Trends in Neonatal Ophthalmic Screening Methods
Abstract
:1. Introduction
2. Full-Term Healthy Newborns
2.1. Selected Ocular Congenital Diseases
2.2. New Trends in Neonatal Ophthalmic Screening
2.3. Additional Possible Screening Enhancements
3. Preterm Newborns
3.1. ROP Risk Factors
3.2. ROP Screening
3.3. International Classification of ROP
3.4. Predictive ROP Models
3.5. Imaging Systems
3.5.1. Fundus Imaging
3.5.2. Fluorescein Angiography
3.5.3. Spectral-Domain Optical Coherence Tomography
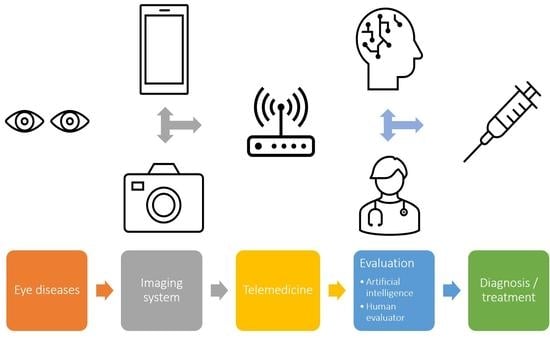
3.6. Artificial Intelligence and Telemedicine
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Azad, A.D.; Al-Moujahed, A.; Ludwig, C.A.; Vail, D.; Callaway, N.F.; Rosenblatt, T.R.; Kumm, J.; Moshfeghi, D.M. The utility of universal newborn eye screening: A review. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, S6–S16. [Google Scholar] [CrossRef]
- Toli, A.; Perente, A.; Labiris, G. Evaluation of the red reflex: An overview for the pediatrician. World J. Methodol. 2021, 11, 263–277. [Google Scholar] [CrossRef]
- Ludwig, C.A.; Callaway, N.F.; Blumenkranz, M.S.; Fredrick, D.R.; Moshfeghi, D.M. Validity of the red reflex exam in the newborn eye screening test cohort. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 103–110. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Li, S. A meta-analysis of prognostic biomarkers in neonatal retinal hemorrhage. Int. Ophthalmol. 2022, 42, 677–688. [Google Scholar] [CrossRef]
- Wood, E.H.; Capone, A., Jr.; Drenser, K.A.; Berrocal, A.; Hubbard, G.B.; Callaway, N.F.; Kychenthal, A.; Ells, A.; Harper, C.A., 3rd; Besirli, C.G.; et al. Referable macular hemorrhage-A clinically meaningful screening target in newborn infants. Position statement of the association of pediatric retina surgeons. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Augestad, L.B.; Klingenberg, O.; Fosse, P. Braille use among Norwegian children from 1967 to 2007: Trends in the underlying causes. Acta Ophthalmol. 2012, 90, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Glatz, M.; Riedl, R.; Glatz, W.; Schneider, M.; Wedrich, A.; Bolz, M.; Strauss, R.W. Blindness and visual impairment in Central Europe. PLoS ONE 2022, 17, e0261897. [Google Scholar] [CrossRef]
- Aiello, L.P. Vascular endothelial growth factor and the eye. Past, present and future. Arch. Ophthalmol. 1996, 114, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.M.; Leech, J.; Diaz, C.M.; Penfold, P.L.; Stone, J.; Keshet, E. Development of the human retinal vasculature: Cellular relations and VEGF expression. Exp. Eye Res. 1997, 65, 555–568. [Google Scholar] [CrossRef]
- Andersen, C.C.; Phelps, D.L. Peripheral retinal ablation for threshold retinopathy of prematurity in preterm infants. Cochrane. Database Syst. Rev. 2000, 1999, CD001693. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Penn, J.S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Fierson, W.M.; Chiang, M.F.; Good, W.; Phelps, D.; Reynolds, J.; Robbins, S.L.; Karr, D.J.; Bradford, G.E.; Nischal, K.; Roarty, J.; et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, J.D.; Dobson, V.; Quinn, G.E.; Fielder, A.R.; Palmer, E.A.; Saunders, R.A.; Hardy, R.J.; Phelps, D.L.; Baker, J.D.; Trese, M.T.; et al. Evidence-based screening criteria for retinopathy of prematurity: Natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch. Ophthalmol. 2002, 120, 1470–1476. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. the international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Paul Chan, R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Peter Campbell, J.; Capone, A., Jr.; et al. International classification of retinopathy of prematurity, third edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef]
- Lofqvist, C.; Andersson, E.; Sigurdsson, J.; Engstrom, E.; Hard, A.L.; Niklasson, A.; Smith, L.E.; Hellstrom, A. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch. Ophthalmol. 2006, 124, 1711–1718. [Google Scholar] [CrossRef] [Green Version]
- Hellstrom, A.; Engstrom, E.; Hard, A.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Lofqvist, C.; Svensson, E.; Holm, S.; Ewald, U.; et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003, 112, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.H.; Wagner, B.D.; Cerda, A.; McCourt, E.A.; Palestine, A.; Enzenauer, R.W.; Braverman, R.S.; Wong, R.K.; Tsui, I.; Gore, C.; et al. Colorado retinopathy of prematurity model: A multi-institutional validation study. J. AAPOS 2016, 20, 220–225. [Google Scholar] [CrossRef]
- Binenbaum, G.; Ying, G.S.; Quinn, G.E.; Huang, J.; Dreiseitl, S.; Antigua, J.; Foroughi, N.; Abbasi, S. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch. Ophthalmol. 2012, 130, 1560–1565. [Google Scholar] [CrossRef]
- Hutchinson, A.K.; Melia, M.; Yang, M.B.; VanderVeen, D.K.; Wilson, L.B.; Lambert, S.R. Clinical models and algorithms for the prediction of retinopathy of prematurity: A report by the american academy of ophthalmology. Ophthalmology 2016, 123, 804–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.C.; Bitoque, D.B.; Martins, C.; Coelho, C.; Borrego, L.M.; Silva, G.A. Serum levels of placental growth factor reflect the severity of retinopathy of prematurity. Acta Paediatr. 2021, 110, 2778–2779. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H.; Urs, R.; Jokl, D.H.; Pinto, L.; Coki, O.; Sahni, R.; Horowitz, J.D.; Brooks, S.E. Ocular blood flow in preterm neonates: A preliminary report. Transl. Vis. Sci. Technol. 2021, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Meng, X.; Xu, N.; Li, J.; Cheng, Y.; Chen, Y.; Huang, L. Ocular phenotype and genetical analysis in patients with retinopathy of prematurity. BMC Ophthalmol. 2022, 22, 22. [Google Scholar] [CrossRef]
- Lorenz, B.; Spasovska, K.; Elflein, H.; Schneider, N. Wide-field digital imaging based telemedicine for screening for acute retinopathy of prematurity (ROP). Six-year results of a multicentre field study. Graefes. Arch. Clin. Exp. Ophthalmol. 2009, 247, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Wood, E.H.; Moshfeghi, A.A.; Nudleman, E.D.; Moshfeghi, D.M. Evaluation of visunex medical’s PanoCam(TM) LT and PanoCam(TM) pro wide-field imaging systems for the screening of ROP in newborn infants. Expert. Rev. Med. Devices 2016, 13, 705–712. [Google Scholar] [CrossRef]
- Dhami, A.; Gupta, G.; Dhami, N.B.; Arora, N.; Dhami, G.S. Analysis of the parental satisfaction for retinopathy of prematurity screening using binocular indirect ophthalmoscopy versus wide field retinal imaging. Indian J. Ophthalmol. 2021, 69, 2142–2145. [Google Scholar] [CrossRef]
- Goyal, A.; Gopalakrishnan, M.; Anantharaman, G.; Chandrashekharan, D.P.; Thachil, T.; Sharma, A. Smartphone guided wide-field imaging for retinopathy of prematurity in neonatal intensive care unit—A smart ROP (SROP) initiative. Indian J. Ophthalmol. 2019, 67, 840–845. [Google Scholar] [CrossRef]
- Vural, A.; Ekinci, D.Y.; Onur, I.U.; Hergunsel, G.O.; Yigit, F.U. Comparison of fluorescein angiographic findings in type 1 and type 2 retinopathy of prematurity with intravitreal bevacizumab monotherapy and spontaneous regression. Int. Ophthalmol. 2019, 39, 2267–2274. [Google Scholar] [CrossRef]
- Vural, A.; Demirayak, B.; Ozbas, M.; Onur, I.U.; Celik, G. Comparison of fluorescein angiography findings in stage 3 retinopathy of prematurity in zone II treated with or without Anti-VEGF. Eur. J. Ophthalmol. 2022, 11206721221076691. [Google Scholar] [CrossRef]
- Mansukhani, S.A.; Hutchinson, A.K.; Neustein, R.; Schertzer, J.; Allen, J.C.; Hubbard, G.B. Fluorescein angiography in retinopathy of prematurity: Comparison of infants treated with bevacizumab to those with spontaneous regression. Ophthalmol. Retina 2019, 3, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Shao, Y.; Lao, J.; Yu, X.; Chen, Y.; Zhang, C.; Li, H.; Shen, L. Ultra-wide-field imaging and intravenous fundus fluorescein angiography in infants with retinopathy of prematurity. Retina 2020, 40, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.S.; Toth, C.A. Optical coherence tomography in retinopathy of prematurity: Looking beyond the vessels. Clin. Perinatol. 2013, 40, 271–296. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.S.; O’Connell, R.V.; Sarin, N.; Freedman, S.F.; Wallace, D.K.; Cotten, C.M.; Winter, K.P.; Stinnett, S.; Chiu, S.J.; Izatt, J.A.; et al. Dynamics of human foveal development after premature birth. Ophthalmology 2011, 118, 2315–2325. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, M.T.; Maldonado, R.S.; Toth, C.A.; O’Connell, R.V.; Chen, B.B.; Chiu, S.J.; Farsiu, S.; Wallace, D.K.; Stinnett, S.S.; Panayotti, G.M.; et al. Subfoveal fluid in healthy full-term newborns observed by handheld spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2012, 153, 167–175.e163. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mangalesh, S.; Tran-Viet, D.; Freedman, S.F.; Vajzovic, L.; Toth, C.A. Fluorescein angiographic characteristics of macular edema during infancy. JAMA Ophthalmol. 2018, 136, 538–542. [Google Scholar] [CrossRef]
- Chen, X.; Prakalapakorn, S.G.; Freedman, S.F.; Vajzovic, L.; Toth, C.A. Differentiating retinal detachment and retinoschisis using handheld optical coherence tomography in stage 4 retinopathy of prematurity. JAMA Ophthalmol. 2020, 138, 81–85. [Google Scholar] [CrossRef]
- Chen, X.; Mangalesh, S.; Dandridge, A.; Tran-Viet, D.; Wallace, D.K.; Freedman, S.F.; Toth, C.A. Spectral-domain OCT findings of retinal vascular-avascular junction in infants with retinopathy of prematurity. Ophthalmol. Retina 2018, 2, 963–971. [Google Scholar] [CrossRef]
- Cernichiaro-Espinosa, L.A.; Olguin-Manriquez, F.J.; Henaine-Berra, A.; Garcia-Aguirre, G.; Quiroz-Mercado, H.; Martinez-Castellanos, M.A. New insights in diagnosis and treatment for Retinopathy of Prematurity. Int. Ophthalmol. 2016, 36, 751–760. [Google Scholar] [CrossRef]
- Bao, Y.; Ming, W.K.; Mou, Z.W.; Kong, Q.H.; Li, A.; Yuan, T.F.; Mi, X.S. Current application of digital diagnosing systems for retinopathy of prematurity. Comput. Methods Programs Biomed. 2021, 200, 105871. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Mitsuhashi, T.; Matsuo, T. Accuracy of deep learning algorithms for the diagnosis of retinopathy of prematurity by fundus images: A systematic review and meta-analysis. J. Ophthalmol. 2021, 2021, 8883946. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yang, H.C.; Poly, T.N.; Jian, W.S.; Jack Li, Y.C. Deep learning algorithms for detection of diabetic retinopathy in retinal fundus photographs: A systematic review and meta-analysis. Comput. Methods Programs Biomed. 2020, 191, 105320. [Google Scholar] [CrossRef]
- Lu, W.; Tong, Y.; Yu, Y.; Xing, Y.; Chen, C.; Shen, Y. Applications of artificial intelligence in ophthalmology: General overview. J. Ophthalmol. 2018, 2018, 5278196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranak, Z.; Pencak, M.; Veith, M. Arteficial intelligence in diabetic retinopathy screening. A review. Cesk Slov. Oftalmol. 2021, 77, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Attallah, O. DIAROP: Automated deep learning-based diagnostic tool for retinopathy of prematurity. Diagnostics 2021, 11, 2034. [Google Scholar] [CrossRef]
- Wang, J.; Ji, J.; Zhang, M.; Lin, J.W.; Zhang, G.; Gong, W.; Cen, L.P.; Lu, Y.; Huang, X.; Huang, D.; et al. Automated explainable multidimensional deep learning platform of retinal images for retinopathy of prematurity screening. JAMA Netw. Open 2021, 4, e218758. [Google Scholar] [CrossRef]

| Anatomical Part of the Eye | Disease Detectable with the Screening | Immaging Technique | Approximate Incidience | Usual Treatment Timing |
|---|---|---|---|---|
| Anterior segment | dysgenesis of the anterior segment | RRT, WFDI | 4:100,000 | depends on degree, first months of life |
| congenital glaucoma | RRT, WFDI | 2–10:100,000 | first months of life | |
| congenital cataract | RRT, WFDI | 18–36:100,000 | 4–8 weeks of life | |
| uveitis | RRT, WFDI | heterogenous group | according to activity (from immediate to no treatment) | |
| Posterior segment | persistent fetal vasculature | RRT, WFDI | 3–7:100,000 | depends on degree, 4–8 weeks of life |
| vitreous hemorrhage | WFDI; RRT in advanced cases | heterogenous group | depends on degree, within weeks | |
| uveitis | WFDI; RRT in advanced cases | heterogenous group | according to activity (from immediate to no treatment) | |
| retinoblastoma | WFDI; RRT in advanced cases | 5:100,000 | immediate after diagnosis | |
| retinal/macular hemorrhage | WFDI; RRT in advanced cases | heterogenous group | observation, treatment in indicated cases during weeks | |
| retinal detachment | WFDI; RRT in advanced cases | rare, heterogenous group | only in selected cases (often impossible to treat) | |
| Coat´s disease | WFDI; RRT in advanced cases | 0.09:100,000 | observation, treatment in indicated cases (activity) | |
| chorioretinal coloboma | WFDI; RRT in advanced cases | 5–22:100,000 | no treatment required |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hložánek, M.; Straňák, Z.; Terešková, Z.; Mareš, J.; Krejčířová, I.; Česká Burdová, M. Trends in Neonatal Ophthalmic Screening Methods. Diagnostics 2022, 12, 1251. https://doi.org/10.3390/diagnostics12051251
Hložánek M, Straňák Z, Terešková Z, Mareš J, Krejčířová I, Česká Burdová M. Trends in Neonatal Ophthalmic Screening Methods. Diagnostics. 2022; 12(5):1251. https://doi.org/10.3390/diagnostics12051251
Chicago/Turabian StyleHložánek, Martin, Zbyněk Straňák, Zuzana Terešková, Jan Mareš, Inka Krejčířová, and Marie Česká Burdová. 2022. "Trends in Neonatal Ophthalmic Screening Methods" Diagnostics 12, no. 5: 1251. https://doi.org/10.3390/diagnostics12051251