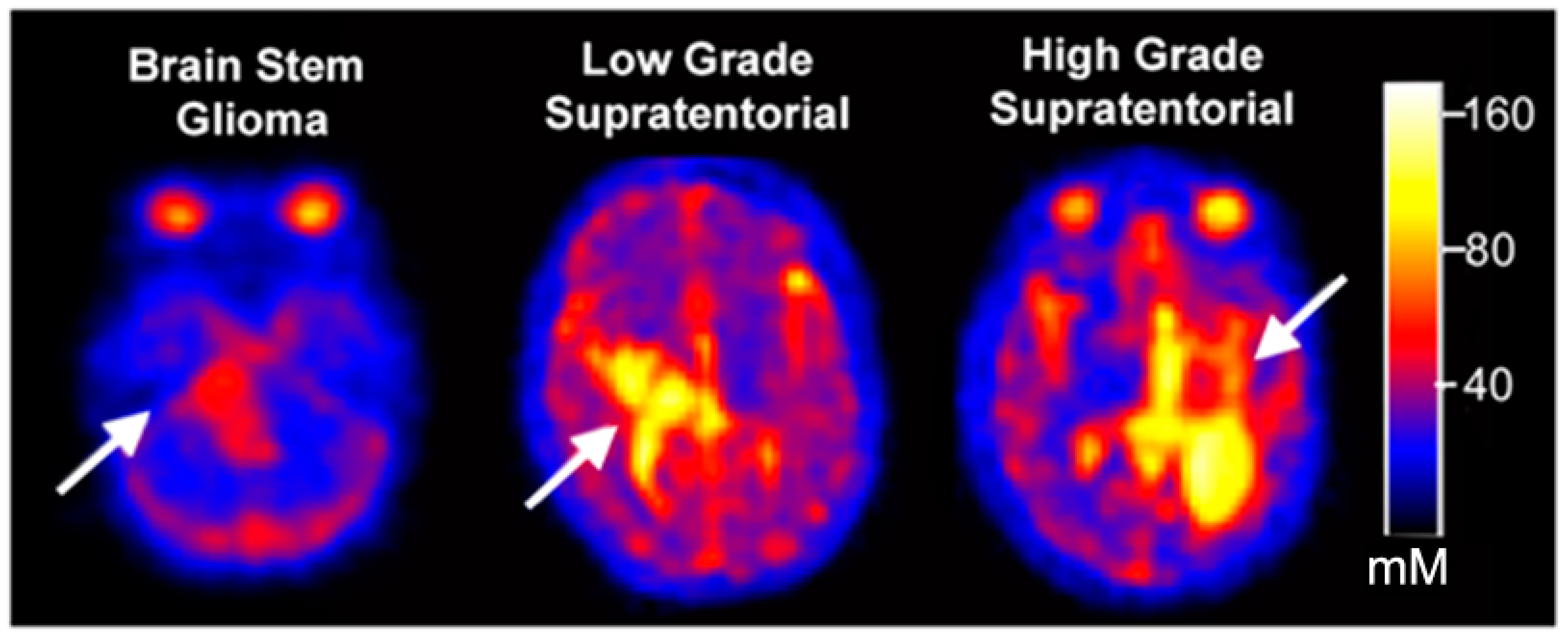
3.2. Quantitative Total Sodium Concentration of Normal Brain and Brain Tumors
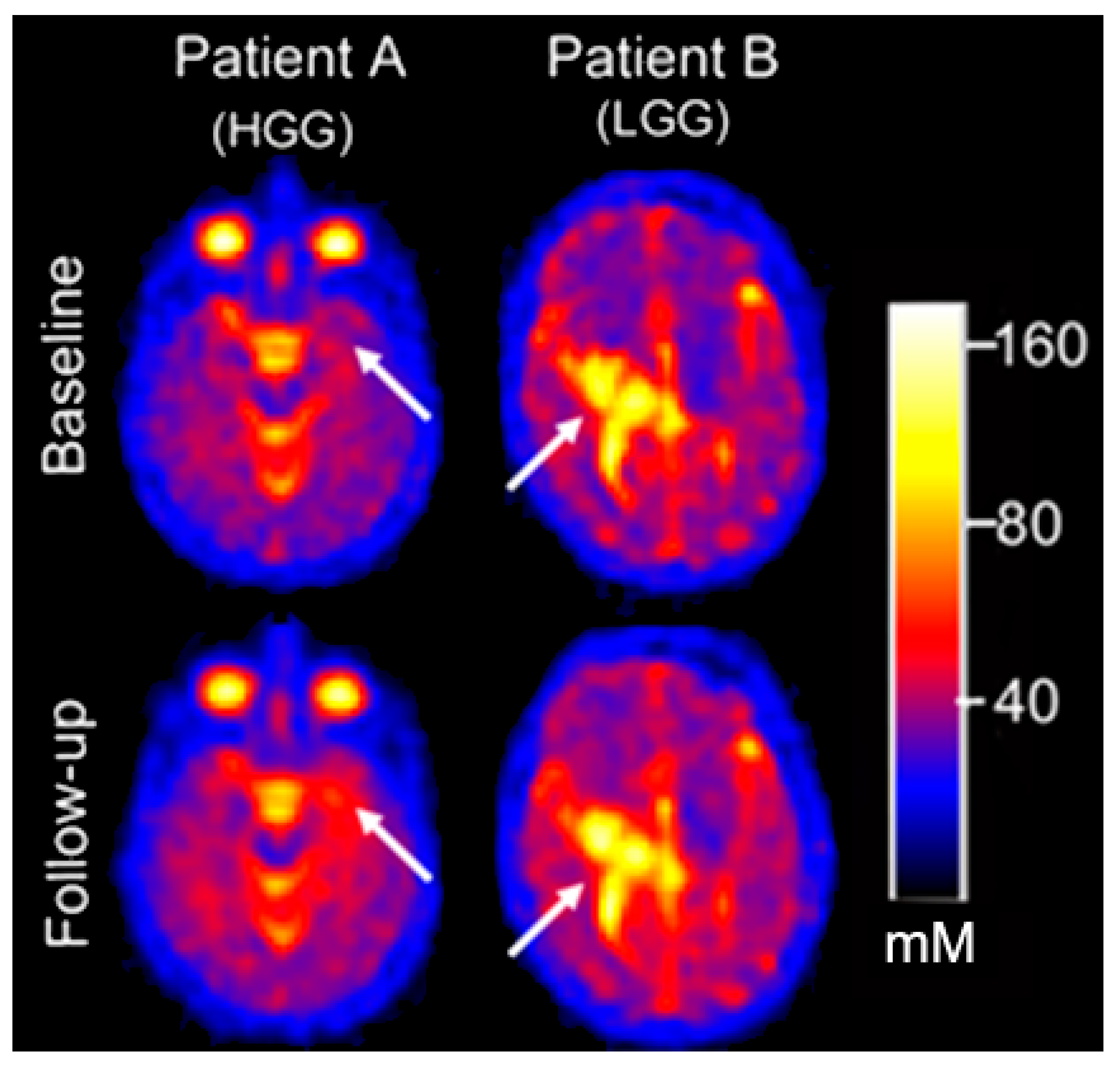
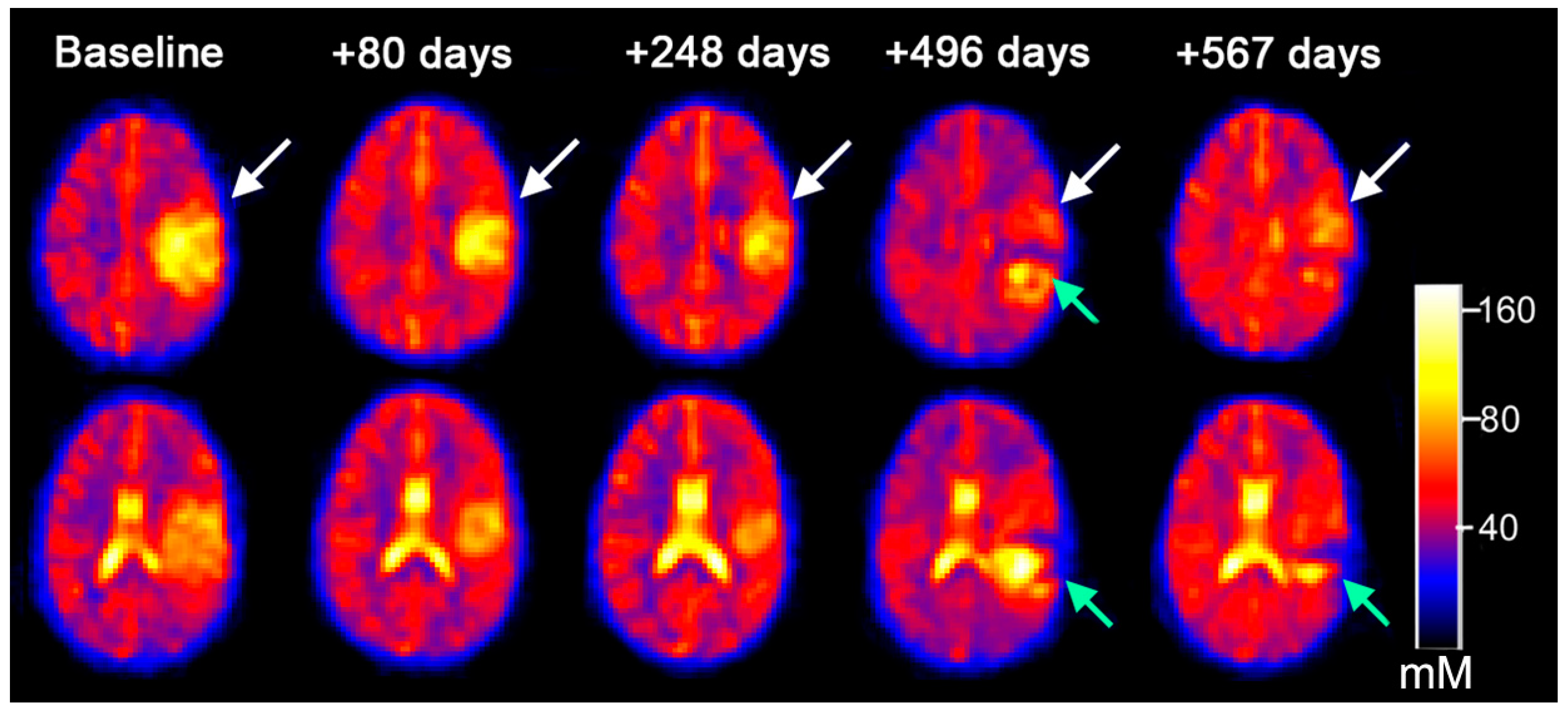
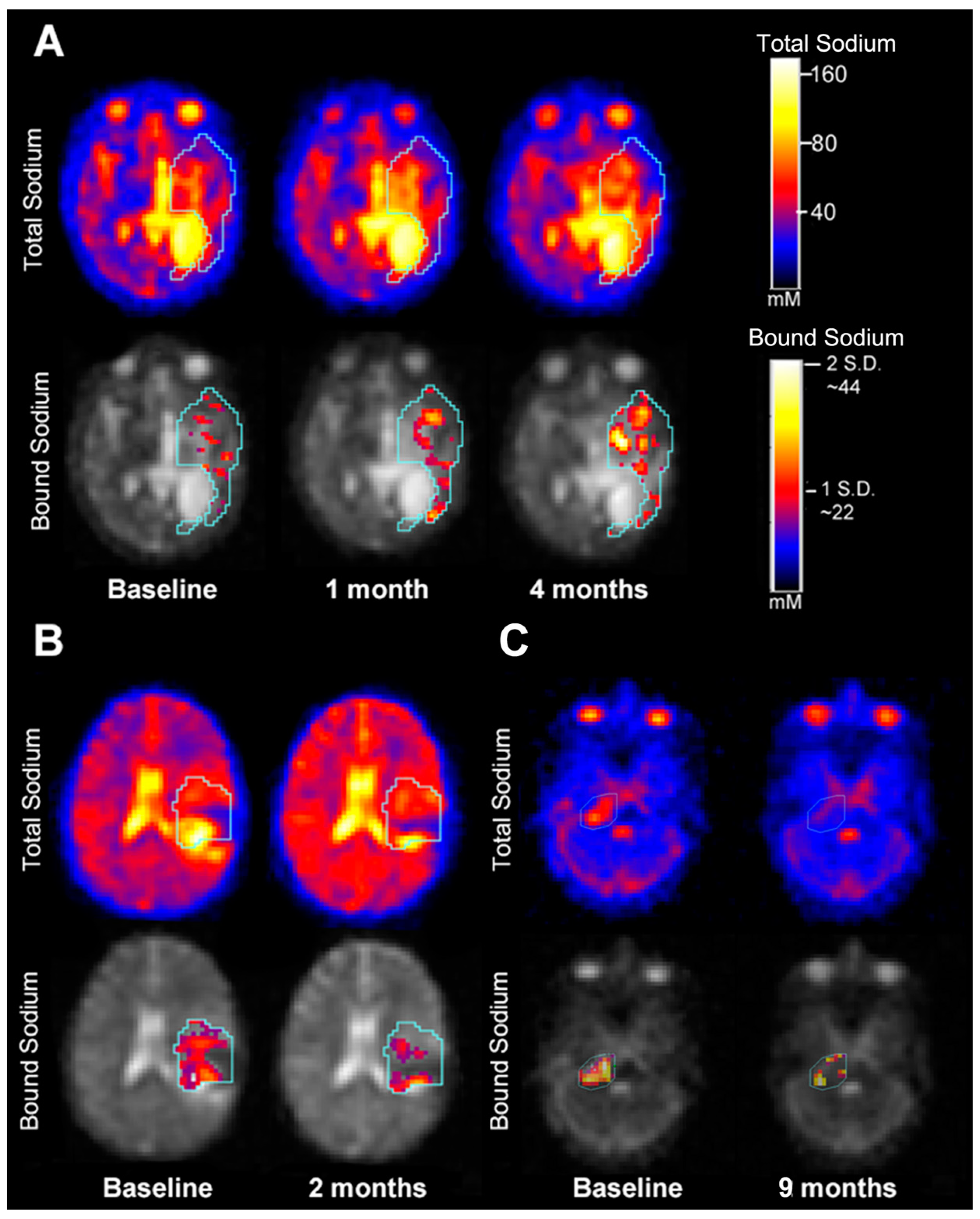
3.3. Serial Quantitative Intra-Tumoral Total Sodium Concentration of Pediatric Glioma
3.4. Quantitative Intra-Tumoral Bound Sodium Concentration of Pediatric Gliomas Including Serial Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BSC | bound sodium concentration |
| vBSC | volume-fraction weighted bound sodium concentration |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DIPG | diffuse intrinsic brainstem glioma |
| FLAIR | fluid-attenuated inversion recovery |
| GAMs | glioma-associated microglia and monocyte-derived macrophages |
| GM | gray matter |
| HGG | high-grade gliomas |
| IDH | isocitrate dehydrogenase |
| ISC | intracellular sodium concentration |
| LGG | low-grade gliomas |
| MIPAV | medical imaging processing, analysis, and visualization |
| MRI | magnetic resonance imaging |
| RAPNO | Response Assessment in Pediatric Neuro-Oncology |
| ROI | region of interest |
| RT | radiotherapy |
| TPI | twisted projection imaging |
| TSC | total sodium concentration |
| WM | white matter |
Appendix A. Quantification of Sodium Concentration
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Blank, P.M.; Ostrom, Q.T.; Rouse, C.; Wolinsky, Y.; Kruchko, C.; Salcido, J.; Barnholtz-Sloan, J.S. Years of life lived with disease and years of potential life lost in children who die of cancer in the United States, 2009. Cancer Med. 2015, 4, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Schepkin, V.D.; Ross, B.D.; Chenevert, T.L.; Rehemtulla, A.; Sharma, S.; Kumar, M.; Stojanovska, J. Sodium magnetic resonance imaging of chemotherapeutic response in a rat glioma. Magn. Reson. Med. 2004, 53, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulborn, K.R.; Davis, D.; Adams, H.; Gindin, T.; Zhou, J. Quantitative tissue sodium concentration mapping of the growth of focal cerebral tumors with sodium magnetic resonance imaging. Magn. Reson. Med. 1999, 41, 351–359. [Google Scholar] [CrossRef]
- Thulborn, K.R. Quantitative sodium MR imaging: A review of its evolving role in medicine. NeuroImage 2018, 168, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, R. Sodium Magnetic Resonance Imaging: From Research to Clinical Use. J. Am. Coll. Radiol. 2007, 4, 739–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouwerkerk, R.; Bleich, K.B.; Gillen, J.S.; Pomper, M.G.; Bottomley, P.A. Tissue Sodium Concentration in Human Brain Tumors as Measured with23Na MR Imaging. Radiology 2003, 227, 529–537. [Google Scholar] [CrossRef]
- Boada, F.E.; Gillen, J.S.; Shen, G.X.; Chang, S.Y.; Thulborn, K. Fast three dimensional sodium imaging. Magn. Reson. Med. 1997, 37, 706–715. [Google Scholar] [CrossRef]
- Qian, Y.; Panigrahy, A.; Laymon, C.M.; Lee, V.K.; Drappatz, J.; Lieberman, F.S.; Boada, F.E.; Mountz, J.M. Short-T2imaging for quantifying concentration of sodium (23Na) of bi-exponential T(2)relaxation. Magn. Reson. Med. 2014, 74, 162–174. [Google Scholar] [CrossRef]
- Riemer, F.; McHugh, D.; Zaccagna, F.; Lewis, D.; McLean, M.A.; Graves, M.J.; Gilbert, F.J.; Parker, G.J.; Gallagher, F.A. Measuring tissue sodium concentration: Cross-vendor repeatability and reproducibility of 23 Na-MRI across two sites. J. Magn. Reson. Imaging 2019, 50, 1278–1284. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.M.; Haneder, S.; Konstandin, S.; Budjan, J.; Morelli, J.N.; Schad, L.R.; Kerl, H.U.; Schoenberg, S.O.; Kabbasch, C. Repeatability and reproducibility of cerebral 23Na imaging in healthy subjects. BMC Med. Imaging 2019, 19, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deen, S.S.; Riemer, F.; McLean, M.A.; Gill, A.B.; Kaggie, J.D.; Grist, J.T.; Crawford, R.; Latimer, J.; Baldwin, P.; Earl, H.M.; et al. Sodium MRI with 3D-cones as a measure of tumour cellularity in high grade serous ovarian cancer. Eur. J. Radiol. Open 2019, 6, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetterling, F.; Gallagher, L.; Mullin, J.; Holmes, W.M.; McCabe, C.; Macrae, I.M.; Fagan, A.J. Sodium-23 Magnetic Resonance Imaging Has Potential for Improving Penumbra Detection but Not for Estimating Stroke Onset Time. J. Cereb. Blood Flow Metab. 2014, 35, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.S.; Stobbe, R.W.; Bhagat, Y.A.; Emery, D.; Butcher, K.S.; Manawadu, D.; Rizvi, N.; Maheshwari, P.; Scozzafava, J.; Shuaib, A.; et al. Sodium imaging intensity increases with time after human ischemic stroke. Ann. Neurol. 2009, 66, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Paling, D.; Solanky, B.S.; Riemer, F.; Tozer, D.J.; Wheeler-Kingshott, C.A.M.; Kapoor, R.; Golay, X.; Miller, D.H. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain 2013, 136, 2305–2317. [Google Scholar] [CrossRef]
- Grapperon, A.-M.; Ridley, B.; Verschueren, A.; Maarouf, A.; Confort-Gouny, S.; Fortanier, E.; Schad, L.; Guye, M.; Ranjeva, J.-P.; Attarian, S.; et al. Quantitative Brain Sodium MRI Depicts Corticospinal Impairment in Amyotrophic Lateral Sclerosis. Radiology 2019, 292, 422–428. [Google Scholar] [CrossRef]
- Maarouf, A.; Audoin, B.; Pariollaud, F.; Gherib, S.; Rico, A.; Soulier, E.; Confort-Gouny, S.; Guye, M.; Schad, L.; Pelletier, J.; et al. Increased total sodium concentration in gray matter better explains cognition than atrophy in MS. Neurology 2016, 88, 289–295. [Google Scholar] [CrossRef]
- Meyer, M.M.; Schmidt, A.; Benrath, J.; Konstandin, S.; Pilz, L.R.; Harrington, M.G.; Budjan, J.; Meyer, M.; Schad, L.R.; Schoenberg, S.O.; et al. Cerebral sodium (23Na) magnetic resonance imaging in patients with migraine—A case-control study. Eur. Radiol. 2019, 29, 7055–7062. [Google Scholar] [CrossRef]
- Inglese, M.; Madelin, G.; Oesingmann, N.; Babb, J.; Wu, W.; Stoeckel, B.; Herbert, J.; Johnson, G. Brain tissue sodium concentration in multiple sclerosis: A sodium imaging study at 3 tesla. Brain 2010, 133, 847–857. [Google Scholar] [CrossRef]
- Cameron, I.L.; Smith, N.K.; Pool, T.B.; Sparks, R.L. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980, 40, 1493–1500. [Google Scholar]
- Nagel, A.M.; Bock, M.; Hartmann, C.; Gerigk, L.; Neumann, J.-O.; Weber, M.-A.; Bendszus, M.; Radbruch, A.; Wick, W.; Schlemmer, H.-P.; et al. The Potential of Relaxation-Weighted Sodium Magnetic Resonance Imaging as Demonstrated on Brain Tumors. Investig. Radiol. 2011, 46, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Kline, R.; Walvick, R.; Regatte, R. A method for estimating intracellular sodium concentration and extracellular volume fraction in brain in vivo using sodium magnetic resonance imaging. Sci. Rep. 2014, 4, 4763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N-Used | R2 | Coeff | DoF | F-Value | p-Value * | |
|---|---|---|---|---|---|---|
| CSF | 26 | 0.0448 | 14.8171 | 2/25 | 0.54 | 0.5901 |
| Vitreous Humor | 26 | 0.0351 | 14.2010 | 2/25 | 0.42 | 0.6634 |
| GM | 26 | 0.2611 | 11.6462 | 2/25 | 4.06 | 0.0308 * |
| WM | 26 | 0.1803 | 12.5907 | 2/25 | 2.53 | 0.1016 |
| Tumor | 26 | 0.4926 | 24.1698 | 2/25 | 11.17 | 0.0004 * |
| ID with Scan# | Tumor Total Sodium Concentration TSC, mM | Change in TSC | Conventional Radiographic Response to Treatment at Time of Follow-Up Imaging | Volume (mm3) | Volume Change (Absolute in mm3/% Change) | Concordant/Discordant (between Changes on Na MRI and Conventional MRI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Mean | Std.Dev. | Change | % Change | Direction * | |||||
| 4002-1 | DIPG | 54.5052 | 13.373 | 6.6723 | 12 | No change | Stable, No tumor progression. | 20.8 | ||
| 4002-2 | 61.1775 | 12.1866 | 25.7 | 4.9/19% | Concordant | |||||
| 4006-1 | DIPG | 51.1066 | 11.9815 | 2.0928 | 4 | No change | Stable, No tumor progression | 41.3 | ||
| 4006-2 | 53.1994 | 12.2965 | 46.4 | 5.1/11% | Concordant | |||||
| 4020-1 | DIPG | 33.7777 | 5.1005 | 22.9931 | 68 | Increase | Stable, No tumor progression, increase in necrosis within tumor | 24.7 | ||
| 4020-2 | 56.7708 | 14.3001 | 58.9 | 34.2/58% | Concordant | |||||
| 4017-1 | LGG | 101.9751 | 15.2094 | −2.7367 | −3 | No change | Stable, No tumor progression | 85.7 | ||
| 4017-2 | 99.2384 | 15.4893 | 84.6 | −1.1/−1.3% | Concordant | |||||
| 4010-1 | HGG | 92.5898 | 12.3779 | First serial scan showed decrease in size of the tumor. The second serial scan showed a decrease in size of the tumor The third serial scan showed necrosis with increase in size of the tumor as well as new enhancing lesion The fourth serial scan showed no change in size of the tumor | 163.6 | |||||
| 4010-2 | 73.9051 | 15.4974 | −18.6847 | −20 | Decrease | 120.7 | −42.9/−36% | Concordant | ||
| 4010-3 | 60.0018 | 14.2077 | −13.9033 | −19 | Decrease | 45.3 | −75.4/−166% | Concordant | ||
| 4010-4 | 67.2067 | 20.0919 | 7.2049 | 12 | No change | 43.5 | −1.8/−4.1% | Discordant | ||
| 4010-5 | 62.0964 | 13.6286 | −5.1103 | −8 | No change in TSC; decrease in BSC including recurrent lesion between last two dual echo scans | 33.8 | −9.7/−28.7% | Concordant | ||
| 4023-1 | HGG | 49.477 | 3.7975 | 7.7313 | 16 | Increase | Interval increase in non-enhancing edema. Infiltrating abnormal signal intensity representing tumor progression | 19.2 | ||
| 4023-2 | 57.2083 | 4.3588 | 39.4 | 20.2/51.3% | Concordant | |||||
| 4025-1 | HGG | 47.0821 | 8.8937 | −11.7503 | −25 | Decrease in TSC and decrease in BSC | Interval increase in size and enhancement of tumor representing recurrence | 26.8 | ||
| 4025-2 | 35.3318 | 5.7061 | 20.4 | −6.4/−31.4% | Concordant | |||||
| 4027-1 | HGG | 77.1108 | 13.6791 | No change in TSC on both serial scans; increase in BSC in recurrent lesion between last two dual echo scans | Progressive increase in size of tumor representing tumor progression | 1 | ||||
| 4027-2 | 81.9984 | 17.2532 | 4.8876 | 6 | 12.9 | 11.9/92.2% | Discordant | |||
| 4027-3 | 78.9372 | 18.4989 | −3.0612 | −4 | 48.6 | 35.7/73.5% | Discordant | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, A.; Lee, V.K.; Qian, Y.; Paldino, M.J.; Ceschin, R.; Hect, J.; Mountz, J.M.; Sun, D.; Kohanbash, G.; Pollack, I.F.; et al. Quantitative Sodium (23Na) MRI in Pediatric Gliomas: Initial Experience. Diagnostics 2022, 12, 1223. https://doi.org/10.3390/diagnostics12051223
Bhatia A, Lee VK, Qian Y, Paldino MJ, Ceschin R, Hect J, Mountz JM, Sun D, Kohanbash G, Pollack IF, et al. Quantitative Sodium (23Na) MRI in Pediatric Gliomas: Initial Experience. Diagnostics. 2022; 12(5):1223. https://doi.org/10.3390/diagnostics12051223
Chicago/Turabian StyleBhatia, Aashim, Vincent Kyu Lee, Yongxian Qian, Michael J. Paldino, Rafael Ceschin, Jasmine Hect, James M. Mountz, Dandan Sun, Gary Kohanbash, Ian F. Pollack, and et al. 2022. "Quantitative Sodium (23Na) MRI in Pediatric Gliomas: Initial Experience" Diagnostics 12, no. 5: 1223. https://doi.org/10.3390/diagnostics12051223






