Abstract
The objective of this review was to summarize published radiomics studies dealing with infradiaphragmatic cancers, blood malignancies, melanoma, and musculoskeletal cancers, and assess their quality. PubMed database was searched from January 1990 to February 2022 for articles performing radiomics on PET imaging of at least 1 specified tumor type. Exclusion criteria includd: non-oncological studies; supradiaphragmatic tumors; reviews, comments, cases reports; phantom or animal studies; technical articles without a clinically oriented question; studies including <30 patients in the training cohort. The review database contained PMID, first author, year of publication, cancer type, number of patients, study design, independent validation cohort and objective. This database was completed twice by the same person; discrepant results were resolved by a third reading of the articles. A total of 162 studies met inclusion criteria; 61 (37.7%) studies included >100 patients, 13 (8.0%) were prospective and 61 (37.7%) used an independent validation set. The most represented cancers were esophagus, lymphoma, and cervical cancer (n = 24, n = 24 and n = 19 articles, respectively). Most studies focused on 18F-FDG, and prognostic and response to treatment objectives. Although radiomics and artificial intelligence are technically challenging, new contributions and guidelines help improving research quality over the years and pave the way toward personalized medicine.
1. Introduction
In the recent years, radiomics has represented one of the major axes of development in medical imaging research. Similarly to all its sister disciplines (for example, genomics, proteomics, and metabolomics), this field seeks to optimize the process of discovering new disease biomarkers through a quantitative approach to medical imaging and offers an instrument to potentially build a new combination of parameters to guide patient-tailored treatment. Radiomics relies on the mathematical extraction of the spatial distribution of signal intensities and pixel interrelationships that are translated in a large number of quantitative features, the most statistically relevant parameters being then selected to deduce the purpose of the study. Thus, disease-specific textural information that are hidden to the human eye become accessible thanks to mathematical extraction. Traditional statistical approaches may have difficulties in handling such big amount of data. On the other hand, Artificial Intelligence (AI), with its ability to identify patterns within the massive dataset, has been proven very useful for this task [1].
However, though AI and radiomics are high potential-carrying techniques, they rely on rigorous processing chains and good quality training bases [2]. Yet, the quality of radiomics publications is often questioned [3], both in terms of number of patients included and lack of dedicated validation cohorts. Moreover, missing information in those studies often undermine the possibility for other researchers to replicate, and therefore externally validate, radiomics-based protocols, thus delaying the application of radiomic models in clinical practice.
As the number of articles on radiomics in oncological Positron Emission Tomography (PET) imaging exponentially increases, we here provide a systematic review, with a particular focus the quality of radiomics studies conducted on several malignancies: infradiaphragmatic cancers including gastrointestinal and genitourinary tumors; blood cancers; musculo-skeletal and skin (MSS) neoplasia.
2. Materials and Methods
This systematic review of published literature was performed according to the reporting standards of the PRISMA-P statement [4]. It was not registered.
2.1. Search Strategy, Inclusion and Exclusion Criteria
We performed a literature search in the PubMed database to identify all eligible articles using the following formula:
(“PET” OR “positron”) AND (“radiomics” OR “radiomic” OR “texture” OR “textural”)
Results were admitted from 1 January 1990, up to and including 18 February 2022. Reviews were automatically identified using the article type options and removed from the extracted database.
Inclusion criteria were: (1) studies based on human data, (2) studies specifying at least one non-supradiaphragmatic tumor type, (3) studies performing radiomics on PET imaging. Exclusion criteria were: (1) studies not related to medical topics, (2) reviews, posters, editorials, comments, cases reports, (3) duplicates, (4) studies outside the oncological field or radiomics not performed on PET, (5) studies only based on phantom or animal data, (6) technical articles (optimization, robustness), without a clinically-oriented question, (7) studies including less than 30 patients in the training cohort (for studies including multiple types of cancers, each cancer type was considered separately), (8) strictly supradiaphragmatic cancers (for example, esophagus was included in this study) (9) studies not written in English, (10) full text not available (Table 1).

Table 1.
Inclusion and exclusion criteria (PICOS systematization).
2.2. Quality Assessment
Studies were assessed for quality based on 3 items:
- The number of patients, estimating the risk of bias and overfitting: less than 50 patients (score 0), 50 to 100 patients (score 1), more than 100 patients (score 2);
- The retrospective (score 0) or prospective (score 2) nature of the collection of data;
- The use of a completely independent cohort for validation: no (score 0), partition of the cohort between training and test set, excluding k-folding (score 1), external validation cohort (score 2).
A simple quality score (QS), consisting in the sum of the 3 previously stated items, was calculated. A maximum possible score of 6 meant high quality study design of the article. Mean and 95% confidence intervals (CI) of the quality scores were calculated for all of the database articles divided by year of publication.
2.3. Data Collection and Review
An Excel review database was generated. The following parameters were extracted from each article:
- PMID, first author, year of publication;
- Organ/type of cancer;
- Quality data: number of patients, retrospective or prospective nature, validation, quality score;
- Objective of the study.
The database was completely filled in twice by the same author, with a one-week interval between the two. Any discrepancies were corrected by a third reading.
3. Results
3.1. Discrepancies between the Two Reading Sessions
Six discrepancies between the 2 reading sessions of the database were encountered and led to a third reading: 1 was misclassified regarding cancer subtype, 5 discrepancies concerned patient number or validation cohort presence.
3.2. Searching Results
A total of 1180 studies were identified in the PubMed database, 239 of which were reviews and therefore automatically excluded. Of the remaining 941 studies, 537 were excluded as 111 of them were off topic, 57 articles corresponded to undetected reviews or editorials, 7 were duplicates, 176 did not deal with oncological or PET-based radiomics, 27 articles were not human-based, 89 were technical articles, and 70 studies included less than 30 patients in the training cohort. A total of 404 articles were sought for retrieval: 5 were not written in English, 17 had no full text available and 220 studies dealt with supradiaphragmatic malignancies and were therefore excluded. Finally, 162 studies were included in the review (Figure 1). Study characteristics table is available in a separate file Table S1.
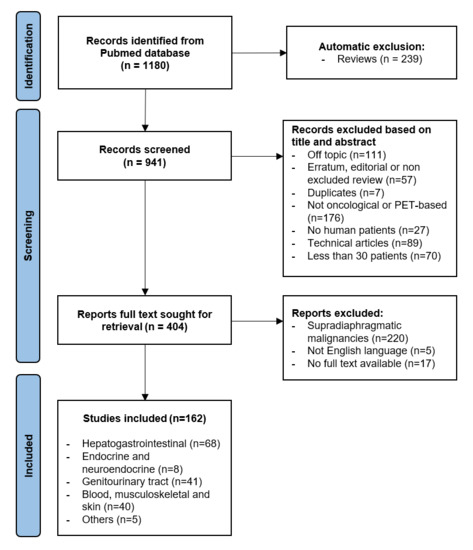
Figure 1.
Flowchart of literature search and article selection.
3.3. Quality Assessments
Mean quality score of the articles was 1.78/6, with a tendency towards constant improvement over the years (Table 1). A total of 61 (37.7%) studies included more than 100 patients each, 13 studies (8.0%) were prospectively based on acquired data, 61 (37.7%) articles described an independent validation set. The number of publications was found to be increasing each year (Table 2).

Table 2.
Mean quality scores and number of publications per year on PET(/CT) radiomics.
3.4. Gastroenteric Tract Cancers (Neuroendocrine Tumors Excluded)
3.4.1. Esophageal and Gastric Cancers
Twenty-four studies on esophageal cancer were included in this review [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]; 1 used 18F-FDG and 18F-FLT, reporting no significant results regarding 18F-FLT [10]. The remaining 23 studies employed only 18F-FDG. The average number of patients included was 114.5 (range 30–449), with 6/24 (25.0%) studies including more than 100 patients. Moreover, 8 studies (33.3%) used an independent validation dataset and 2/24 (16.7%) were prospectively designed. Prognosis and treatment response prediction were the main investigated subjects, gathering 21/24 (87.5%) studies.
An externally validated study, conducted by Zhang et al. [19] on 190 patients, aimed at predicting lymph node metastases using pre-treatment PET radiomics of the primary tumor, achieving an AUC of 0.69 on the validation cohort. The question of overall survival prediction was raised by Foley et al. [13], however, the prognostic model developed on his cohort of 449 patients (training n = 302, internal validation n = 101, external validation n = 46) failed to be transposable to the validation groups, even after PET harmonization. Some data were oriented towards the ability of PET radiomics to predict treatment response to concurrent chemo-radiotherapy, such as in the study by Cao et al. [12], that included 159 patients with thoracic esophagus squamous cell carcinoma (AUC of 0.835 on the validation dataset).
A total of 7 studies on gastric cancer were found [29,30,31,32,33,34,35], all using 18F-FDG as radiopharmaceutical. The average number of patients included was 163.7 (range 79–214), with 5/7 (71.4%) studies including more than 100 patients, 5/7 (71.4%) using a separate validation dataset and 1/7 (14.3%) using prospective data.
Furthermore, 4 studies were conducted for diagnostic purposes: 2 for nodal involvement prediction (AUC between 0.74 and 0.81) [29,35], 1 for peritoneal involvement prediction (AUC 0.88 in the validation cohorts) [30] and 1 to differentiate between gastric cancer and primary gastric lymphoma (AUC 0.77) [31]. The remaining three were prognosis-oriented [32,33,34].
3.4.2. Colorectal and anal Cancers
A total of 19 studies on colorectal cancer were included [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], all using 18F-FDG except for one [37], that employed both 18F-FLT and 18F-FDG without any reported added value on prognosis prediction. On average, 118.7 (ranging from 37 to 381) patients were included, with 7/19 (36.8%) studies including more than 100 patients, 1 using prospectively acquired data and 5/19 using a validation cohort (26.3%). Most of these studies dealt with prognosis and treatment response prediction (15/19–78.9%). The largest one, by Kang et al. [51] (training set n = 228; validation set n = 153) developed a prognosis nomogram: the radiomics signature was significantly associated with progression free survival both in training and validation sets (p < 0.001).
Only 1 study was conducted on patients with anal cancer (n = 189) and found that the inclusion of PET textural parameters might provide superior prediction of PFS than existing methods designed without it [55].
3.4.3. Pancreatic Cancers
Pancreatic cancer was featured in 13 studies based on 18F-FDG [56,57,58,59,60,61,62,63,64,65,66,67,68]. An average of 110.7 patients was included (range: 48–198, 8 studies with more than 100 patients) with 1 (7.7%) prospective study and 4 (30.8%) using a validation cohort. A total of 8 studies (61.5%) focused on prognosis, while the remaining 5 dealt with diagnostic issues and histological characterization. Promising results were found in terms of grade of tumoral differentiation prediction [62] with a model based on a twelve-feature-combined radiomics signature that could stratify pancreatic ductal adenocarcinoma patients into grade G1 and grade G2/3 groups, with an AUC of 0.994 in the training set and 0.921 in the validation set.
3.4.4. Liver Cancers
Moreover, 4 retrospective studies were identified for liver cancer [69,70,71,72] with an average of 65.5 included patients (range 47–99). Among them, 1 used a separate validation cohort [69]. Two studies focused on the response prediction of 90Y-transarterial radioembolization treatment, one using 18F-FDG [70], the other using post therapy 90Y PET [72]. One study aimed at differentiating between hepatic lymphoma and hepatocellular carcinoma (AUC 0.87 on the training set, no validation cohort) [71]. The last 1 [69] used radiomics for microvascular invasion and prognosis prediction in early-stage hepatocellular carcinoma (AUC 0.69 on the validation cohort).
3.5. Genitourinary Tract Cancers
3.5.1. Cervical and Endometrial Cancers
A total of 22 publications on cervical cancer were retrieved; 19 of them exclusively dealt with cervical cancer [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91], while the remaining 3 described multiple types of cancers, including cervical cancer [92,93,94]. All of the studies were retrospective and employed 18F-FDG. The average number of patients included in the 19 studies on cervical cancer was 105.2 (range 42–190), with 9/19 (47%) studies including more than 100 patients; 10/18 (55.6%) used dedicated validation cohorts (the remaining 1 being a validation study). Most of these studies were aimed at investigating the prognosis and disease-free survival of patients with cervical cancer. In a PET/MRI radiomics study including 102 patients with locally advanced cervical cancer (69 for the training set and 33 for the testing set), Lucia et al. [84] showed that radiomics features such as Grey Level Non-Uniformity in PET were independent prognostic factors for the outcome of patients treated with chemoradiotherapy. These findings were then successfully validated in another study using French and Canadian cohorts [77], though higher accuracy of the model was found dependent from harmonization of the radiomic features deriving from the three centers involved. In another work including 170 patients with FIGO stage IB-IVA cervical cancer, Shen et al. [76] noted that radiomics could predict pelvic or para-aortic lymph node metastases and histology.
A total of 5 studies on endometrial cancer were identified [95,96,97,98,99], all using 18F-FDG, with an average number of 121.0 (range 53–170) patients. Moreover 4 out of 5 studies (80.0%) included more than 100 patients and were validated on an independent set. No prospective studies were found. Two studies successfully used image parameters derived from the primary tumor to increase nodal staging accuracy [98,99]. Wang et al. [95] tried to use radiomics to differentiate endometrial precancerous lesions and early-stage carcinoma, however, only SUV values had high predictive diagnostic value. Finally, two articles found radiomics patterns that may orient toward underlying Lynch syndrome [96] or refine prognosis [97].
3.5.2. Vulvar and Ovarian Cancers
Only preliminary studies were available in these cases, focusing on prognosis. Only one study reported applying radiomics to vulvar cancer [100]. It had a retrospective design, 40 patients included (which is not exactly a low number, vulvar cancer being part of the rare tumors family), and no validation cohort. Although the identified radiomics features did not correlate strongly with tumor biology, Moran’s I was found to predict patients’ prognosis. The only article found on advanced high-grade serous ovarian cancer [101] was retrospectively designed, it included 261 patients, and it had a separate validation set. Results from this study reported a higher prognostic performance of the investigated model combining clinical data with 18F-FDG PET radiomic features compared to other models of clinical variables alone.
3.5.3. Prostate Cancer
We included 11 studies of prostate cancer radiomics [102,103,104,105,106,107,108,109,110,111,112], 8 employing 68Ga-PSMA, 2 Choline (1 18F-Fluoroethilcholine and 1 11C-Choline) and 1 using 18F-DCFPyl. An average of 71.3 patients was included (range 41–101); 2 of the studies were prospective and 4/11 studies used a validation cohort. Five studies were conducted for prognosis and treatment response prediction purposes. Interestingly, the explorative study conducted by Mazemi et al. [102] on 83 patients used a machine-learning approach on 68Ga-PSMA PET/CT to predict 177Lu-PSMA response and obtained an AUC of 80%.
3.5.4. Renal Cancer
Only 1 study [113] was available on renal cancer PET radiomics and it used 18F-FDG texture analysis to predict the pathological Fuhrman nuclear grade of clear cell renal cell carcinoma. In the prospective validation cohort, the PET/CT texture parameter model had a good predictive ability, with an AUC of 0.792.
3.6. Neuroendocrine and Adrenal Tumors
Seven studies were found to be conducted on neuroendocrine tumors [114,115,116,117,118,119,120], half of which on pancreatic neuroendocrine tumors. The radiotracers used were 68Ga-DOTA-peptides (6/7) and 18F-FDG (1/7). Four studies aimed at predicting prognosis and four were conducted for diagnostic purposes, particularly for Ki67 prediction. Bevilacqua et al. [114] developed a model to predict grade 1 and grade 2 pancreatic neuroendocrine tumors, obtaining an AUC > 0.8.
A study [121] retrospectively performed on 49 patients with pheochromocytoma used PET textural features combined with MTV to better differentiate between sporadic and mutated tumors, and found 18F-FDG PET/CT to provide evidences for a genetic predisposition when combined with radiomics biomarkers.
3.7. Blood Malignancies
A total of 24 articles on lymphomas were included in this review [122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145], 13 of which studying diffuse large B-cell lymphoma (including 2 studies on gastro-intestinal lymphoma), 3 on follicular lymphoma, 3 on Hodgkin’s lymphoma, 2 on mantle cell lymphoma and 3 on other sub-types of lymphoma. 18F-FDG was the only tracer employed and all studies built radiomic models on baseline, pre-treatment PET images, often including clinical parameters and international prognostic indices. The average number of patients included was 124.7 (range 30–383), with 11/24 (45.8%) studies including more than 100 patients, 12/24 (50.0%) using a validation cohort and 3/24 (12.5%) using prospective data. Main objectives of the studies included prognosis and treatment response prediction (19/24, 79.2%) and bone marrow involvement prediction (3/24, 12.5%), with encouraging results. Among the most interesting findings, a prospective and validated study conducted by Ceriani et al. [134] on 133 patients with diffuse large B-cell lymphoma derived a radiomics score to predict progression free survival (AUC 0.706 on test data) and overall survival (AUC 0.703 on test data).
Finally, two studies used radiomics to predict bone marrow involvement in patients with suspected relapsed acute leukemia [146] and progression to symptomatic multiple myeloma [147].
3.8. Musculo-Skeletal and Skin Cancers
A total of 12 studies focused 18F-FDG PET radiomics on sarcomas [148,149,150,151,152,153,154,155,156,157,158,159], including 6 on osteosarcoma. On average, 72.4 patients were included (range 35–197), the higher numbers corresponding to studies including sarcomas regardless of their subtypes. Only 1 study had prospectively acquired data and 3/12 (25.0%) used validation data. Seven aimed at predicting prognosis or treatment response, 3/12 used radiomics to predict distant metastases, 2/12 were conducted for differential diagnosis purposes.
Two studies involved patients with melanomas, one of which used radiomics to differentiate pseudo progression from progression under immune checkpoint inhibition (AUC 0.82–no validation set) [160] and the other to predict BRAFV600 mutation, however, unsuccessfully [161].
3.9. Others
The remaining five studies included 1 study with unknown primary tumor [162], 1 study on malignant peripheral nerve sheath tumors [163] and 3 studies on liver and spinal metastases [164,165,166], with 3/5 (60.0%) prognostic studies and 2/5 (40.0%) diagnostic studies.
4. Discussion
4.1. Quality Assessment
In this work, we extracted 162 publications related to radiomics. Our composite score for the evaluation of the quality of the publications was low, estimated at 1.78/6 on average, in good agreement with previous work reporting low quality of radiomics publications [3].
Radiomics is dependent on the size of the reconstructed voxels and images post-filtering [167]. The retrospective nature of most of the available studies (>90%) and the lack of conservation of raw data prevent the performance of a standardized dedicated reconstruction protocol for radiomic purposes [2,168] and may limit the external validity of the proposed models. However, some solutions such as the ComBat harmonization method are starting to be used, with positive results [169].
The second most common obstacle to the achievement of higher quality in radiomic articles is the overfitting phenomenon. Overfitting is encountered when training is performed on too homogeneous population sets (for example, learning performed on a monocentric database with a single imaging system) or within limited data: the generated model will too closely correspond to a particular set of data and will fail to reliably predict outcomes in populations with far different characteristics [170]. Many studies have limited cohort sizes, often less than 100 patients (62.3% in our review). This low number may furthermore prevent the constitution of validation cohorts that are independent from the training base, as usually recommended [171].
Despite this, we observe an improvement in the quality of articles over the years on our composite criterion combining the number of patients, the presence of a validation cohort and the presence of prospective data.
4.2. Trends and Topics
The number of studies on radiomics is exponentially increasing, relying both on machine learning and deep learning approaches. In this systematic review Part 2, the most studied cancers were found to be, in order of frequency, esophageal cancer, lymphoma and cervical cancer. Most studies focused on prognostic and treatment response objectives. 18F-FDG remains the most studied tracer. Among the 21 primary tumor subtypes identified in this review, 14 were described in less than 10 publications, leaving room for future developments.
With regards to gastroenteric tumors, PET radiomics and AI analysis should be evaluated for wider application, as it has demonstrated considerable prognostic predictive validity in different settings. Interestingly, esophageal and pancreatic cancers were studied in several papers [13,65,146]: given their poor prognosis, PET radiomics and AI could offer a valid tool to personalize treatment regimens and increase precision medicine. Gastric and colo-rectal cancers could benefit from a quantitative approach both for diagnostic and prognostic purposes [34,49]. PET radiomics and AI analysis in liver cancer should be further evaluated with more specific tracers other than 18F-FDG, such as perfusion tracers and new ones such as 18F/68Ga- FAPI.
Regarding genito-urinary tumors, PET radiomics and AI analysis could help in the outcome prediction of cases with highly aggressive disease. Urinary tract cancers are less commonly studied with 18F-FDG PET/CT, even if they find its useful applicability in staging and restaging of metastatic aggressive diseases. Prostate cancer scans could be performed with different PET radiopharmaceuticals for radiomics and AI analysis purposes, such as 11C/18F-Choline and 68Ga/18F-PSMA. For PET of pelvic tumors, the interference of radioactive urine should be kept in mind in the contouring phase of radiomics and AI protocols.
Only few studies evaluated PET radiomics and AI analysis in neuroendocrine tumors [114,115,116,117,118,119,120]. In low-grade disease, 68Ga-labelled somatostatin analogues are used for staging and restaging purposes: radiomics and AI analysis could provide more information in metastatic stable disease treated with cold somatostatin analogues. In high-grade disease, the prediction of relapsing and progressive disease by 18F-FDG PET/CT could be a useful tool for personalized medicine.
Several studies evaluated the role of PET radiomics and AI analysis in blood malignancies [122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145]. Lymphoma radiomics on 18F-FDG PET/CT was more often assessed on patients with DLBCL, in retrospective cohorts and for prognostic purposes. Further studies in larger prospective cohorts and in different histotypes of lymphoma are needed. Nevertheless, other aggressive diseases such as leukemia and myeloma could take advantage of PET radiomics and AI analysis, also with different PET tracers such as aminoacidic tracers and the immuno-PET tracer 68Ga-Pentixafor.
Few studies evaluated muscolo-skeletal [148,149,150,151,152,153,154,155,156,157,158,159] and skin tumors [160,161]. These are usually aggressive neoplasias, for which new prognostic models derived by PET radiomics and AI analysis could help clinicians and patients to improve survival outcomes. Immunotherapies in metastatic melanoma could be better evaluated with new immuno-PET radiomics and AI tools, to avoid misinterpretations in stable disease as pseudo-progression due to inflammatory reactions.
4.3. Limitations
We applied an arbitrary threshold of 30 patients to eliminate studies that were too exposed to overfitting bias. One of the disadvantages of this selection is the potential elimination of rare pathologies from this review, as previously reported [168].
The articles were read by only one person, which exposes the risk of error in data collection. However, data collection was performed twice in order to limit this risk.
Finally, the scale used to assess the quality of the articles was practical but rather simplistic. We did not thoroughly evaluate the methodological aspects of each study. In particular, we did not check whether a satisfactory description of the factors of variability of the radiomic analyses was systematically given, namely and not exhaustively: the type of contouring used, the resampling and discretization parameters [172,173].
5. Conclusions
PET radiomics and AI analysis in infradiaphragmatic cancers, blood malignancies, melanoma, and musculo-skeletal cancers are an upcoming field in nuclear oncology and the number of related publications is increasing every year. Limitations encountered in the past, such as small sample size of studied populations or lack of validation cohorts, are progressively being corrected and promise further advancement towards personalized medicine.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics12061330/s1, Table S1: Study characteristics p2.xlsx’.
Author Contributions
Conceptualization, D.M. and E.K.A.T.; methodology, D.M.; validation, D.M., E.K.A.T., L.B., R.G., D.P. and S.A.; resources, S.A.; data curation, D.M.; writing—original draft preparation, D.M.; writing—review and editing, E.K.A.T., L.B., R.G., D.P. and S.A.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Papadimitroulas, P.; Brocki, L.; Christopher Chung, N.; Marchadour, W.; Vermet, F.; Gaubert, L.; Eleftheriadis, V.; Plachouris, D.; Visvikis, D.; Kagadis, G.C.; et al. Artificial Intelligence: Deep Learning in Oncological Radiomics and Challenges of Interpretability and Data Harmonization. Phys. Med. 2021, 83, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Cheze Le Rest, C.; Antonorsi, N.; Tixier, F.; Tankyevych, O.; Jaouen, V.; Lucia, F.; Bourbonne, V.; Schick, U.; Badic, B.; et al. Radiomics in PET/CT: Current Status and Future AI-Based Evolutions. Semin. Nucl. Med. 2021, 51, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of Science and Reporting of Radiomics in Oncologic Studies: Room for Improvement According to Radiomics Quality Score and TRIPOD Statement. Eur. Radiol. 2020, 30, 523–536. [Google Scholar] [CrossRef] [PubMed]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, N.; Rossi, G.; Benetti, G.; Zuffante, M.; Micera, R.; Pavarana, M.; Guariglia, S.; Zivelonghi, E.; Mengardo, V.; Weindelmayer, J.; et al. (18)F-FDG PET/CT Metrics Are Correlated to the Pathological Response in Esophageal Cancer Patients Treated With Induction Chemotherapy Followed by Neoadjuvant Chemo-Radiotherapy. Front. Oncol. 2020, 10, 599907. [Google Scholar] [CrossRef] [PubMed]
- Karahan Şen, N.P.; Aksu, A.; Çapa Kaya, G. A Different Overview of Staging PET/CT Images in Patients with Esophageal Cancer: The Role of Textural Analysis with Machine Learning Methods. Ann. Nucl. Med. 2021, 35, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Beck, M.; Päßler, T.; Lili, C.; Hua, W.; Mai, H.D.; Amthauer, H.; Biebl, M.; Thuss-Patience, P.C.; Berger, J.; et al. A FDG-PET Radiomics Signature Detects Esophageal Squamous Cell Carcinoma Patients Who Do Not Benefit from Chemoradiation. Sci. Rep. 2020, 10, 17671. [Google Scholar] [CrossRef]
- Beukinga, R.J.; Wang, D.; Karrenbeld, A.; Dijksterhuis, W.P.M.; Faber, H.; Burgerhof, J.G.M.; Mul, V.E.M.; Slart, R.H.J.A.; Coppes, R.P.; Plukker, J.T.M. Addition of HER2 and CD44 to (18)F-FDG PET-Based Clinico-Radiomic Models Enhances Prediction of Neoadjuvant Chemoradiotherapy Response in Esophageal Cancer. Eur. Radiol. 2021, 31, 3306–3314. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lue, K.-H.; Chu, S.-C.; Chang, B.-S.; Wang, L.-Y.; Liu, D.-W.; Liu, S.-H.; Chao, Y.-K.; Chan, S.-C. Combining the Radiomic Features and Traditional Parameters of (18)F-FDG PET with Clinical Profiles to Improve Prognostic Stratification in Patients with Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy and Surgery. Ann. Nucl. Med. 2019, 33, 657–670. [Google Scholar] [CrossRef]
- Ma, C.; Li, D.; Yin, Y.; Cao, J. Comparison of Characteristics of 18F-Fluorodeoxyglucose and 18F-Fluorothymidine PET during Staging of Esophageal Squamous Cell Carcinoma. Nucl. Med. Commun. 2015, 36, 1181–1186. [Google Scholar] [CrossRef]
- Foley, K.G.; Hills, R.K.; Berthon, B.; Marshall, C.; Parkinson, C.; Lewis, W.G.; Crosby, T.D.L.; Spezi, E.; Roberts, S.A. Development and Validation of a Prognostic Model Incorporating Texture Analysis Derived from Standardised Segmentation of PET in Patients with Oesophageal Cancer. Eur. Radiol. 2018, 28, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Li, Y.; Li, Z.; An, D.; Li, B.; Lin, Q. Development and Validation of a Radiomics Signature on Differentially Expressed Features of (18)F-FDG PET to Predict Treatment Response of Concurrent Chemoradiotherapy in Thoracic Esophagus Squamous Cell Carcinoma. Radiother. Oncol. 2020, 146, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.G.; Shi, Z.; Whybra, P.; Kalendralis, P.; Larue, R.; Berbee, M.; Sosef, M.N.; Parkinson, C.; Staffurth, J.; Crosby, T.D.L.; et al. External Validation of a Prognostic Model Incorporating Quantitative PET Image Features in Oesophageal Cancer. Radiother. Oncol. 2019, 133, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Desbordes, P.; Ruan, S.; Modzelewski, R.; Vauclin, S.; Vera, P.; Gardin, I. Feature Selection for Outcome Prediction in Oesophageal Cancer Using Genetic Algorithm and Random Forest Classifier. Comput. Med. Imaging Graph. 2017, 60, 42–49. [Google Scholar] [CrossRef]
- Tixier, F.; Le Rest, C.C.; Hatt, M.; Albarghach, N.; Pradier, O.; Metges, J.-P.; Corcos, L.; Visvikis, D. Intratumor Heterogeneity Characterized by Textural Features on Baseline 18F-FDG PET Images Predicts Response to Concomitant Radiochemotherapy in Esophageal Cancer. J. Nucl. Med. 2011, 52, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Beukinga, R.J.; Hulshoff, J.B.; van Dijk, L.V.; Muijs, C.T.; Burgerhof, J.G.M.; Kats-Ugurlu, G.; Slart, R.H.J.A.; Slump, C.H.; Mul, V.E.M.; Plukker, J.T.M. Predicting Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer with Textural Features Derived from Pretreatment (18)F-FDG PET/CT Imaging. J. Nucl. Med. 2017, 58, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Ypsilantis, P.-P.; Siddique, M.; Sohn, H.-M.; Davies, A.; Cook, G.; Goh, V.; Montana, G. Predicting Response to Neoadjuvant Chemotherapy with PET Imaging Using Convolutional Neural Networks. PLoS ONE 2015, 10, e0137036. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawahara, D.; Tani, S.; Kubo, K.; Katsuta, T.; Imano, N.; Takeuchi, Y.; Nishibuchi, I.; Saito, A.; Nagata, Y. Predicting the Local Response of Esophageal Squamous Cell Carcinoma to Neoadjuvant Chemoradiotherapy by Radiomics with a Machine Learning Method Using (18)F-FDG PET Images. Diagnostics 2021, 11, 1049. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Z.; Kalendralis, P.; Whybra, P.; Parkinson, C.; Berbee, M.; Spezi, E.; Roberts, A.; Christian, A.; Lewis, W.; et al. Prediction of Lymph Node Metastases Using Pre-Treatment PET Radiomics of the Primary Tumour in Esophageal Adenocarcinoma: An External Validation Study. Br. J. Radiol. 2021, 94, 20201042. [Google Scholar] [CrossRef]
- Beukinga, R.J.; Hulshoff, J.B.; Mul, V.E.M.; Noordzij, W.; Kats-Ugurlu, G.; Slart, R.H.J.A.; Plukker, J.T.M. Prediction of Response to Neoadjuvant Chemotherapy and Radiation Therapy with Baseline and Restaging (18)F-FDG PET Imaging Biomarkers in Patients with Esophageal Cancer. Radiology 2018, 287, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Desbordes, P.; Ruan, S.; Modzelewski, R.; Pineau, P.; Vauclin, S.; Gouel, P.; Michel, P.; Di Fiore, F.; Vera, P.; Gardin, I. Predictive Value of Initial FDG-PET Features for Treatment Response and Survival in Esophageal Cancer Patients Treated with Chemo-Radiation Therapy Using a Random Forest Classifier. PLoS ONE 2017, 12, e0173208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rishi, A.; Zhang, G.G.; Yuan, Z.; Sim, A.J.; Song, E.Y.; Moros, E.G.; Tomaszewski, M.R.; Latifi, K.; Pimiento, J.M.; Fontaine, J.-P.; et al. Pretreatment CT and (18) F-FDG PET-Based Radiomic Model Predicting Pathological Complete Response and Loco-Regional Control Following Neoadjuvant Chemoradiation in Oesophageal Cancer. J. Med. Imaging Radiat. Oncol. 2021, 65, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Coroller, T.P.; Sanford, N.N.; Mamon, H.; Aerts, H.J.W.L.; Berbeco, R.I. Relationship between the Temporal Changes in Positron-Emission-Tomography-Imaging-Based Textural Features and Pathologic Response and Survival in Esophageal Cancer Patients. Front. Oncol. 2016, 6, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatt, M.; Tixier, F.; Cheze Le Rest, C.; Pradier, O.; Visvikis, D. Robustness of Intratumour 18F-FDG PET Uptake Heterogeneity Quantification for Therapy Response Prediction in Oesophageal Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Jinguji, M.; Nakabeppu, Y.; Nakajo, M.; Higashi, R.; Fukukura, Y.; Sasaki, K.; Uchikado, Y.; Natsugoe, S.; Yoshiura, T. Texture Analysis of (18)F-FDG PET/CT to Predict Tumour Response and Prognosis of Patients with Esophageal Cancer Treated by Chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 206–214. [Google Scholar] [CrossRef]
- van Rossum, P.S.N.; Fried, D.V.; Zhang, L.; Hofstetter, W.L.; van Vulpen, M.; Meijer, G.J.; Court, L.E.; Lin, S.H. The Incremental Value of Subjective and Quantitative Assessment of 18F-FDG PET for the Prediction of Pathologic Complete Response to Preoperative Chemoradiotherapy in Esophageal Cancer. J. Nucl. Med. 2016, 57, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Yu, W.; Ma, J.; Ren, Y.; Fu, X.; Zhao, J. The Role of PET-Based Radiomic Features in Predicting Local Control of Esophageal Cancer Treated with Concurrent Chemoradiotherapy. Sci. Rep. 2018, 8, 9902. [Google Scholar] [CrossRef]
- Dong, X.; Xing, L.; Wu, P.; Fu, Z.; Wan, H.; Li, D.; Yin, Y.; Sun, X.; Yu, J. Three-Dimensional Positron Emission Tomography Image Texture Analysis of Esophageal Squamous Cell Carcinoma: Relationship between Tumor 18F-Fluorodeoxyglucose Uptake Heterogeneity, Maximum Standardized Uptake Value, and Tumor Stage. Nucl. Med. Commun. 2013, 34, 40–46. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Xin, B.; Sun, Y.; Feng, D.; Fulham, M.J.; Wang, X.; Song, S. (18)F-FDG PET/CT Radiomics for Preoperative Prediction of Lymph Node Metastases and Nodal Staging in Gastric Cancer. Front. Oncol. 2021, 11, 723345. [Google Scholar] [CrossRef]
- Xue, B.; Jiang, J.; Chen, L.; Wu, S.; Zheng, X.; Zheng, X.; Tang, K. Development and Validation of a Radiomics Model Based on (18)F-FDG PET of Primary Gastric Cancer for Predicting Peritoneal Metastasis. Front. Oncol. 2021, 11, 740111. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Ji, C.-F.; Wang, H.; He, J.; Liu, S.; Ge, Y.; Zhou, Z.-Y. Differentiating Gastric Cancer and Gastric Lymphoma Using Texture Analysis (TA) of Positron Emission Tomography (PET). Chin. Med. J. 2020, 134, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, J.; Zhang, H.; Yin, H.; Zhang, R.; Zhang, J.; Chen, X. Machine Learning Analysis for the Noninvasive Prediction of Lymphovascular Invasion in Gastric Cancer Using PET/CT and Enhanced CT-Based Radiomics and Clinical Variables. Abdom. Radiol. N. Y. 2022, 47, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kang, S.Y.; Yoon, J.; Kim, T.-Y.; Cheon, G.J.; Oh, D.-Y. Prospective Evaluation of Metabolic Intratumoral Heterogeneity in Patients with Advanced Gastric Cancer Receiving Palliative Chemotherapy. Sci. Rep. 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, Q.; Lv, W.; Xi, S.; Huang, W.; Sun, Z.; Chen, H.; Zhao, L.; Liu, W.; Hu, Y.; et al. Radiomic Signature of (18)F Fluorodeoxyglucose PET/CT for Prediction of Gastric Cancer Survival and Chemotherapeutic Benefits. Theranostics 2018, 8, 5915–5928. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-Q.; Yu, W.-J.; Shao, X.-L.; Li, X.-F.; Niu, R.; Zhang, F.-F.; Shi, Y.-M.; Wang, Y.-T. Radiomics Model Based on Preoperative 18F-Fluorodeoxyglucose PET Predicts N2-3b Lymph Node Metastasis in Gastric Cancer Patients. Nucl. Med. Commun. 2022, 43, 340–349. [Google Scholar] [CrossRef]
- Lovinfosse, P.; Koopmansch, B.; Lambert, F.; Jodogne, S.; Kustermans, G.; Hatt, M.; Visvikis, D.; Seidel, L.; Polus, M.; Albert, A.; et al. (18)F-FDG PET/CT Imaging in Rectal Cancer: Relationship with the RAS Mutational Status. Br. J. Radiol. 2016, 89, 20160212. [Google Scholar] [CrossRef] [Green Version]
- Nakajo, M.; Kajiya, Y.; Tani, A.; Jinguji, M.; Nakajo, M.; Kitazono, M.; Yoshiura, T. A Pilot Study for Texture Analysis of (18)F-FDG and (18)F-FLT-PET/CT to Predict Tumor Recurrence of Patients with Colorectal Cancer Who Received Surgery. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2158–2168. [Google Scholar] [CrossRef]
- Martin-Gonzalez, P.; de Mariscal, E.G.; Martino, M.E.; Gordaliza, P.M.; Peligros, I.; Carreras, J.L.; Calvo, F.A.; Pascau, J.; Desco, M.; Muñoz-Barrutia, A. Association of Visual and Quantitative Heterogeneity of 18F-FDG PET Images with Treatment Response in Locally Advanced Rectal Cancer: A Feasibility Study. PLoS ONE 2020, 15, e0242597. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Boimel, P.; Janopaul-Naylor, J.; Zhong, H.; Xiao, Y.; Ben-Josef, E.; Fan, Y. Collaborative clustering of subjects and radiomic features for predicting clinical outcomes of rectal cancer patients. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging, Venice, Italy, 8–11 April 2019; pp. 1303–1306. [Google Scholar] [CrossRef]
- Li, H.; Boimel, P.; Janopaul-Naylor, J.; Zhong, H.; Xiao, Y.; Ben-Josef, E.; Fan, Y. Deep convolutional neural networks for imaging data based survival analysis of rectal cancer. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging, Venice, Italy, 8–11 April 2019; pp. 846–849. [Google Scholar] [CrossRef]
- Lovinfosse, P.; Polus, M.; Van Daele, D.; Martinive, P.; Daenen, F.; Hatt, M.; Visvikis, D.; Koopmansch, B.; Lambert, F.; Coimbra, C.; et al. FDG PET/CT Radiomics for Predicting the Outcome of Locally Advanced Rectal Cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 365–375. [Google Scholar] [CrossRef]
- Chen, S.-W.; Shen, W.-C.; Chen, W.T.-L.; Hsieh, T.-C.; Yen, K.-Y.; Chang, J.-G.; Kao, C.-H. Metabolic Imaging Phenotype Using Radiomics of [(18)F]FDG PET/CT Associated with Genetic Alterations of Colorectal Cancer. Mol. Imaging Biol. 2019, 21, 183–190. [Google Scholar] [CrossRef]
- Shen, W.-C.; Chen, S.-W.; Wu, K.-C.; Lee, P.-Y.; Feng, C.-L.; Hsieh, T.-C.; Yen, K.-Y.; Kao, C.-H. Predicting Pathological Complete Response in Rectal Cancer after Chemoradiotherapy with a Random Forest Using (18)F-Fluorodeoxyglucose Positron Emission Tomography and Computed Tomography Radiomics. Ann. Transl. Med. 2020, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.-I.; Ha, S.; Kang, S.-B.; Lee, K.-W.; Lee, H.-S.; Kim, J.-S.; Oh, H.-K.; Lee, H.-Y.; Kim, S.E. Prediction of Neoadjuvant Radiation Chemotherapy Response and Survival Using Pretreatment [(18)F]FDG PET/CT Scans in Locally Advanced Rectal Cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 422–431. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Q.; Zhang, Y.; Wu, H.; Zhou, Y.; Zhao, S. Preoperative Prediction of Regional Lymph Node Metastasis of Colorectal Cancer Based on (18)F-FDG PET/CT and Machine Learning. Ann. Nucl. Med. 2021, 35, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Frazer, M.; Rishi, A.; Latifi, K.; Tomaszewski, M.R.; Moros, E.G.; Feygelman, V.; Felder, S.; Sanchez, J.; Dessureault, S.; et al. Pretreatment CT and PET Radiomics Predicting Rectal Cancer Patients in Response to Neoadjuvant Chemoradiotherapy. Rep. Pract. Oncol. Radiother. 2021, 26, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Minamimoto, R.; Gohda, Y.; Miwa, K.; Otani, K.; Kiyomatsu, T.; Yano, H. Prognostic Value of (18)F-FDG PET/CT with Texture Analysis in Patients with Rectal Cancer Treated by Surgery. Ann. Nucl. Med. 2021, 35, 843–852. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Xin, B.; Hao, Y.; Wang, L.; Song, S.; Xu, J.; Wang, X. Quantitative Prediction of Microsatellite Instability in Colorectal Cancer With Preoperative PET/CT-Based Radiomics. Front. Oncol. 2021, 11, 702055. [Google Scholar] [CrossRef]
- Lv, L.; Xin, B.; Hao, Y.; Yang, Z.; Xu, J.; Wang, L.; Wang, X.; Song, S.; Guo, X. Radiomic Analysis for Predicting Prognosis of Colorectal Cancer from Preoperative (18)F-FDG PET/CT. J. Transl. Med. 2022, 20, 66. [Google Scholar] [CrossRef]
- van Helden, E.J.; Vacher, Y.J.L.; van Wieringen, W.N.; van Velden, F.H.P.; Verheul, H.M.W.; Hoekstra, O.S.; Boellaard, R.; Menke-van der Houven van Oordt, C.W. Radiomics Analysis of Pre-Treatment [(18)F]FDG PET/CT for Patients with Metastatic Colorectal Cancer Undergoing Palliative Systemic Treatment. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2307–2317. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Lee, J.-H.; Lee, H.S.; Cho, E.-S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Park, C.; Yeu, Y.; Clemenceau, J.R.; et al. Radiomics Features of (18)F-Fluorodeoxyglucose Positron-Emission Tomography as a Novel Prognostic Signature in Colorectal Cancer. Cancers 2021, 13, 392. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Habes, M.; Li, Y.; Boimel, P.; Janopaul-Naylor, J.; Xiao, Y.; Ben-Josef, E.; Fan, Y. Robust Collaborative Clustering of Subjects and Radiomic Features for Cancer Prognosis. IEEE Trans. Biomed. Eng. 2020, 67, 2735–2744. [Google Scholar] [CrossRef]
- Schurink, N.W.; van Kranen, S.R.; Berbee, M.; van Elmpt, W.; Bakers, F.C.H.; Roberti, S.; van Griethuysen, J.J.M.; Min, L.A.; Lahaye, M.J.; Maas, M.; et al. Studying Local Tumour Heterogeneity on MRI and FDG-PET/CT to Predict Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Eur. Radiol. 2021, 31, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Karahan Şen, N.P.; Aksu, A.; Kaya, G.Ç. Value of Volumetric and Textural Analysis in Predicting the Treatment Response in Patients with Locally Advanced Rectal Cancer. Ann. Nucl. Med. 2020, 34, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Zhong, J.; Frood, R.; Currie, S.; Gilbert, A.; Appelt, A.L.; Sebag-Montefiore, D.; Scarsbrook, A. Prediction of Outcome in Anal Squamous Cell Carcinoma Using Radiomic Feature Analysis of Pre-Treatment FDG PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2790–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Gu, B.; Song, S.; Zhang, B.; Wang, W.; Xu, J.; Yu, X.; Shi, S. A Novel Validated Recurrence Stratification System Based on (18)F-FDG PET/CT Radiomics to Guide Surveillance After Resection of Pancreatic Cancer. Front. Oncol. 2021, 11, 650266. [Google Scholar] [CrossRef]
- Lim, C.H.; Cho, Y.S.; Choi, J.Y.; Lee, K.-H.; Lee, J.K.; Min, J.H.; Hyun, S.H. Imaging Phenotype Using (18)F-Fluorodeoxyglucose Positron Emission Tomography-Based Radiomics and Genetic Alterations of Pancreatic Ductal Adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2113–2122. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, H.S.; Choi, S.H.; Choi, D.W.; Lee, J.K.; Lee, K.H.; Park, J.O.; Lee, K.-H.; Kim, B.-T.; Choi, J.Y. Intratumoral Heterogeneity of (18)F-FDG Uptake Predicts Survival in Patients with Pancreatic Ductal Adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1461–1468. [Google Scholar] [CrossRef]
- Wang, G.; Dang, H.; Yu, P.; Liu, H.; Wu, Y.; Yao, S.; Tian, J.; Ye, H.; Xu, B. Multiparameter Analysis Using (18)F-FDG PET/CT in the Differential Diagnosis of Pancreatic Cystic Neoplasms. Contrast Media Mol. Imaging 2021, 2021, 6658644. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.-H.; Ahn, H.; Lee, S.M.; Jang, S.J. Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT. Cancers 2021, 13, 3563. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kang, S.Y.; Cheon, G.J.; Oh, D.-Y.; Bang, Y.-J. Predictive Role of Temporal Changes in Intratumoral Metabolic Heterogeneity During Palliative Chemotherapy in Patients with Advanced Pancreatic Cancer: A Prospective Cohort Study. J. Nucl. Med. 2020, 61, 33–39. [Google Scholar] [CrossRef]
- Xing, H.; Hao, Z.; Zhu, W.; Sun, D.; Ding, J.; Zhang, H.; Liu, Y.; Huo, L. Preoperative Prediction of Pathological Grade in Pancreatic Ductal Adenocarcinoma Based on (18)F-FDG PET/CT Radiomics. EJNMMI Res. 2021, 11, 19. [Google Scholar] [CrossRef]
- Yoo, M.Y.; Yoon, Y.-S.; Suh, M.S.; Cho, J.Y.; Han, H.-S.; Lee, W.W. Prognosis Prediction of Pancreatic Cancer after Curative Intent Surgery Using Imaging Parameters Derived from F-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Medicine 2020, 99, e21829. [Google Scholar] [CrossRef] [PubMed]
- Toyama, Y.; Hotta, M.; Motoi, F.; Takanami, K.; Minamimoto, R.; Takase, K. Prognostic Value of FDG-PET Radiomics with Machine Learning in Pancreatic Cancer. Sci. Rep. 2020, 10, 17024. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, J.; Pollom, E.; Alagappan, M.; Shirato, H.; Chang, D.T.; Koong, A.C.; Li, R. Quantitative Analysis of (18)F-Fluorodeoxyglucose Positron Emission Tomography Identifies Novel Prognostic Imaging Biomarkers in Locally Advanced Pancreatic Cancer Patients Treated With Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, C.; Liu, Z.; Wang, L.; Pan, G.; Sun, G.; Chang, Y.; Zuo, C.; Yang, X. Radiomics Analysis for the Differentiation of Autoimmune Pancreatitis and Pancreatic Ductal Adenocarcinoma in (18) F-FDG PET/CT. Med. Phys. 2019, 46, 4520–4530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, M.; Zuo, C.; Yang, Z.; Yang, X.; Ren, S.; Peng, Y.; Sun, G.; Shen, J.; Cheng, C.; et al. Radiomics Model of Dual-Time 2-[(18)F]FDG PET/CT Imaging to Distinguish between Pancreatic Ductal Adenocarcinoma and Autoimmune Pancreatitis. Eur. Radiol. 2021, 31, 6983–6991. [Google Scholar] [CrossRef]
- Mori, M.; Passoni, P.; Incerti, E.; Bettinardi, V.; Broggi, S.; Reni, M.; Whybra, P.; Spezi, E.; Vanoli, E.G.; Gianolli, L.; et al. Training and Validation of a Robust PET Radiomic-Based Index to Predict Distant-Relapse-Free-Survival after Radio-Chemotherapy for Locally Advanced Pancreatic Cancer. Radiother. Oncol. 2020, 153, 258–264. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Fang, Q.; Zhang, X.; Hou, P.; Wu, H.; Wang, X. Radiomics Analysis of [(18)F]FDG PET/CT for Microvascular Invasion and Prognosis Prediction in Very-Early- and Early-Stage Hepatocellular Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2599–2614. [Google Scholar] [CrossRef]
- Blanc-Durand, P.; Van Der Gucht, A.; Jreige, M.; Nicod-Lalonde, M.; Silva-Monteiro, M.; Prior, J.O.; Denys, A.; Depeursinge, A.; Schaefer, N. Signature of Survival: A (18)F-FDG PET Based Whole-Liver Radiomic Analysis Predicts Survival after (90)Y-TARE for Hepatocellular Carcinoma. Oncotarget 2018, 9, 4549–4558. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Guo, W.; Cui, X.; Zhuo, H.; Xiao, Y.; Ou, X.; Zhao, Y.; Zhang, T.; Ma, X. Three-Dimensional Texture Analysis Based on PET/CT Images to Distinguish Hepatocellular Carcinoma and Hepatic Lymphoma. Front. Oncol. 2019, 9, 844. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Cui, C.; Xu, J.; Kaza, R.; El Naqa, I.; Dewaraja, Y.K. Tumor Response Prediction in (90)Y Radioembolization with PET-Based Radiomics Features and Absorbed Dose Metrics. EJNMMI Phys. 2020, 7, 74. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Rahman, T.; Yamamoto, M.; Yamada, S.; Tsuyoshi, H.; Kiyono, Y.; Kimura, H.; Yoshida, Y.; Okazawa, H. (18)F-FDG PET Radiomics Approaches: Comparing and Clustering Features in Cervical Cancer. Ann. Nucl. Med. 2017, 31, 678–685. [Google Scholar] [CrossRef]
- Mu, W.; Liang, Y.; Hall, L.O.; Tan, Y.; Balagurunathan, Y.; Wenham, R.; Wu, N.; Tian, J.; Gillies, R.J. (18)F-FDG PET/CT Habitat Radiomics Predicts Outcome of Patients with Cervical Cancer Treated with Chemoradiotherapy. Radiol. Artif. Intell. 2020, 2, e190218. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Lovinfosse, P.; Hermesse, J.; Decuypere, M.; Rousseau, C.; Lucia, F.; Schick, U.; Reinhold, C.; Robin, P.; Hatt, M.; et al. [(18)F]FDG PET Radiomics to Predict Disease-Free Survival in Cervical Cancer: A Multi-Scanner/Center Study with External Validation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3432–3443. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Chen, S.-W.; Liang, J.-A.; Hsieh, T.-C.; Yen, K.-Y.; Kao, C.-H. [18]Fluorodeoxyglucose Positron Emission Tomography for the Textural Features of Cervical Cancer Associated with Lymph Node Metastasis and Histological Type. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Lucia, F.; Visvikis, D.; Vallières, M.; Desseroit, M.-C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A.; et al. External Validation of a Combined PET and MRI Radiomics Model for Prediction of Recurrence in Cervical Cancer Patients Treated with Chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef]
- Altazi, B.A.; Fernandez, D.C.; Zhang, G.G.; Hawkins, S.; Naqvi, S.M.; Kim, Y.; Hunt, D.; Latifi, K.; Biagioli, M.; Venkat, P.; et al. Investigating Multi-Radiomic Models for Enhancing Prediction Power of Cervical Cancer Treatment Outcomes. Phys. Medica 2018, 46, 180–188. [Google Scholar] [CrossRef]
- Nakajo, M.; Jinguji, M.; Tani, A.; Yano, E.; Hoo, C.K.; Hirahara, D.; Togami, S.; Kobayashi, H.; Yoshiura, T. Machine Learning Based Evaluation of Clinical and Pretreatment (18)F-FDG-PET/CT Radiomic Features to Predict Prognosis of Cervical Cancer Patients. Abdom. Radiol. N. Y. 2022, 47, 838–847. [Google Scholar] [CrossRef]
- Li, X.-R.; Jin, J.-J.; Yu, Y.; Wang, X.-H.; Guo, Y.; Sun, H.-Z. PET-CT Radiomics by Integrating Primary Tumor and Peritumoral Areas Predicts E-Cadherin Expression and Correlates with Pelvic Lymph Node Metastasis in Early-Stage Cervical Cancer. Eur. Radiol. 2021, 31, 5967–5979. [Google Scholar] [CrossRef]
- Chong, G.O.; Park, S.-H.; Jeong, S.Y.; Kim, S.J.; Park, N.J.-Y.; Lee, Y.H.; Lee, S.-W.; Hong, D.G.; Park, J.Y.; Han, H.S. Prediction Model for Tumor Budding Status Using the Radiomic Features of F-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Cervical Cancer. Diagnostics 2021, 11, 1517. [Google Scholar] [CrossRef]
- Reuzé, S.; Orlhac, F.; Chargari, C.; Nioche, C.; Limkin, E.; Riet, F.; Escande, A.; Haie-Meder, C.; Dercle, L.; Gouy, S.; et al. Prediction of Cervical Cancer Recurrence Using Textural Features Extracted from 18F-FDG PET Images Acquired with Different Scanners. Oncotarget 2017, 8, 43169–43179. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, C.; Yu, Y.; Guo, Y.; Sun, H. Prediction of Lymphovascular Space Invasion Using a Combination of Tenascin-C, Cox-2, and PET/CT Radiomics in Patients with Early-Stage Cervical Squamous Cell Carcinoma. BMC Cancer 2021, 21, 866. [Google Scholar] [CrossRef] [PubMed]
- Lucia, F.; Visvikis, D.; Desseroit, M.-C.; Miranda, O.; Malhaire, J.-P.; Robin, P.; Pradier, O.; Hatt, M.; Schick, U. Prediction of Outcome Using Pretreatment (18)F-FDG PET/CT and MRI Radiomics in Locally Advanced Cervical Cancer Treated with Chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 768–786. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Young, L.; Grigsby, P. Predictive Value of Standardized Intratumoral Metabolic Heterogeneity in Locally Advanced Cervical Cancer Treated With Chemoradiation. Int. J. Gynecol. Cancer 2016, 26, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Maquilan, G.M.; Thomas, K.; Wachsmann, J.; Wang, J.; Folkert, M.R.; Albuquerque, K. Quantitative PET Imaging and Clinical Parameters as Predictive Factors for Patients With Cervical Carcinoma: Implications of a Prediction Model Generated Using Multi-Objective Support Vector Machine Learning. Technol. Cancer Res. Treat. 2020, 19, 1533033820983804. [Google Scholar] [CrossRef] [PubMed]
- Roman-Jimenez, G.; Acosta, O.; Leseur, J.; Devillers, A.; Der Sarkissian, H.; Guzman, L.; Grossiord, E.; Ospina, J.-D.; De Crevoisier, R. Random Forests to Predict Tumor Recurrence Following Cervical Cancer Therapy Using Pre- and per-Treatment (18)F-FDG PET Parameters. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2444–2447. [Google Scholar] [CrossRef]
- Jin, J.; Wu, K.; Li, X.; Yu, Y.; Wang, X.; Sun, H. Relationship between Tumor Heterogeneity and Volume in Cervical Cancer: Evidence from Integrated Fluorodeoxyglucose 18 PET/MR Texture Analysis. Nucl. Med. Commun. 2021, 42, 545–552. [Google Scholar] [CrossRef]
- Mu, W.; Chen, Z.; Liang, Y.; Shen, W.; Yang, F.; Dai, R.; Wu, N.; Tian, J. Staging of Cervical Cancer Based on Tumor Heterogeneity Characterized by Texture Features on (18)F-FDG PET Images. Phys. Med. Biol. 2015, 60, 5123–5139. [Google Scholar] [CrossRef] [Green Version]
- Pedraza, S.; Seiffert, A.P.; Sarandeses, P.; Muñoz-Lopez, B.; Gómez, E.J.; Sánchez-González, P.; Pérez-Regadera, J.F. The Value of Metabolic Parameters and Textural Analysis in Predicting Prognosis in Locally Advanced Cervical Cancer Treated with Chemoradiotherapy. Strahlenther. Onkol. 2022, 1–10. [Google Scholar] [CrossRef]
- Li, K.; Sun, H.; Lu, Z.; Xin, J.; Zhang, L.; Guo, Y.; Guo, Q. Value of [(18)F]FDG PET Radiomic Features and VEGF Expression in Predicting Pelvic Lymphatic Metastasis and Their Potential Relationship in Early-Stage Cervical Squamous Cell Carcinoma. Eur. J. Radiol. 2018, 106, 160–166. [Google Scholar] [CrossRef]
- Novikov, M. Multiparametric Quantitative and Texture (18)F-FDG PET/CT Analysis for Primary Malignant Tumour Grade Differentiation. Eur. Radiol. Exp. 2019, 3, 48. [Google Scholar] [CrossRef]
- Hao, H.; Zhou, Z.; Li, S.; Maquilan, G.; Folkert, M.R.; Iyengar, P.; Westover, K.D.; Albuquerque, K.; Liu, F.; Choy, H.; et al. Shell Feature: A New Radiomics Descriptor for Predicting Distant Failure after Radiotherapy in Non-Small Cell Lung Cancer and Cervix Cancer. Phys. Med. Biol. 2018, 63, 095007. [Google Scholar] [CrossRef]
- Wu, J.; Lian, C.; Ruan, S.; Mazur, T.R.; Mutic, S.; Anastasio, M.A.; Grigsby, P.W.; Vera, P.; Li, H. Treatment Outcome Prediction for Cancer Patients Based on Radiomics and Belief Function Theory. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, H.; Guo, Y.; Zou, L. (18)F-FDG PET/CT Quantitative Parameters and Texture Analysis Effectively Differentiate Endometrial Precancerous Lesion and Early-Stage Carcinoma. Mol. Imaging 2019, 18, 1536012119856965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wu, K.; Li, X.; Jin, J.; Yu, Y.; Sun, H. Additional Value of PET/CT-Based Radiomics to Metabolic Parameters in Diagnosing Lynch Syndrome and Predicting PD1 Expression in Endometrial Carcinoma. Front. Oncol. 2021, 11, 595430. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Jinguji, M.; Tani, A.; Kikuno, H.; Hirahara, D.; Togami, S.; Kobayashi, H.; Yoshiura, T. Application of a Machine Learning Approach for the Analysis of Clinical and Radiomic Features of Pretreatment [(18)F]-FDG PET/CT to Predict Prognosis of Patients with Endometrial Cancer. Mol. Imaging Biol. 2021, 23, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Crivellaro, C.; Landoni, C.; Elisei, F.; Buda, A.; Bonacina, M.; Grassi, T.; Monaco, L.; Giuliani, D.; Gotuzzo, I.; Magni, S.; et al. Combining Positron Emission Tomography/Computed Tomography, Radiomics, and Sentinel Lymph Node Mapping for Nodal Staging of Endometrial Cancer Patients. Int. J. Gynecol. Cancer 2020, 30, 378–382. [Google Scholar] [CrossRef] [PubMed]
- De Bernardi, E.; Buda, A.; Guerra, L.; Vicini, D.; Elisei, F.; Landoni, C.; Fruscio, R.; Messa, C.; Crivellaro, C. Radiomics of the Primary Tumour as a Tool to Improve (18)F-FDG-PET Sensitivity in Detecting Nodal Metastases in Endometrial Cancer. EJNMMI Res. 2018, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Collarino, A.; Garganese, G.; Fragomeni, S.M.; Pereira Arias-Bouda, L.M.; Ieria, F.P.; Boellaard, R.; Rufini, V.; de Geus-Oei, L.-F.; Scambia, G.; Valdés Olmos, R.A.; et al. Radiomics in Vulvar Cancer: First Clinical Experience Using (18)F-FDG PET/CT Images. J. Nucl. Med. 2018, 60, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, Z. Radiomics Analysis of PET and CT Components of (18)F-FDG PET/CT Imaging for Prediction of Progression-Free Survival in Advanced High-Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 638124. [Google Scholar] [CrossRef]
- Moazemi, S.; Erle, A.; Khurshid, Z.; Lütje, S.; Muders, M.; Essler, M.; Schultz, T.; Bundschuh, R.A. Decision-Support for Treatment with (177)Lu-PSMA: Machine Learning Predicts Response with High Accuracy Based on PSMA-PET/CT and Clinical Parameters. Ann. Transl. Med. 2021, 9, 818. [Google Scholar] [CrossRef]
- Aksu, A.; Vural Topuz, Ö.; Yılmaz, G.; Çapa Kaya, G.; Yılmaz, B. Dual Time Point Imaging of Staging PSMA PET/CT Quantification; Spread and Radiomic Analyses. Ann. Nucl. Med. 2022, 36, 310–318. [Google Scholar] [CrossRef]
- Erle, A.; Moazemi, S.; Lütje, S.; Essler, M.; Schultz, T.; Bundschuh, R.A. Evaluating a Machine Learning Tool for the Classification of Pathological Uptake in Whole-Body PSMA-PET-CT Scans. Tomogr. Ann Arbor Mich 2021, 7, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, S.; Pandey, A.K.; Arora, G.; Sharma, A.; Seth, A.; Kumar, R. Haralick Texture Features Extracted from Ga-68 PSMA PET/CT to Differentiate Normal Prostate from Prostate Cancer: A Feasibility Study. Nucl. Med. Commun. 2021, 42, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Cysouw, M.C.F.; Jansen, B.H.E.; van de Brug, T.; Oprea-Lager, D.E.; Pfaehler, E.; de Vries, B.M.; van Moorselaar, R.J.A.; Hoekstra, O.S.; Vis, A.N.; Boellaard, R. Machine Learning-Based Analysis of [(18)F]DCFPyL PET Radiomics for Risk Stratification in Primary Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Hu, S.; Lin, X.; Zou, Q.; Zou, M.; Zhang, Z.; Xu, L.; Jiang, N.; Zhang, Y. Machine Learning-Based Prediction of Invisible Intraprostatic Prostate Cancer Lesions on (68) Ga-PSMA-11 PET/CT in Patients with Primary Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1523–1534. [Google Scholar] [CrossRef]
- Alongi, P.; Stefano, A.; Comelli, A.; Laudicella, R.; Scalisi, S.; Arnone, G.; Barone, S.; Spada, M.; Purpura, P.; Bartolotta, T.V.; et al. Radiomics Analysis of 18F-Choline PET/CT in the Prediction of Disease Outcome in High-Risk Prostate Cancer: An Explorative Study on Machine Learning Feature Classification in 94 Patients. Eur. Radiol. 2021, 31, 4595–4605. [Google Scholar] [CrossRef]
- Khurshid, Z.; Ahmadzadehfar, H.; Gaertner, F.C.; Papp, L.; Zsóter, N.; Essler, M.; Bundschuh, R.A. Role of Textural Heterogeneity Parameters in Patient Selection for 177Lu-PSMA Therapy via Response Prediction. Oncotarget 2018, 9, 33312–33321. [Google Scholar] [CrossRef] [Green Version]
- Papp, L.; Spielvogel, C.P.; Grubmüller, B.; Grahovac, M.; Krajnc, D.; Ecsedi, B.; Sareshgi, R.A.M.; Mohamad, D.; Hamboeck, M.; Rausch, I.; et al. Supervised Machine Learning Enables Non-Invasive Lesion Characterization in Primary Prostate Cancer with [(68)Ga]Ga-PSMA-11 PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1795–1805. [Google Scholar] [CrossRef]
- Solari, E.L.; Gafita, A.; Schachoff, S.; Bogdanović, B.; Villagrán Asiares, A.; Amiel, T.; Hui, W.; Rauscher, I.; Visvikis, D.; Maurer, T.; et al. The Added Value of PSMA PET/MR Radiomics for Prostate Cancer Staging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 527–538. [Google Scholar] [CrossRef]
- Tu, S.-J.; Tran, V.T.; Teo, J.M.; Chong, W.C.; Tseng, J.-R. Utility of Radiomic Zones for Risk Classification and Clinical Outcome Predictions Using Supervised Machine Learning during Simultaneous (11) C-Choline PET/MRI Acquisition in Prostate Cancer Patients. Med. Phys. 2021, 48, 5192–5201. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Jiang, H.; Zhao, H.; Han, W.; Wang, M.; Fu, P. (18)F-FDG Texture Analysis Predicts the Pathological Fuhrman Nuclear Grade of Clear Cell Renal Cell Carcinoma. Abdom. Radiol. N. Y. 2021, 46, 5618–5628. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Calabrò, D.; Malavasi, S.; Ricci, C.; Casadei, R.; Campana, D.; Baiocco, S.; Fanti, S.; Ambrosini, V. A [68Ga]Ga-DOTANOC PET/CT Radiomic Model for Non-Invasive Prediction of Tumour Grade in Pancreatic Neuroendocrine Tumours. Diagnostics 2021, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Metovic, J.; Papotti, M.; Volante, M.; Molinari, F.; et al. Diagnostic Value of Conventional PET Parameters and Radiomic Features Extracted from 18F-FDG-PET/CT for Histologic Subtype Classification and Characterization of Lung Neuroendocrine Neoplasms. Biomedicines 2021, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Pasetto, S.; Rancoita, P.M.V.; Muffatti, F.; Bettinardi, V.; Presotto, L.; Andreasi, V.; et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET/Computed Tomography Radiomics in Pancreatic Neuroendocrine Neoplasms: An Endearing Tool for Preoperative Risk Assessment. Nucl. Med. Commun. 2020, 41, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsótér, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-Therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.; Ganeshan, B.; Endozo, R.; Wan, S.; Aldridge, M.D.; Groves, A.M.; Bomanji, J.B.; Gaze, M.N. Radiomics-Based Texture Analysis of (68)Ga-DOTATATE Positron Emission Tomography and Computed Tomography Images as a Prognostic Biomarker in Adults With Neuroendocrine Cancers Treated With (177)Lu-DOTATATE. Front. Oncol. 2021, 11, 686235. [Google Scholar] [CrossRef]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival Prediction in Patients Undergoing Radionuclide Therapy Based on Intratumoral Somatostatin-Receptor Heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Textural Analysis of Hybrid DOTATOC-PET/MRI and Its Association with Histological Grading in Patients with Liver Metastases from Neuroendocrine Tumors. Nucl. Med. Commun. 2020, 41, 363–369. [Google Scholar] [CrossRef]
- Ansquer, C.; Drui, D.; Mirallié, E.; Renaudin-Autain, K.; Denis, A.; Gimenez-Roqueplo, A.-P.; Leux, C.; Toulgoat, F.; Kraeber-Bodéré, F.; Carlier, T. Usefulness of FDG-PET/CT-Based Radiomics for the Characterization and Genetic Orientation of Pheochromocytomas Before Surgery. Cancers 2020, 12, 2424. [Google Scholar] [CrossRef]
- Eertink, J.J.; van de Brug, T.; Wiegers, S.E.; Zwezerijnen, G.J.C.; Pfaehler, E.A.G.; Lugtenburg, P.J.; van der Holt, B.; de Vet, H.C.W.; Hoekstra, O.S.; Boellaard, R.; et al. (18)F-FDG PET Baseline Radiomics Features Improve the Prediction of Treatment Outcome in Diffuse Large B-Cell Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 932–942. [Google Scholar] [CrossRef]
- Parvez, A.; Tau, N.; Hussey, D.; Maganti, M.; Metser, U. (18)F-FDG PET/CT Metabolic Tumor Parameters and Radiomics Features in Aggressive Non-Hodgkin’s Lymphoma as Predictors of Treatment Outcome and Survival. Ann. Nucl. Med. 2018, 32, 410–416. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Riedl, C.C.; Kumar, A.; Dogan, A.; Gibbs, P.; Weber, M.; Staber, P.B.; Huicochea Castellanos, S.; Schöder, H. [18F]FDG-PET/CT Radiomics for Prediction of Bone Marrow Involvement in Mantle Cell Lymphoma: A Retrospective Study in 97 Patients. Cancers 2020, 12, 1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.; Jiang, H.; He, X.; Feng, L.; Ni, M.; Ma, M.; Wang, J.; Zhang, T.; Wu, S.; et al. A Novel Analytic Approach for Outcome Prediction in Diffuse Large B-Cell Lymphoma by [(18)F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, S.A.; Elhalawani, H.; Lee, J.; Wang, Q.; Mohamed, A.S.R.; Dabaja, B.S.; Pinnix, C.C.; Gunther, J.R.; Court, L.; Rao, A.; et al. A PET Radiomics Model to Predict Refractory Mediastinal Hodgkin Lymphoma. Sci. Rep. 2019, 9, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Bouallègue, F.; Tabaa, Y.A.; Kafrouni, M.; Cartron, G.; Vauchot, F.; Mariano-Goulart, D. Association between Textural and Morphological Tumor Indices on Baseline PET-CT and Early Metabolic Response on Interim PET-CT in Bulky Malignant Lymphomas. Med. Phys. 2017, 44, 4608–4619. [Google Scholar] [CrossRef]
- Aide, N.; Fruchart, C.; Nganoa, C.; Gac, A.-C.; Lasnon, C. Baseline (18)F-FDG PET Radiomic Features as Predictors of 2-Year Event-Free Survival in Diffuse Large B Cell Lymphomas Treated with Immunochemotherapy. Eur. Radiol. 2020, 30, 4623–4632. [Google Scholar] [CrossRef]
- Coskun, N.; Okudan, B.; Uncu, D.; Kitapci, M.T. Baseline 18F-FDG PET Textural Features as Predictors of Response to Chemotherapy in Diffuse Large B-Cell Lymphoma. Nucl. Med. Commun. 2021, 42, 1227–1232. [Google Scholar] [CrossRef]
- Han, E.J.; O, J.H.; Yoon, H.; Ha, S.; Yoo, I.R.; Min, J.W.; Choi, J.-I.; Choi, B.-O.; Park, G.; Lee, H.H.; et al. Comparison of FDG PET/CT and Bone Marrow Biopsy Results in Patients with Diffuse Large B Cell Lymphoma with Subgroup Analysis of PET Radiomics. Diagnostics 2022, 12, 222. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Li, L.; Tian, R. Development and Validation of an (18)F-FDG PET Radiomic Model for Prognosis Prediction in Patients with Nasal-Type Extranodal Natural Killer/T Cell Lymphoma. Eur. Radiol. 2020, 30, 5578–5587. [Google Scholar] [CrossRef]
- Aide, N.; Talbot, M.; Fruchart, C.; Damaj, G.; Lasnon, C. Diagnostic and Prognostic Value of Baseline FDG PET/CT Skeletal Textural Features in Diffuse Large B Cell Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Faudemer, J.; Aide, N.; Gac, A.-C.; Damaj, G.; Vilque, J.-P.; Lasnon, C. Diagnostic Value of Baseline (18)FDG PET/CT Skeletal Textural Features in Follicular Lymphoma. Sci. Rep. 2021, 11, 23812. [Google Scholar] [CrossRef]
- Ceriani, L.; Milan, L.; Cascione, L.; Gritti, G.; Dalmasso, F.; Esposito, F.; Pirosa, M.C.; Schär, S.; Bruno, A.; Dirnhofer, S.; et al. Generation and Validation of a PET Radiomics Model That Predicts Survival in Diffuse Large B Cell Lymphoma Treated with R-CHOP14: A SAKK 38/07 Trial Post-Hoc Analysis. Hematol. Oncol. 2022, 40, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.E.; Dai, D.; Xu, G.; Zhao, R.; Li, T.; Pan, T.; Wang, L.; Lin, Y.; Wang, Z.; Jaffray, D.; et al. Lesion-Based Radiomics Signature in Pretherapy 18F-FDG PET Predicts Treatment Response to Ibrutinib in Lymphoma. Clin. Nucl. Med. 2022, 47, 209–218. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, F.M.; Yin, Y.; Mantzorou-Kyriaki, E.; Kahle, X.U.; de Haas, R.J.; Yakar, D.; Glaudemans, A.W.J.M.; Noordzij, W.; Kwee, T.C.; Nijland, M. Machine Learning in the Differentiation of Follicular Lymphoma from Diffuse Large B-Cell Lymphoma with Radiomic [(18)F]FDG PET/CT Features. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1535–1543. [Google Scholar] [CrossRef]
- Jiang, C.; Li, A.; Teng, Y.; Huang, X.; Ding, C.; Chen, J.; Xu, J.; Zhou, Z. Optimal PET-Based Radiomic Signature Construction Based on the Cross-Combination Method for Predicting the Survival of Patients with Diffuse Large B-Cell Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, X.-L.; Pu, L.-T.; Zhou, R.-F.; Ou, X.-J.; Tian, R. Prediction of Overall Survival and Progression-Free Survival by the (18)F-FDG PET/CT Radiomic Features in Patients with Primary Gastric Diffuse Large B-Cell Lymphoma. Contrast Media Mol. Imaging 2019, 2019, 5963607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lue, K.-H.; Wu, Y.-F.; Lin, H.-H.; Hsieh, T.-C.; Liu, S.-H.; Chan, S.-C.; Chen, Y.-H. Prognostic Value of Baseline Radiomic Features of (18)F-FDG PET in Patients with Diffuse Large B-Cell Lymphoma. Diagnostics 2020, 11, 36. [Google Scholar] [CrossRef]
- Lue, K.-H.; Wu, Y.-F.; Liu, S.-H.; Hsieh, T.-C.; Chuang, K.-S.; Lin, H.-H.; Chen, Y.-H. Prognostic Value of Pretreatment Radiomic Features of 18F-FDG PET in Patients With Hodgkin Lymphoma. Clin. Nucl. Med. 2019, 44, e559–e565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, Y.; Chen, Z.; Li, J.; Sang, S.; Deng, S. Radiomic Features of (18)F-FDG PET in Hodgkin Lymphoma Are Predictive of Outcomes. Contrast Media Mol. Imaging 2021, 2021, 6347404. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Riedl, C.C.; Kumar, A.; Gibbs, P.; Weber, M.; Tal, I.; Schilksy, J.; Schöder, H. Radiomic Features of Glucose Metabolism Enable Prediction of Outcome in Mantle Cell Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2760–2769. [Google Scholar] [CrossRef] [Green Version]
- Lippi, M.; Gianotti, S.; Fama, A.; Casali, M.; Barbolini, E.; Ferrari, A.; Fioroni, F.; Iori, M.; Luminari, S.; Menga, M.; et al. Texture Analysis and Multiple-Instance Learning for the Classification of Malignant Lymphomas. Comput. Methods Programs Biomed. 2020, 185, 105153. [Google Scholar] [CrossRef]
- Sun, Y.; Qiao, X.; Jiang, C.; Liu, S.; Zhou, Z. Texture Analysis Improves the Value of Pretreatment (18)F-FDG PET/CT in Predicting Interim Response of Primary Gastrointestinal Diffuse Large B-Cell Lymphoma. Contrast Media Mol. Imaging 2020, 2020, 2981585. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Isohashi, K.; Matsunaga, K.; Watabe, T.; Kato, H.; Kanakura, Y.; Hatazawa, J. Volumetric and Texture Analysis on FDG PET in Evaluating and Predicting Treatment Response and Recurrence after Chemotherapy in Follicular Lymphoma. Int. J. Clin. Oncol. 2019, 24, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, C.; Xin, B.; Zheng, C.; Zhao, Y.; Hao, K.; Wang, Q.; Wahl, R.L.; Wang, X.; Zhou, Y. (18)F-FDG PET/CT Radiomic Analysis with Machine Learning for Identifying Bone Marrow Involvement in the Patients with Suspected Relapsed Acute Leukemia. Theranostics 2019, 9, 4730–4739. [Google Scholar] [CrossRef] [PubMed]
- Ripani, D.; Caldarella, C.; Za, T.; Rossi, E.; De Stefano, V.; Giordano, A. Progression to Symptomatic Multiple Myeloma Predicted by Texture Analysis-Derived Parameters in Patients Without Focal Disease at (18)F-FDG PET/CT. Clin. Lymphoma Myeloma Leuk. 2021, 21, 536–544. [Google Scholar] [CrossRef]
- Vallières, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A Radiomics Model from Joint FDG-PET and MRI Texture Features for the Prediction of Lung Metastases in Soft-Tissue Sarcomas of the Extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Yamamoto, M.; Shono, K.; Yamada, S.; Tsuyoshi, H.; Kiyono, Y.; Kimura, H.; Okazawa, H.; Yoshida, Y. Assessment of Intratumor Heterogeneity in Mesenchymal Uterine Tumor by an (18)F-FDG PET/CT Texture Analysis. Ann. Nucl. Med. 2017, 31, 752–757. [Google Scholar] [CrossRef]
- Song, H.; Jiao, Y.; Wei, W.; Ren, X.; Shen, C.; Qiu, Z.; Yang, Q.; Wang, Q.; Luo, Q.-Y. Can Pretreatment (18)F-FDG PET Tumor Texture Features Predict the Outcomes of Osteosarcoma Treated by Neoadjuvant Chemotherapy? Eur. Radiol. 2019, 29, 3945–3954. [Google Scholar] [CrossRef]
- Peng, Y.; Bi, L.; Guo, Y.; Feng, D.; Fulham, M.; Kim, J. Deep Multi-Modality Collaborative Learning for Distant Metastases Predication in PET-CT Soft-Tissue Sarcoma Studies. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, July 23–27 2019; pp. 3658–3688. [Google Scholar] [CrossRef]
- Sheen, H.; Kim, W.; Byun, B.H.; Kong, C.-B.; Song, W.S.; Cho, W.H.; Lim, I.; Lim, S.M.; Woo, S.-K. Metastasis Risk Prediction Model in Osteosarcoma Using Metabolic Imaging Phenotypes: A Multivariable Radiomics Model. PLoS ONE 2019, 14, e0225242. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Wang, G.; Guo, J. PET/MR Fusion Texture Analysis for the Clinical Outcome Prediction in Soft-Tissue Sarcoma. Cancer Imaging 2022, 22, 7. [Google Scholar] [CrossRef]
- Wolsztynski, E.; O’Sullivan, F.; Keyes, E.; O’Sullivan, J.; Eary, J.F. Positron Emission Tomography-Based Assessment of Metabolic Gradient and Other Prognostic Features in Sarcoma. J. Med. Imaging Bellingham Wash 2018, 5, 024502. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, W.; Byun, B.H.; Kong, C.-B.; Song, W.S.; Lim, I.; Lim, S.M.; Woo, S.-K. Prediction of Chemotherapy Response of Osteosarcoma Using Baseline (18)F-FDG Textural Features Machine Learning Approaches with PCA. Contrast Media Mol. Imaging 2019, 2019, 3515080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jeong, S.Y.; Kim, B.-C.; Byun, B.-H.; Lim, I.; Kong, C.-B.; Song, W.S.; Lim, S.M.; Woo, S.-K. Prediction of Neoadjuvant Chemotherapy Response in Osteosarcoma Using Convolutional Neural Network of Tumor Center (18)F-FDG PET Images. Diagnostics 2021, 11, 1976. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-C.; Kim, J.; Kim, K.; Byun, B.H.; Lim, I.; Kong, C.-B.; Song, W.S.; Koh, J.-S.; Woo, S.-K. Preliminary Radiogenomic Evidence for the Prediction of Metastasis and Chemotherapy Response in Pediatric Patients with Osteosarcoma Using (18)F-FDF PET/CT, EZRIN and KI67. Cancers 2021, 13, 2671. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kido, S.; Suga, K.; Hirano, Y.; Tachibana, R.; Muramatsu, K.; Chagawa, K.; Tanaka, S. Texture Analysis on (18)F-FDG PET/CT Images to Differentiate Malignant and Benign Bone and Soft-Tissue Lesions. Ann. Nucl. Med. 2014, 28, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Sharma, A.; Sharma, A.; Bakhshi, S.; Patel, C.; Pandey, A.K.; Dhamija, E.; Batra, A.; Kumar, R. Utility of 18F-FDG-PET/CT in Management and Prognostication of Treatment Naïve Late-Stage Soft Tissue Sarcomas. Nucl. Med. Commun. 2021, 42, 818–825. [Google Scholar] [CrossRef]
- Basler, L.; Gabryś, H.S.; Hogan, S.A.; Pavic, M.; Bogowicz, M.; Vuong, D.; Tanadini-Lang, S.; Förster, R.; Kudura, K.; Huellner, M.W.; et al. Radiomics, Tumor Volume, and Blood Biomarkers for Early Prediction of Pseudoprogression in Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibition. Clin. Cancer Res. 2020, 26, 4414–4425. [Google Scholar] [CrossRef] [Green Version]
- Saadani, H.; van der Hiel, B.; Aalbersberg, E.A.; Zavrakidis, I.; Haanen, J.B.A.G.; Hoekstra, O.S.; Boellaard, R.; Stokkel, M.P.M. Metabolic Biomarker-Based BRAFV600 Mutation Association and Prediction in Melanoma. J. Nucl. Med. 2019, 60, 1545–1552. [Google Scholar] [CrossRef]
- Ishiwata, Y.; Kaneta, T.; Nawata, S.; Iizuka, H.; Utsunomiya, D. Feasibility of Prognosis Assessment for Cancer of Unknown Primary Origin Using Texture Analysis of 18F-Fluorodeoxyglucose PET/Computed Tomography Images of Largest Metastatic Lymph Node. Nucl. Med. Commun. 2021, 42, 86–92. [Google Scholar] [CrossRef]
- Yadav, D.; Shamim, S.A.; Rastogi, S.; Upadhyay, D.M.R.; Pandey, A.K.; Kumar, R. Role of 18F-FDG PET/Computed Tomography in Prognostication and Management of Malignant Peripheral Nerve Sheath Tumors. Nucl. Med. Commun. 2020, 41, 924–932. [Google Scholar] [CrossRef]
- Rahmim, A.; Bak-Fredslund, K.P.; Ashrafinia, S.; Lu, L.; Schmidtlein, C.R.; Subramaniam, R.M.; Morsing, A.; Keiding, S.; Horsager, J.; Munk, O.L. Prognostic Modeling for Patients with Colorectal Liver Metastases Incorporating FDG PET Radiomic Features. Eur. J. Radiol. 2019, 113, 101–109. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, H.; Yin, Y.; Zhang, J.; Yang, M.; Qin, S.; Zhang, X.; Yu, F. Texture Analysis of (18)F-FDG PET/CT for Differential Diagnosis Spinal Metastases. Front. Med. 2020, 7, 605746. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.; Bundschuh, L.; Zsótér, N.; Essler, M.; Bundschuh, R.A. Tumor Heterogeneity for Differentiation between Liver Tumors and Normal Liver Tissue in 18F-FDG PET/CT. Nukl. Nucl. Med. 2021, 60, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.A.; Hwang, D.; Cen, S.Y.; Levy, J.; Liu, D.; Lau, C.; Rivas, M.; Desai, B.; Goodenough, D.J.; Duddalwar, V.A. Reliability of CT-based Texture Features: Phantom Study. J. Appl. Clin. Med. Phys. 2019, 20, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Fiel, M.; Moscoso, A.; Pubul, V.; Ruibal, Á.; Silva-Rodríguez, J.; Aguiar, P. A Systematic Review of PET Textural Analysis and Radiomics in Cancer. Diagnostics 2021, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Da-Ano, R.; Lucia, F.; Masson, I.; Abgral, R.; Alfieri, J.; Rousseau, C.; Mervoyer, A.; Reinhold, C.; Pradier, O.; Schick, U.; et al. A Transfer Learning Approach to Facilitate ComBat-Based Harmonization of Multicentre Radiomic Features in New Datasets. PLoS ONE 2021, 16, e0253653. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Bousabarah, K.; Hoevels, M.; Treuer, H. Radiomics in Radiation Oncology—Basics, Methods, and Limitations. Strahlenther. Onkol. 2020, 196, 848–855. [Google Scholar] [CrossRef]
- Bluemke, D.A.; Moy, L.; Bredella, M.A.; Ertl-Wagner, B.B.; Fowler, K.J.; Goh, V.J.; Halpern, E.F.; Hess, C.P.; Schiebler, M.L.; Weiss, C.R. Assessing Radiology Research on Artificial Intelligence: A Brief Guide for Authors, Reviewers, and Readers—From the Radiology Editorial Board. Radiology 2020, 294, 487–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, S.A.; Ibrahim, A.; Woodruff, H.C.; Andrearczyk, V.; Müller, H.; Primakov, S.; Salahuddin, Z.; Chatterjee, A.; Lambin, P. Making Radiomics More Reproducible across Scanner and Imaging Protocol Variations: A Review of Harmonization Methods. J. Pers. Med. 2021, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Nyflot, M.J.; Yang, F.; Byrd, D.; Bowen, S.R.; Sandison, G.A.; Kinahan, P.E. Quantitative Radiomics: Impact of Stochastic Effects on Textural Feature Analysis Implies the Need for Standards. J. Med. Imaging 2015, 2, 041002. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).