Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging
Abstract
1. Introduction
2. Type of Resections
3. Complications
3.1. Early Postoperative Complications
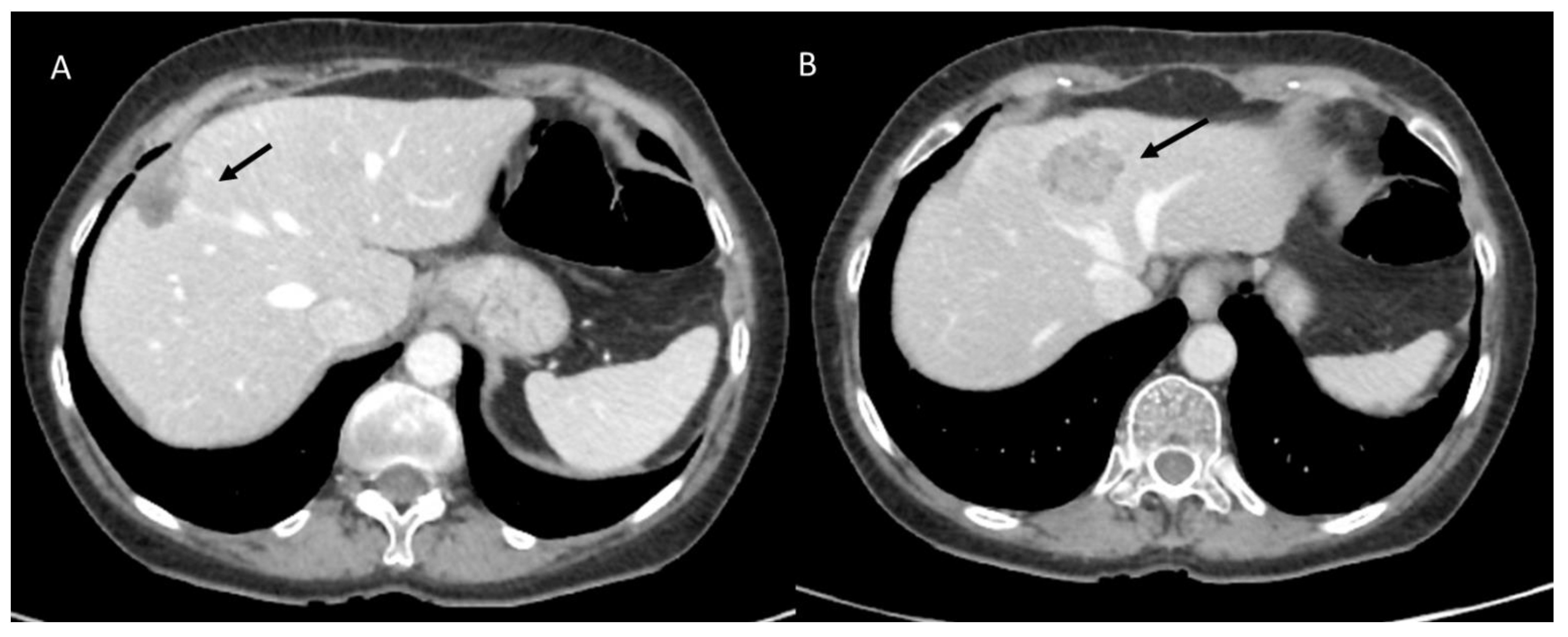
3.1.1. Fluid Collection
3.1.2. Posthepatectomy Hemorrhage
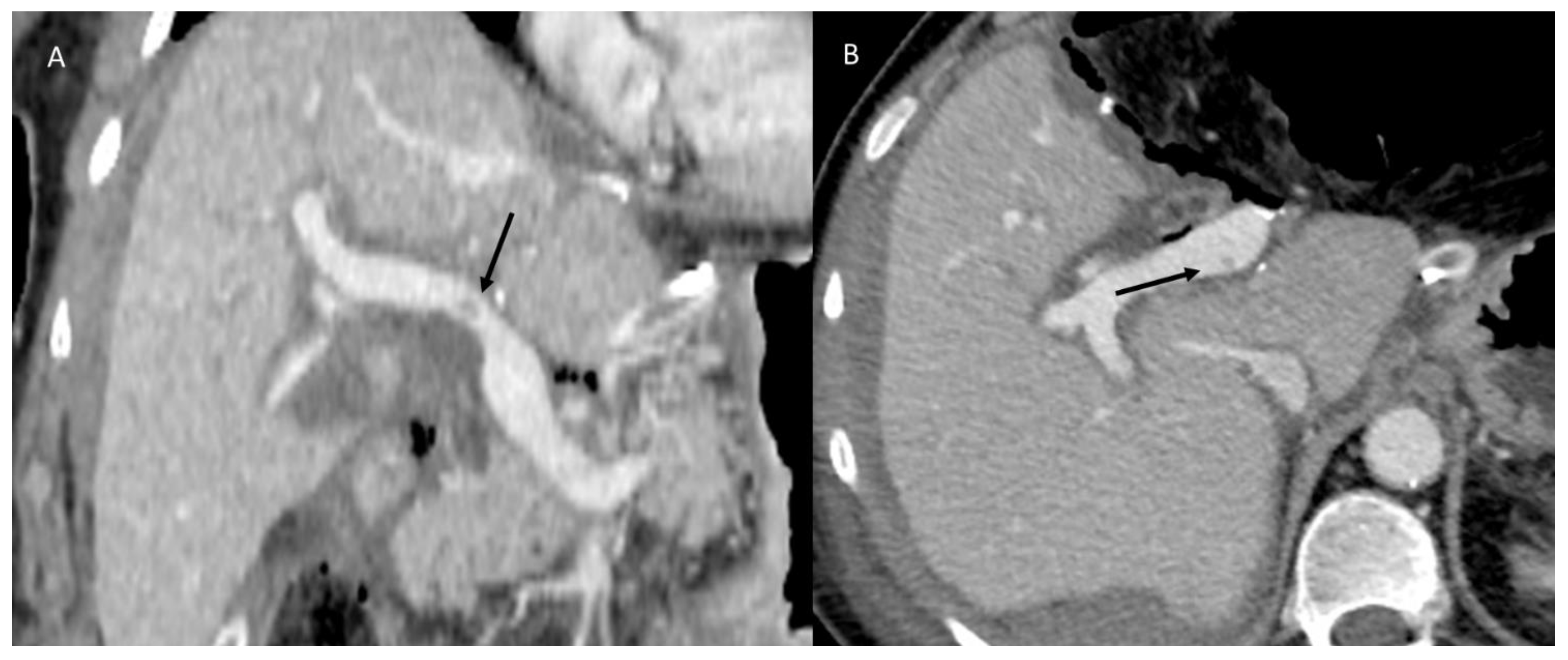
3.1.3. Vascular Thrombosis
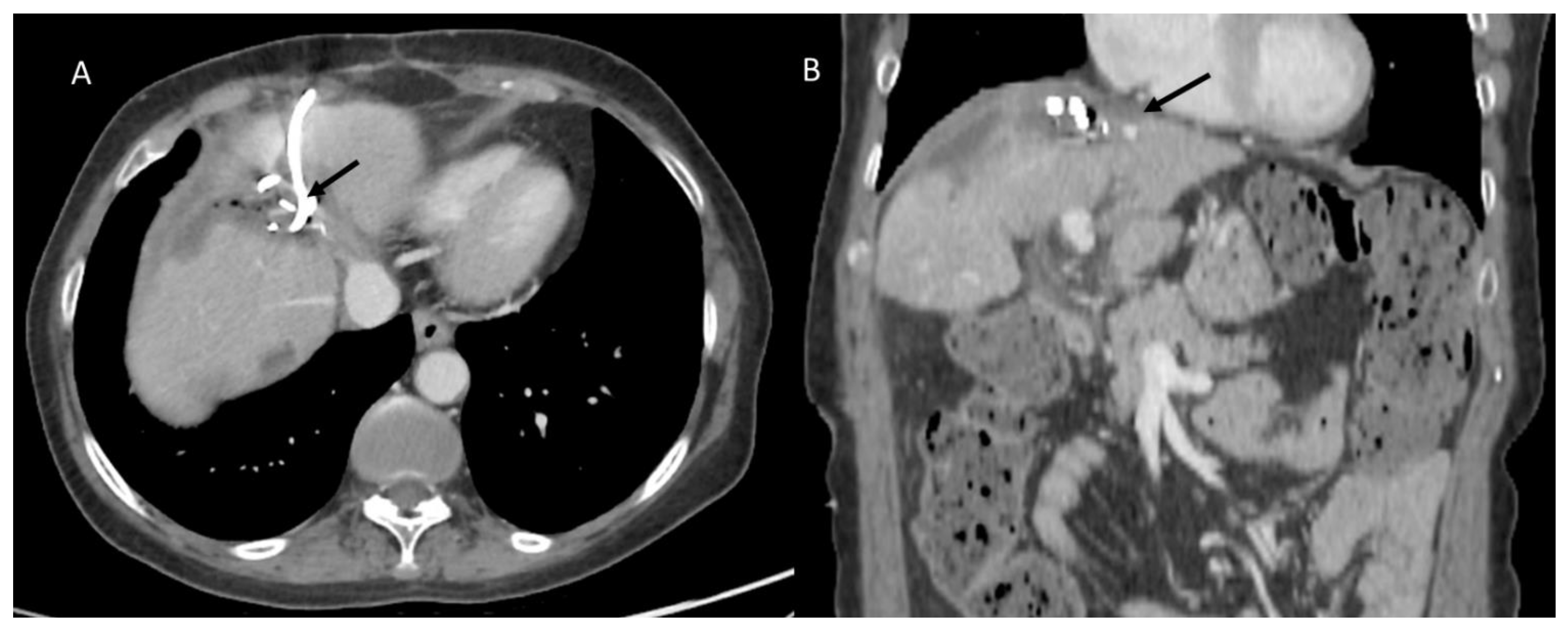
3.1.4. Biliary Injuries
3.2. Late Postoperative Complications
3.2.1. Disease Recurrence
3.2.2. Late Strictures and Ischemic Cholangitis
4. Multidisciplinary Assessment
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafaro, K.J.; Stewart, C.; Fong, A.; Fong, Y. Robotic Liver Resection. Surg. Clin. N. Am. 2020, 100, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Divatia, J.V. Enhanced recovery after surgery in liver resection: Current concepts and controversies. Korean J. Anesthesiol. 2019, 72, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Piccirillo, M.; Palaia, R.; Nasti, G.; Petrillo, A.; Izzo, F. A radiologist’s point of view in the presurgical and intraoperative setting of colorectal liver metastases. Future Oncol. 2018, 14, 2189–2206. [Google Scholar] [CrossRef]
- Fanelli, F.; Cannavale, A.; Chisci, E.; Citone, M.; Falcone, G.M.; Michelagnoli, S.; Miele, V. Direct percutaneous embolization of aneurysm sac: A safe and effective procedure to treat post-EVAR type II endoleaks. Radiol. Med. 2021, 126, 258–263. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous ablation therapy of hepatocellular carcinoma with irreversible electroporation: MRI findings. AJR Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Palaia, R.; Botti, G.; Tatangelo, F.; Granata, F.; Cascella, M.; Izzo, F.; et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE 2017, 12, e0179951. [Google Scholar] [CrossRef]
- Dimick, J.B.; Wainess, R.M.; Cowan, J.A.; Upchurch, G.R.; Knol, J.A.; Colletti, L.M. National trends in the use and outcomes of hepatic resection. J. Am. Coll. Surg. 2004, 199, 31–38. [Google Scholar] [CrossRef]
- Giurazza, F.; Contegiacomo, A.; Calandri, M.; Mosconi, C.; Modestino, F.; Corvino, F.; Scrofani, A.R.; Marra, P.; Coniglio, G.; Failla, G.; et al. IVC filter retrieval: A multicenter proposal of two score systems to predict application of complex technique and procedural outcome. Radiol. Med. 2021, 126, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Asiyanbola, B.; Chang, D.; Gleisner, A.L.; Nathan, H.; Choti, M.A.; Schulick, R.D.; Pawlik, T.M. Operative mortality after hepatic resection: Are literature-based rates broadly applicable? J. Gastrointest. Surg. 2008, 12, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; Ribero, D.; Reddy, S.K.; Donadon, M.; Zorzi, D.; Gautam, S.; Abdalla, E.K.; Curley, S.A.; Capussotti, L.; Clary, B.M.; et al. Hepatic insufficiency and mortality in 1059 noncirrhotic patients undergoing major hepatectomy. J. Am. Coll. Surg. 2007, 204, 854–862. [Google Scholar] [CrossRef]
- Barabino, M.; Gurgitano, M.; Fochesato, C.; Angileri, S.A.; Franceschelli, G.; Santambrogio, R.; Mariani, N.M.; Opocher, E.; Carrafiello, G. LI-RADS to categorize liver nodules in patients at risk of HCC: Tool or a gadget in daily practice? Radiol. Med. 2021, 126, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.K.; Ghaferi, A.A.; Osborne, N.H.; Pawlik, T.M.; Campbell, D.A.; Englesbe, M.J.; Welling, T.H. Body mass index and adverse perioperative outcomes following hepatic resection. J. Gastrointest. Surg. 2010, 14, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Benzoni, E.; Cojutti, A.; Lorenzin, D.; Adani, G.L.; Baccarani, U.; Favero, A.; Zompicchiati, A.; Bresadola, F.; Uzzau, A. Liver resective surgery: A multivariate analysis of postoperative outcome and complication. Langenbecks Arch. Surg. 2007, 392, 45–54. [Google Scholar] [CrossRef]
- Sadamori, H.; Yagi, T.; Shinoura, S.; Umeda, Y.; Yoshida, R.; Satoh, D.; Nobuoka, D.; Utsumi, M.; Fujiwara, T. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br. J. Surg. 2013, 100, 122–129. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Venanzio Setola, S.; Mattace Raso, M.; Avallone, A.; De Stefano, A.; Nasti, G.; Palaia, R.; Delrio, P.; Petrillo, A.; et al. Liver radiologic findings of chemotherapy-induced toxicity in liver colorectal metastases patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9697–9706. [Google Scholar] [CrossRef]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.; et al. Critical role of bevacizumab scheduling in combination with pre-surgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the BRANCH trial. Oncotarget 2015, 6, 30394–30407. [Google Scholar] [CrossRef]
- Hu, H.T.; Shan, Q.Y.; Chen, S.L.; Li, B.; Feng, S.T.; Xu, E.J.; Li, X.; Long, J.Y.; Xie, X.Y.; Lu, M.D.; et al. CT-based radiomics for preoperative prediction of early recurrent hepatocellular carcinoma: Technical reproducibility of acquisition and scanners. Radiol. Med. 2020, 125, 697–705. [Google Scholar] [CrossRef]
- Gabelloni, M.; Di Nasso, M.; Morganti, R.; Faggioni, L.; Masi, G.; Falcone, A.; Neri, E. Application of the ESR iGuide clinical decision support system to the imaging pathway of patients with hepatocellular carcinoma and cholangiocarcinoma: Preliminary findings. Radiol. Med. 2020, 125, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Calandri, M.; Bergamasco, L.; Darvizeh, F.; Grazioli, L.; Inchingolo, R.; Ippolito, D.; Rousset, S.; Veltri, A.; Fonio, P.; et al. Characterization of the arterial enhancement pattern of focal liver lesions by multiple arterial phase magnetic resonance imaging: Comparison between hepatocellular carcinoma and focal nodular hyperplasia. Radiol. Med. 2020, 125, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; de Lutio di Castelguidone, E.; Avallone, A.; Palaia, R.; Delrio, P.; Tatangelo, F.; Botti, G.; Grassi, R.; Izzo, F.; et al. Diagnostic performance of gadoxetic acid-enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol. 2019, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: Theoretical principles and clinical applications. Radiol. Med. 2019, 124, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, E.; Barugola, G.; Nicosia, L.; Mazzola, R.; Ricchetti, F.; Dell’Abate, P.; Alongi, F.; Ruffo, G. A comparative analysis between radiation dose intensification and conventional fractionation in neoadjuvant locally advanced rectal cancer: A monocentric prospective observational study. Radiol. Med. 2020, 125, 990–998. [Google Scholar] [CrossRef]
- Agostini, A.; Floridi, C.; Borgheresi, A.; Badaloni, M.; Esposto Pirani, P.; Terilli, F.; Ottaviani, L.; Giovagnoni, A. Proposal of a low-dose, long-pitch, dual-source chest CT protocol on third-generation dual-source CT using a tin filter for spectral shaping at 100 kVp for CoronaVirus Disease 2019 (COVID-19) patients: A feasibility study. Radiol. Med. 2020, 125, 365–373. [Google Scholar] [CrossRef]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. Radiol. Med. 2020, 125, 384–397. [Google Scholar] [CrossRef]
- Bottari, A.; Silipigni, S.; Carerj, M.L.; Cattafi, A.; Maimone, S.; Marino, M.A.; Mazziotti, S.; Pitrone, A.; Squadrito, G.; Ascenti, G. Dual-source dual-energy CT in the evaluation of hepatic fractional extracellular space in cirrhosis. Radiol. Med. 2020, 125, 7–14. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Cereser, L.; Girometti, R.; Da Re, J.; Marchesini, F.; Como, G.; Zuiani, C. Inter-reader agreement of high-resolution computed tomography findings in patients with COVID-19 pneumonia: A multi-reader study. Radiol. Med. 2021, 126, 577–584. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Dell’ Aversana, F.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Costa, M.; Picone, C.; Cozzi, D.; Moroni, C.; La Casella, G.V.; Montanino, A.; Monti, R.; Mazzoni, F.; et al. Preliminary Report on Computed Tomography Radiomics Features as Biomarkers to Immunotherapy Selection in Lung Adenocarcinoma Patients. Cancers 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gregucci, F.; Bonaparte, I.; Vitulano, N.; Surgo, A.; Mazzola, R.; Di Monaco, A.; Carbonara, R.; Alongi, F.; Langialonga, T.; et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2021, 126, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-generation iterative reconstruction on a dual-source, high-pitch, low-dose chest CT protocol with tin filter for spectral shaping at 100 kV: A study on a small series of COVID-19 patients. Radiol. Med. 2021, 126, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’ Aversana, F.; Ottaiano, A.; Avallone, A.; Nasti, G.; Grassi, F.; et al. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers 2022, 14, 1110. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB-MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef]
- Compagnone, G.; Padovani, R.; D’Ercole, L.; Orlacchio, A.; Bernardi, G.; D’Avanzo, M.A.; Grande, S.; Palma, A.; Campanella, F.; Rosi, A. Provision of Italian diagnostic reference levels for diagnostic and interventional radiology. Radiol. Med. 2021, 126, 99–105. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef]
- Esposito, A.; Buscarino, V.; Raciti, D.; Casiraghi, E.; Manini, M.; Biondetti, P.; Forzenigo, L. Characterization of liver nodules in patients with chronic liver disease by MRI: Performance of the Liver Imaging Reporting and Data System (LI-RADS v.2018) scale and its comparison with the Likert scale. Radiol. Med. 2020, 125, 15–23. [Google Scholar] [CrossRef]
- Orsatti, G.; Zucchetta, P.; Varotto, A.; Crimì, F.; Weber, M.; Cecchin, D.; Bisogno, G.; Spimpolo, A.; Giraudo, C.; Stramare, R. Volumetric histograms-based analysis of apparent diffusion coefficients and standard uptake values for the assessment of pediatric sarcoma at staging: Preliminary results of a PET/MRI study. Radiol. Med. 2021, 126, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Setola, S.V.; de Lutio di Castelguidone, E.; Piccirillo, M.; Palaia, R.; Grassi, R.; Granata, F.; Izzo, F.; et al. Multidetector computer tomography in the pancreatic adenocarcinoma assessment: An update. Infect Agent Cancer 2016, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Berardo, S.; Sukhovei, L.; Andorno, S.; Carriero, A.; Stecco, A. Quantitative bone marrow magnetic resonance imaging through apparent diffusion coefficient and fat fraction in multiple myeloma patients. Radiol. Med. 2021, 126, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Crimì, F.; Capelli, G.; Spolverato, G.; Bao, Q.R.; Florio, A.; Milite Rossi, S.; Cecchin, D.; Albertoni, L.; Campi, C.; Pucciarelli, S.; et al. MRI T2-weighted sequences-based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). Radiol. Med. 2020, 125, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Sansone, M.; Grassi, R.; Maio, F.; Palaia, R.; Tatangelo, F.; Botti, G.; Grimm, R.; Curley, S.; et al. Magnetic resonance imaging in the assessment of pancreatic cancer with quantitative parameter extraction by means of dynamic contrast-enhanced magnetic resonance imaging, diffusion kurtosis imaging and intravoxel incoherent motion diffusion-weighted imaging. Therap. Adv. Gastroenterol. 2020, 13, 1756284819885052. [Google Scholar] [CrossRef] [PubMed]
- Mungai, F.; Verrone, G.B.; Bonasera, L.; Bicci, E.; Pietragalla, M.; Nardi, C.; Berti, V.; Mazzoni, L.N.; Miele, V. Imaging biomarkers in the diagnosis of salivary gland tumors: The value of lesion/parenchyma ratio of perfusion-MR pharmacokinetic parameters. Radiol. Med. 2021, 126, 1345–1355. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Bordonaro, V.; Ciancarella, P.; Ciliberti, P.; Curione, D.; Napolitano, C.; Santangelo, T.P.; Natali, G.L.; Rollo, M.; Guccione, P.; Pasquini, L.; et al. Dynamic contrast-enhanced magnetic resonance lymphangiography in pediatric patients with central lymphatic system disorders. Radiol. Med. 2021, 126, 737–743. [Google Scholar] [CrossRef]
- Quinn, S.F.; Bodne, D.J.; Clark, R.A.; Karl, R.C.; Nicosia, S.V. Upper abdomen: CT findings following partial hepatectomy. Radiology 1988, 168, 879–880. [Google Scholar] [CrossRef]
- Wigham, A.; Alexander Grant, L. Radiologic assessment of hepatobiliary surgical complications. Semin. Ultrasound CT MR 2013, 34, 18–31. [Google Scholar] [CrossRef]
- Romano, S.; Tortora, G.; Scaglione, M.; Lassandro, F.; Guidi, G.; Grassi, R.; Romano, L. MDCT imaging of post interventional liver: A pictorial essay. Eur. J. Radiol. 2005, 53, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Arrive, L.; Hricak, H.; Goldberg, H.I.; Thoeni, R.F.; Margulis, A.R. MR appearance of the liver after partial hepatectomy. Am. J. Roentgenol. 1989, 152, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Molina-Romero, F.X.; Palma-Zamora, E.; Morales-Soriano, R.; Rodríguez-Pino, J.C.; González-Argente, F.X.; Morón-Canis, J.M. Comparison of anatomical resection and non-anatomical resection in patients with hepatocellular carcinoma: Propensity score matching method. Cir. Cir. 2019, 87, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Alonso Casado, O.; González Moreno, S.; Encinas García, S.; Rojo Sebastián, A.; Olavarría Delgado, A. Marcaje de metástasis hepática antes de quimioterapia neoadyuvante para su posterior localización y resección mediante hepatectomía no anatómica [Hepatic metastasis marking before neoadjuvant chemotherapy for their subsequent location and resection using non-anatomical hepatectomy]. Cir. Esp. 2013, 91, 687–689. (In Spanish) [Google Scholar] [CrossRef]
- Lupo, L.; Gallerani, A.; Aquilino, F.; Di Palma, G.; De Fazio, M.; Guglielmi, A.; Memeo, V. Resezione anatomica epatica con termoablazione a radiofrequenza nel trattamento dei tumori primitivi o secondari del fegato [Anatomical hepatic resection using radiofrequency thermoablation in the treatment of primary or secondary liver tumors]. Tumori 2003, 89 (Suppl. 4), 105–106. (In Italian) [Google Scholar]
- Lee, M.K.T.; Gao, F.; Strasberg, S.M. Perceived complexity of various liver resections: Results of a survey of experts with development of a complexity score and classification. J. Am. Coll. Surg. 2015, 220, 64–69. [Google Scholar] [CrossRef]
- Terminology Committee of the International Hepato-Pancreato-Biliary Association. Terminology of liver anatomy and resections. HPB 2000, 2, 333–339. [Google Scholar]
- Zimmitti, G.; Roses, R.E.; Andreou, A.; Shindoh, J.; Curley, S.A.; Aloia, T.A.; Vauthey, J.N. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: An experience with 2628 consecutive resections. J. Gastrointest. Surg. 2013, 17, 57–64. [Google Scholar] [CrossRef]
- Li, G.Z.; Speicher, P.J.; Lidsky, M.E.; Darrabie, M.D.; Scarborough, J.E.; White, R.R.; Turley, R.S.; Clary, B.M. Hepatic resection for hepatocellular carcinoma: Do contemporary morbidity and mortality rates demand a transition to ablation as first-line treatment? J. Am. Coll. Surg. 2014, 218, 827–834. [Google Scholar] [CrossRef]
- Okuda, K. Hepatocellular carcinoma. J. Hepatol. 2000, 32, 225–237. [Google Scholar] [CrossRef]
- Orcutt, S.T.; Anaya, D.A. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control 2018, 25, 1073274817744621. [Google Scholar] [CrossRef] [PubMed]
- Anger, F.; Klein, I.; Löb, S.; Wiegering, A.; Singh, G.; Sperl, D.; Götze, O.; Geier, A.; Lock, J.F. Preoperative Liver Function Guiding HCC Resection in Normal and Cirrhotic Liver. Visc. Med. 2021, 37, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Choi, J.A.; Choi, J.M.; Cho, E.S.; Kim, J.H.; Chung, J.J.; Yu, J.S. Sclerotic changes of cavernous hemangioma in the cirrhotic liver: Long-term follow-up using dynamic contrast-enhanced computed tomography. Radiol. Med. 2020, 125, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Summers, P.E.; Agostini, A.; Ambrosini, R.; Cianci, R.; Cristel, G.; Calistri, L.; Colagrande, S. Dynamic contrast-enhanced MRI in oncology: How we do it. Radiol. Med. 2020, 125, 1288–1300. [Google Scholar] [CrossRef]
- Minutoli, F.; Pergolizzi, S.; Blandino, A.; Mormina, E.; Amato, E.; Gaeta, M. Effect of granulocyte colony-stimulating factor on bone marrow: Evaluation by intravoxel incoherent motion and dynamic contrast-enhanced magnetic resonance imaging. Radiol. Med. 2020, 125, 280–287. [Google Scholar] [CrossRef]
- Ishizawa, T.; Mise, Y.; Aoki, T.; Hasegawa, K.; Beck, Y.; Sugawara, Y.; Kokudo, N. Surgical technique: New advances for expanding indications and increasing safety in liver resection for HCC: The Eastern perspective. J. Hepatobiliary Pancreat Sci. 2010, 17, 389–393. [Google Scholar] [CrossRef]
- Baker, T.; Tabrizian, P.; Zendejas, I.; Gamblin, T.C.; Kazimi, M.; Boudjema, K.; Geller, D.; Salem, R. Conversion to resection post radioembolization in patients with HCC: Recommendations from a multidisciplinary working group. HPB 2021, S1365-182X(21)01739-1. [Google Scholar] [CrossRef]
- Lee, C.W.; Yu, M.C.; Wang, C.C.; Lee, W.C.; Tsai, H.I.; Kuan, F.C.; Chen, C.W.; Hsieh, Y.C.; Chen, H.Y. Liver resection for hepatocellular carcinoma larger than 10 cm: A multi-institution long-term observational study. World J. Gastrointest. Surg. 2021, 13, 476–492. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ahmed, M.; Adam, A.; Arai, Y.; Arellano, R.; de Baère, T.; Bale, R.; Bellera, C.; Binkert, C.A.; Brace, C.L.; et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology 2021, 301, 533–540. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baère, T.; Gervais, D.A.; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Ahmed, M. Technology Assessment Committee of the Society of Interventional Radiology. Image-guided tumor ablation: Standardization of terminology and reporting criteria--a 10-year update: Supplement to the consensus document. J. Vasc. Interv. Radiol. 2014, 25, 1706–1708. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Piccirillo, M.; Pradella, S.; Giordano, M.; Cappabianca, S.; Brunese, L.; et al. Abbreviated MRI Protocol for the Assessment of Ablated Area in HCC Patients. Int. J. Environ. Res. Public Health 2021, 18, 3598. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, M.; Kroehnert, J.A.; Molineus, F.; Kandioler, D.; Schindl, M.; Riss, P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur. Surg. 2018, 50, 256–261. [Google Scholar] [CrossRef]
- Katayama, H.; Kurokawa, Y.; Nakamura, K.; Ito, H.; Kanemitsu, Y.; Masuda, N.; Tsubosa, Y.; Satoh, T.; Yokomizo, A.; Fukuda, H.; et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg. Today 2016, 46, 668–685. [Google Scholar] [CrossRef]
- Khan, M.T.; Akhtar, T.; Yasin, M.A.; Chaudhary, N.A.; Sadiq, A.; Tameez Ud Din, A. A survey of perioperative complications with Clavien-Dindo classification: A cross-sectional study. J. Pak. Med. Assoc. 2021, 71, 572–574. [Google Scholar]
- Giani, A.; Cipriani, F.; Famularo, S.; Donadon, M.; Bernasconi, D.P.; Ardito, F.; Fazio, F.; Nicolini, D.; Perri, P.; Giuffrida, M.; et al. Performance of Comprehensive Complication Index and Clavien-Dindo Complication Scoring System in Liver Surgery for Hepatocellular Carcinoma. Cancers 2020, 12, 3868. [Google Scholar] [CrossRef]
- Ishii, M.; Mizuguchi, T.; Harada, K.; Ota, S.; Meguro, M.; Ueki, T.; Nishidate, T.; Okita, K.; Hirata, K. Comprehensive review of post-liver resection surgical complications and a new universal classification and grading system. World J. Hepatol. 2014, 6, 745–751. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Cassata, A.; Palaia, R.; Delrio, P.; Grassi, R.; Tatangelo, F.; Grazzini, G.; Izzo, F.; et al. Abbreviated MRI protocol for colorectal liver metastases: How the radiologist could work in pre surgical setting. PLoS ONE 2020, 15, e0241431. [Google Scholar] [CrossRef]
- Albano, D.; Stecco, A.; Micci, G.; Sconfienza, L.M.; Colagrande, S.; Reginelli, A.; Grassi, R.; Carriero, A.; Midiri, M.; Lagalla, R.; et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: An Italian survey. Radiol. Med. 2021, 126, 299–305. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef] [PubMed]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.; Rademacher, G.; Ekkernkamp, A.; Güthoff, C.; Mutze, S. Emergency ultrasound-based algorithms for diagnosing blunt abdominal trauma. Cochrane Database Syst. Rev. 2015, 2015, CD004446. [Google Scholar] [CrossRef]
- Abdolrazaghnejad, A.; Banaie, M.; Safdari, M. Ultrasonography in Emergency Department; a Diagnostic Tool for Better Examination and Decision-Making. Adv. J. Emerg. Med. 2017, 2, e7. [Google Scholar] [CrossRef]
- Amini, R.; Wyman, M.T.; Hernandez, N.C.; Guisto, J.A.; Adhikari, S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J. Ultrasound Med. 2017, 36, 913–921. [Google Scholar] [CrossRef]
- Tyler, P.D.; Carey, J.; Stashko, E.; Levenson, R.B.; Shapiro, N.I.; Rosen, C.L. The Potential Role of Ultrasound in the Work-up of Appendicitis in the Emergency Department. J. Emerg. Med. 2019, 56, 191–196. [Google Scholar] [CrossRef]
- Parag, P.; Hardcastle, T.C. Interpretation of emergency CT scans in polytrauma: Trauma surgeon vs. radiologist. Afr. J. Emerg. Med. 2020, 10, 90–94. [Google Scholar] [CrossRef]
- Poletti, P.A.; Andereggen, E.; Rutschmann, O.; de Perrot, T.; Caviezel, A.; Platon, A. Indications au CT low-dose aux urgences [Indications for low-dose CT in the emergency setting]. Rev. Med. Suisse 2009, 5, 1590–1594. [Google Scholar]
- Willemink, M.J.; Schilham, A.M.; Leiner, T.; Mali, W.P.; de Jong, P.A.; Budde, R.P. Iterative reconstruction does not substantially delay CT imaging in an emergency setting. Insights Imaging 2013, 4, 391–397. [Google Scholar] [CrossRef]
- Ternovoy, S.; Ustyuzhanin, D.; Shariya, M.; Shabanova, M.; Gaman, S.; Serova, N.; Mironov, V.; Merkulova, I.; Rienmueller, A.; Meyer, E.L.; et al. Reliability of coronary computed tomography angiography in acute coronary syndrome in an emergency setting. Heliyon 2021, 7, e06075. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Cannizzaro, E.; Bruno, F.; Schicchi, N.; Fogante, M.; Agostini, A.; De Donato, M.C.; De Cataldo, C.; Giovagnoni, A.; Barile, A.; et al. Coronary artery disease (CAD) extension-derived risk stratification for asymptomatic diabetic patients: Usefulness of low-dose coronary computed tomography angiography (CCTA) in detecting high-risk profile patients. Radiol. Med. 2020, 125, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Z.; Gong, L.; Jiang, S.; Wang, L.; Zhang, H. Classification of lung nodules based on CT images using squeeze-and-excitation network and aggregated residual transformations. Radiol. Med. 2020, 125, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Flammia, F.; Chiti, G.; Trinci, M.; Danti, G.; Cozzi, D.; Grassi, R.; Palumbo, P.; Bruno, F.; Agostini, A.; Fusco, R.; et al. Optimization of CT protocol in polytrauma patients: An update. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Yamamoto, H.; Morita, T. Comparison of a Bayesian estimation algorithm and singular value decomposition algorithms for 80-detector row CT perfusion in patients with acute ischemic stroke. Radiol. Med. 2021, 126, 795–803. [Google Scholar] [CrossRef]
- Rampado, O.; Depaoli, A.; Marchisio, F.; Gatti, M.; Racine, D.; Ruggeri, V.; Ruggirello, I.; Darvizeh, F.; Fonio, P.; Ropolo, R. Effects of different levels of CT iterative reconstruction on low-contrast detectability and radiation dose in patients of different sizes: An anthropomorphic phantom study. Radiol. Med. 2021, 126, 55–62. [Google Scholar] [CrossRef]
- Ruys, A.T.; van Beem, B.E.; Engelbrecht, M.R.; Bipat, S.; Stoker, J.; Van Gulik, T.M. Radiological staging in patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2012, 85, 1255–1262. [Google Scholar] [CrossRef]
- Ichikawa, S.; Isoda, H.; Shimizu, T.; Tamada, D.; Taura, K.; Togashi, K.; Onishi, H.; Motosugi, U. Distinguishing intrahepatic mass-forming biliary carcinomas from hepatocellular carcinoma by computed tomography and magnetic resonance imaging using the Bayesian method: A bi-center study. Eur. Radiol. 2020, 30, 5992–6002. [Google Scholar] [CrossRef]
- Chu, H.; Liu, Z.; Liang, W.; Zhou, Q.; Zhang, Y.; Lei, K.; Tang, M.; Cao, Y.; Chen, S.; Peng, S.; et al. Radiomics using CT images for preoperative prediction of futile resection in intrahepatic cholangiocarcinoma. Eur. Radiol. 2021, 31, 2368–2376. [Google Scholar] [CrossRef]
- Megibow, A.J. Clinical abdominal dual-energy CT: 15 years later. Abdom. Radiol. 2020, 45, 1198–1201. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Esposto Pirani, P.; Di Cesare, E.; Giovagnoni, A. The sub-millisievert era in CTCA: The technical basis of the new radiation dose approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Lay Ergün, E.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kim, J.S.; Kim, K.H.; Moon, H.J.; Kim, S. Clinical significance of radiation dose-volume parameters and functional status on the patient-reported quality of life changes after thoracic radiotherapy for lung cancer: A prospective study. Radiol. Med. 2021, 126, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.P.; Sam, M.; Raubenheimer, M.; Patel, V.; Low, G. Hepatic hemangiomas: The various imaging avatars and its mimickers. Radiol. Med. 2020, 125, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Song, J.; Ma, Z.; Chen, T. Combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging to predict neoadjuvant chemotherapy effect in FIGO stage IB2-IIA2 cervical cancers. Radiol. Med. 2020, 125, 1233–1242. [Google Scholar] [CrossRef]
- Sun, N.N.; Ge, X.L.; Liu, X.S.; Xu, L.L. Histogram analysis of DCE-MRI for chemoradiotherapy response evaluation in locally advanced esophageal squamous cell carcinoma. Radiol. Med. 2020, 125, 165–176. [Google Scholar] [CrossRef]
- Shannon, B.A.; Ahlawat, S.; Morris, C.D.; Levin, A.S.; Fayad, L.M. Do contrast-enhanced and advanced MRI sequences improve diagnostic accuracy for indeterminate lipomatous tumors? Radiol. Med. 2022, 127, 90–99. [Google Scholar] [CrossRef]
- Lee, J.; Joo, I.; Lee, D.H.; Jeon, S.K.; Lee, J.M. Clinical outcomes of patients with a high alpha-fetoprotein level but without evident recurrence on CT or MRI in surveillance after curative-intent treatment for hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 597–606. [Google Scholar] [CrossRef]
- Görgec, B.; Hansen, I.; Kemmerich, G.; Syversveen, T.; Abu Hilal, M.; Belt, E.J.T.; Bisschops, R.H.C.; Bollen, T.L.; Bosscha, K.; Burgmans, M.C.; et al. Clinical added value of MRI to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): Study protocol for an international multicentre prospective diagnostic accuracy study. BMC Cancer 2021, 21, 1116. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Kuang, S.; Zhang, Y.; Xie, S.; He, B.; Deng, Y.; Yang, H.; Shan, Q.; Wu, J.; et al. Liver Imaging Reporting and Data System Category 5: MRI Predictors of Microvascular Invasion and Recurrence After Hepatectomy for Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2019, 213, 821–830, Erratum in AJR Am. J. Roentgenol. 2019, 213, 958. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Filice, F.; Leongito, M.; Palaia, R.; Izzo, F.; Petrillo, A. Major and ancillary magnetic resonance features of LI-RADS to assess HCC: An overview and update. Infect. Agent Cancer 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Picone, C.; Vallone, P.; Belli, A.; Incollingo, P.; Albino, V.; Tatangelo, F.; Izzo, F.; et al. Microvascular invasion and grading in hepatocellular carcinoma: Correlation with major and ancillary features according to LIRADS. Abdom. Radiol. 2019, 44, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.H.; Benjaminov, O.; Belinki, A.; Geler, A.; Braun, M.; Knizhnik, M.; Aizner, S.; Shaharabani, E.; Sulkes, J.; Shabtai, E.; et al. Magnetic resonance cholangiopancreatography for the accurate diagnosis of biliary complications after liver transplantation: Comparison with endoscopic retrograde cholangiography and percutaneous transhepatic cholangiography-long-term follow-up. Clin. Transplant. 2010, 24, E163–E169. [Google Scholar] [CrossRef]
- Letourneau, J.G.; Steely, J.W.; Crass, J.R.; Goldberg, M.E.; Grage, T.; Day, D.L. Upper abdomen: CT findings following partial hepatectomy. Radiology 1988, 166 Pt 1, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Patrone, R.; Granata, V.; Belli, A.; Palaia, R.; Albino, V.; Piccirillo, M.; Fusco, R.; Tatangelo, F.; Nasti, G.; Avallone, A.; et al. The safety and efficacy of Glubran 2 as biliostatic agent in liver resection. Infect. Agent Cancer 2021, 16, 19. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Cannataci, C.; Cimo’, B.; Mamone, G.; Tuzzolino, F.; D’Amico, M.; Cortis, K.; Maruzzelli, L.; Miraglia, R. Portal vein puncture-related complications during transjugular intrahepatic portosystemic shunt creation: Colapinto needle set vs. Rösch-Uchida needle set. Radiol. Med. 2021, 126, 1487–1495. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Boullosa Seoane, E.; Cannavale, A.; de Haan, M.W.; Dezman, R.; Kloeckner, R.; O’Sullivan, G.; Ryan, A.; Tsoumakidou, G. CIRSE Clinical Practice Manual. Cardiovasc. Intervent. Radiol. 2021, 44, 1323–1353, Erratum in Cardiovasc. Intervent. Radiol. 2021, 44, 1323–1353. [Google Scholar] [CrossRef]
- Miele, V.; Di Giampietro, I. Diagnostic Imaging in Emergency. Salut. E Soc. 2014, 127–138. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Buffa, V.; Fedeli, S.; Luzietti, M.; Vallone, A.; Ruopoli, R.; Miele, V.; Rengo, M.; Maurizi Enrici, M.; Fina, P.; et al. Preliminary experience with abdominal dual-energy CT (DECT): True versus virtual nonenhanced images of the liver. Radiol. Med. 2010, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, V.; Trinci, M.; van der Byl, G.; Catania, V.D.; Calisti, A.; Miele, V. Ultrasound in newborns and children suffering from non-traumatic acute abdominal pain: Imaging with clinical and surgical correlation. J. Ultrasound 2014, 18, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Palaia, R.; Albino, V.; Amore, A.; di Giacomo, R.; Piccirillo, M.; Leongito, M.; Nasto, A.; Granata, V.; Petrillo, A.; et al. Hepatocellular carcinoma and liver metastases: Clinical data on a new dual-lumen catheter kit for surgical sealant infusion to prevent perihepatic bleeding and dissemination of cancer cells following biopsy and loco-regional treatments. Infect. Agent Cancer 2015, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Maddern, G.; Koch, M.; Hugh, T.J.; Fan, S.T.; Nimura, Y.; Figueras, J.; Vauthey, J.N.; et al. Post-hepatectomy haemorrhage: A definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB 2011, 13, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.; Menias, C.; Rucker, C.; Bhalla, S.; Peterson, C.M.; Wang, L.; Gratz, B. Blood in the belly: CT findings of hemoperitoneum. Radiographics 2007, 27, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Bashir, M.; Robbani, I.; Rasool, S.R.; Shera, F.A.; Hamid, I. Sentinel clot sign in hemoperitoneum. Abdom. Radiol. 2019, 44, 1955–1956. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Mirvis, S.E.; Reaney, S.M. Pictorial review: CT appearances of contrast medium extravasations associated with injury sustained from blunt abdominal trauma. Clin. Radiol. 1995, 50, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Kim, K.W.; Lee, J.; Kwon, H.J.; Kwon, J.H.; Song, G.W.; Lee, S.G. The role of multiphase CT in patients with acute postoperative bleeding after liver transplantation. Abdom. Radiol. 2020, 45, 141–152. [Google Scholar] [CrossRef]
- Di Domenico, S.; Rossini, A.; Petrocelli, F.; Valente, U.; Ferro, C. Recurrent acute Budd-Chiari syndrome after right hepatectomy: US color-Doppler vascular pattern and left hepatic vein stenting for treatment. Abdom. Imaging 2013, 38, 320–323. [Google Scholar] [CrossRef]
- Yoshiya, S.; Shirabe, K.; Nakagawara, H.; Soejima, Y.; Yoshizumi, T.; Ikegami, T.; Yamashita, Y.; Harimoto, N.; Nishie, A.; Yamanaka, T.; et al. Portal vein thrombosis after hepatectomy. World J. Surg. 2014, 38, 1491–1497. [Google Scholar] [CrossRef]
- Cohen, J.; Edelman, R.R.; Chopra, S. Portal vein thrombosis: A review. Am. J. Med. 1992, 92, 173–182. [Google Scholar] [CrossRef]
- Witte, C.L.; Brewer, M.L.; Witte, M.H.; Pond, G.B. Protean manifestations of pylethrombosis. A review of thirty-four patients. Ann. Surg. 1985, 202, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sheen, C.L.; Lamparelli, H.; Milne, A.; Green, I.; Ramage, J.K. Clinical features, diagnosis and outcome of acute portal vein thrombosis. QJM 2000, 93, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, M.; Miyamoto, S.; Nagamatsu, S.; Kayano, S.; Taji, M.; Kinoshita, T.; Kosuge, T.; Kimata, Y. Hepatic artery reconstruction following ablative surgery for hepatobiliary and pancreatic malignancies. Eur. J. Surg. Oncol. 2012, 38, 580–585. [Google Scholar] [CrossRef]
- Silva, M.A.; Jambulingam, P.S.; Gunson, B.K.; Mayer, D.; Buckels, J.A.; Mirza, D.F.; Bramhall, S.R. Hepatic artery thrombosis following orthotopic liver transplantation: A 10-year experience from a single centre in the United Kingdom. Liver Transpl. 2006, 12, 146–151. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Gunson, B.K.; Mirza, D.F.; Mayer, D.A.; Buckels, J.A.; McMaster, P.; Neuberger, J.M. Delayed hepatic artery thrombosis in adult orthotopic liver transplantation—A 12-year experience. Transplantation 2001, 71, 1592–1596. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Abu-Yousef, M.M. Doppler US of the liver made simple. Radiographics 2011, 31, 161–188, Erratum in Radiographics 2011, 31, 904. [Google Scholar] [CrossRef]
- Ralls, P.W.; Johnson, M.B.; Radin, D.R.; Boswell, W.D., Jr.; Lee, K.P.; Halls, J.M. Budd-Chiari syndrome: Detection with color Doppler sonography. AJR Am. J. Roentgenol. 1992, 159, 113–116. [Google Scholar] [CrossRef][Green Version]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Boonstra, E.A.; de Boer, M.T.; Sieders, E.; Peeters, P.M.; de Jong, K.P.; Slooff, M.J.; Porte, R.J. Risk factors for central bile duct injury complicating partial liver resection. Br. J. Surg. 2012, 99, 256–262. [Google Scholar] [CrossRef]
- Hoeffel, C.; Azizi, L.; Lewin, M.; Laurent, V.; Aubé, C.; Arrivé, L.; Tubiana, J.M. Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. Radiographics 2006, 26, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Hamatsu, T.; Rikimaru, T.; Tanaka, S.; Shirabe, K.; Shimada, M.; Sugimachi, K. Bile leakage after hepatic resection. Ann. Surg. 2001, 233, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Togo, S.; Tanaka, K.; Masui, H.; Endo, I.; Sekido, H.; Nagahori, K.; Shimada, H. Risk factors and management of bile leakage after hepatic resection. World J. Surg. 2003, 27, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, K.W.; Yu, J.S.; Kim, M.J.; Kim, K.W.; Lim, J.S.; Cho, E.S.; Yoon, D.S.; Kim, T.K.; Lee, S.I.; et al. Early biliary complications of laparoscopic cholecystectomy: Evaluation on T2-weighted MR cholangiography in conjunction with mangafodipir trisodium-enhanced 3D T1-weighted MR cholangiography. AJR Am. J. Roentgenol. 2004, 183, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Alegre Castellanos, A.; Molina Granados, J.F.; Escribano Fernandez, J.; Gallardo Muñoz, I.; Triviño Tarradas Fde, A. Early phase detection of bile leak after hepatobiliary surgery: Value of Gd-EOB-DTPA-enhanced MR cholangiography. Abdom. Imaging 2012, 37, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Melamud, K.; LeBedis, C.A.; Anderson, S.W.; Soto, J.A. Biliary imaging: Multimodality approach to imaging of biliary injuries and their complications. Radiographics 2014, 34, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Saad, N.E.; Quazi, R.R.; Darcy, M.D.; Picus, D.D.; Menias, C.O. Management of iatrogenic bile duct injuries: Role of the interventional radiologist. Radiographics 2013, 33, 117–134. [Google Scholar] [CrossRef]
- Kaufman, J.A.; Lee, M.J. Vascular and Interventional Radiology; Elsevier Health Sciences: Philadelphia, PA, USA, 2013. [Google Scholar]
- Mulé, S.; Colosio, A.; Cazejust, J.; Kianmanesh, R.; Soyer, P.; Hoeffel, C. Imaging of the postoperative liver: Review of normal appearances and common complications. Abdom. Imaging 2015, 40, 2761–2776. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, S.H.; Lee, M.W.; Lee, J.Y. Small (≤2 cm) hepatocellular carcinoma in patients with chronic liver disease: Comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphasic 64-multirow detector CT. Br. J. Radiol. 2012, 85, e314–e322. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Castelguidone, E.L.D.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: The radiologist’s challenge. Radiol. Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol. Med. 2021, 126, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G.; et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. J. Clin. Med. 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics 2022, 12, 890. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Belli, A.; Borzillo, V.; Palumbo, P.; Bruno, F.; Grassi, R.; Ottaiano, A.; Nasti, G.; Pilone, V.; et al. Conventional, functional and radiomics assessment for intrahepatic cholangiocarcinoma. Infect. Agent Cancer 2022, 17, 13. [Google Scholar] [CrossRef]
- Patrone, R.; Izzo, F.; Palaia, R.; Granata, V.; Nasti, G.; Ottaiano, A.; Pasta, G.; Belli, A. Minimally invasive surgical treatment of intrahepatic cholangiocarcinoma: A systematic review. World J. Gastrointest Oncol. 2021, 13, 2203–2215. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, M.J.; Choi, J.Y.; Park, M.S.; Lim, J.S.; Chung, Y.E.; Kim, K.W. Detection of recurrent hepatocellular carcinoma on post-operative surveillance: Comparison of MDCT and gadoxetic acid-enhanced MRI. Abdom. Imaging 2014, 39, 291–299. [Google Scholar] [CrossRef]
- Sasaki, K.; Matsuda, M.; Ohkura, Y.; Kawamura, Y.; Inoue, M.; Hashimoto, M.; Ikeda, K.; Kumada, H.; Watanabe, G. In hepatocellular carcinomas, any proportion of poorly differentiated components is associated with poor prognosis after hepatectomy. World J. Surg. 2014, 38, 1147–1153. [Google Scholar] [CrossRef]
- Hyder, O.; Hatzaras, I.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013, 153, 811–818. [Google Scholar] [CrossRef]
- Jadvar, H.; Henderson, R.W.; Conti, P.S. [F-18]fluorodeoxyglucose positron emission tomography and positron emission tomography: Computed tomography in recurrent and metastatic cholangiocarcinoma. J. Comput. Assist. Tomogr. 2007, 31, 223–228. [Google Scholar] [CrossRef]
- de Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [PubMed]
- DeMatteo, R.P.; Palese, C.; Jarnagin, W.R.; Sun, R.L.; Blumgart, L.H.; Fong, Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J. Gastrointest Surg. 2000, 4, 178–184. [Google Scholar] [CrossRef]
- Ward, J.; Sheridan, M.B.; Guthrie, J.A.; Davies, M.H.; Millson, C.E.; Lodge, J.P.; Pollard, S.G.; Prasad, K.R.; Toogood, G.J.; Robinson, P.J. Bile duct strictures after hepatobiliary surgery: Assessment with MR cholangiography. Radiology 2004, 231, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.D. What is the upper limit of normal for the common bile duct on ultrasound: How much do you want it to be? Am. J. Gastroenterol. 2000, 95, 897–900. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Filice, S.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Petrillo, A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect. Agent Cancer 2018, 13, 23. [Google Scholar] [CrossRef]
- Granata, V.; Petrillo, M.; Fusco, R.; Setola, S.V.; de Lutio di Castelguidone, E.; Catalano, O.; Piccirillo, M.; Albino, V.; Izzo, F.; Petrillo, A. Surveillance of HCC Patients after Liver RFA: Role of MRI with Hepatospecific Contrast versus Three-Phase CT Scan-Experience of High Volume Oncologic Institute. Gastroenterol. Res. Pract. 2013, 2013, 469097. [Google Scholar] [CrossRef]
- Rega, D.; Pace, U.; Scala, D.; Chiodini, P.; Granata, V.; Fares Bucci, A.; Pecori, B.; Delrio, P. Treatment of splenic flexure colon cancer: A comparison of three different surgical procedures: Experience of a high volume cancer center. Sci. Rep. 2019, 9, 10953. [Google Scholar] [CrossRef]
- Izzo, F.; Piccirillo, M.; Albino, V.; Palaia, R.; Belli, A.; Granata, V.; Setola, S.; Fusco, R.; Petrillo, A.; Orlando, R.; et al. Prospective screening increases the detection of potentially curable hepatocellular carcinoma: Results in 8900 high-risk patients. HPB 2013, 15, 985–990. [Google Scholar] [CrossRef]
- Barretta, M.L.; Catalano, O.; Setola, S.V.; Granata, V.; Marone, U.; D’Errico Gallipoli, A. Gallbladder metastasis: Spectrum of imaging findings. Abdom. Imaging 2011, 36, 729–734. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutolo, C.; De Muzio, F.; Fusco, R.; Simonetti, I.; Belli, A.; Patrone, R.; Grassi, F.; Dell’Aversana, F.; Pilone, V.; Petrillo, A.; et al. Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging. Diagnostics 2022, 12, 1323. https://doi.org/10.3390/diagnostics12061323
Cutolo C, De Muzio F, Fusco R, Simonetti I, Belli A, Patrone R, Grassi F, Dell’Aversana F, Pilone V, Petrillo A, et al. Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging. Diagnostics. 2022; 12(6):1323. https://doi.org/10.3390/diagnostics12061323
Chicago/Turabian StyleCutolo, Carmen, Federica De Muzio, Roberta Fusco, Igino Simonetti, Andrea Belli, Renato Patrone, Francesca Grassi, Federica Dell’Aversana, Vincenzo Pilone, Antonella Petrillo, and et al. 2022. "Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging" Diagnostics 12, no. 6: 1323. https://doi.org/10.3390/diagnostics12061323
APA StyleCutolo, C., De Muzio, F., Fusco, R., Simonetti, I., Belli, A., Patrone, R., Grassi, F., Dell’Aversana, F., Pilone, V., Petrillo, A., Izzo, F., & Granata, V. (2022). Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging. Diagnostics, 12(6), 1323. https://doi.org/10.3390/diagnostics12061323






