Modified Immunoscore Improves Prediction of Survival Outcomes in Patients Undergoing Radical Cystectomy for Bladder Cancer—A Retrospective Digital Pathology Study
Abstract
:1. Introduction
2. Materials and Methods
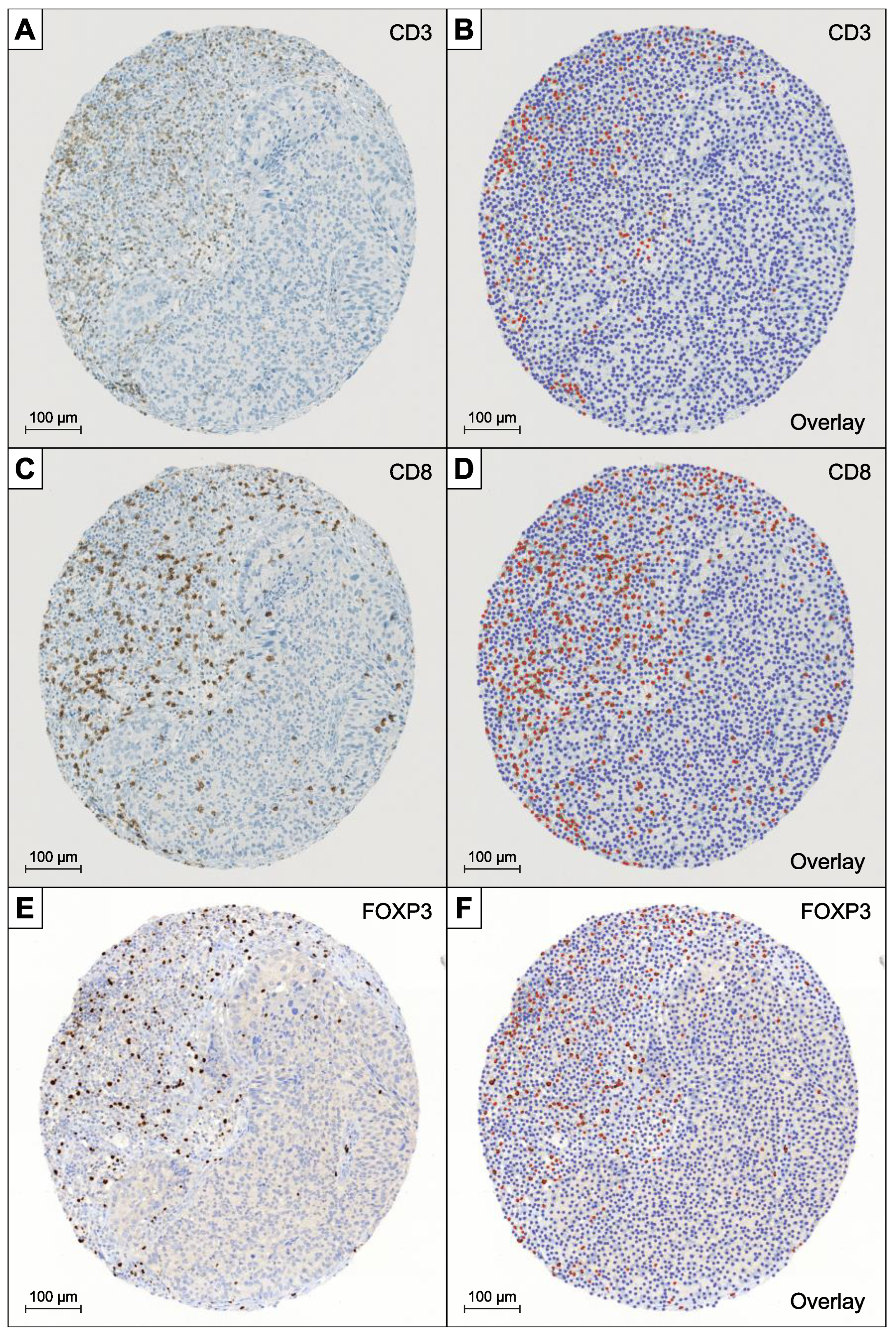
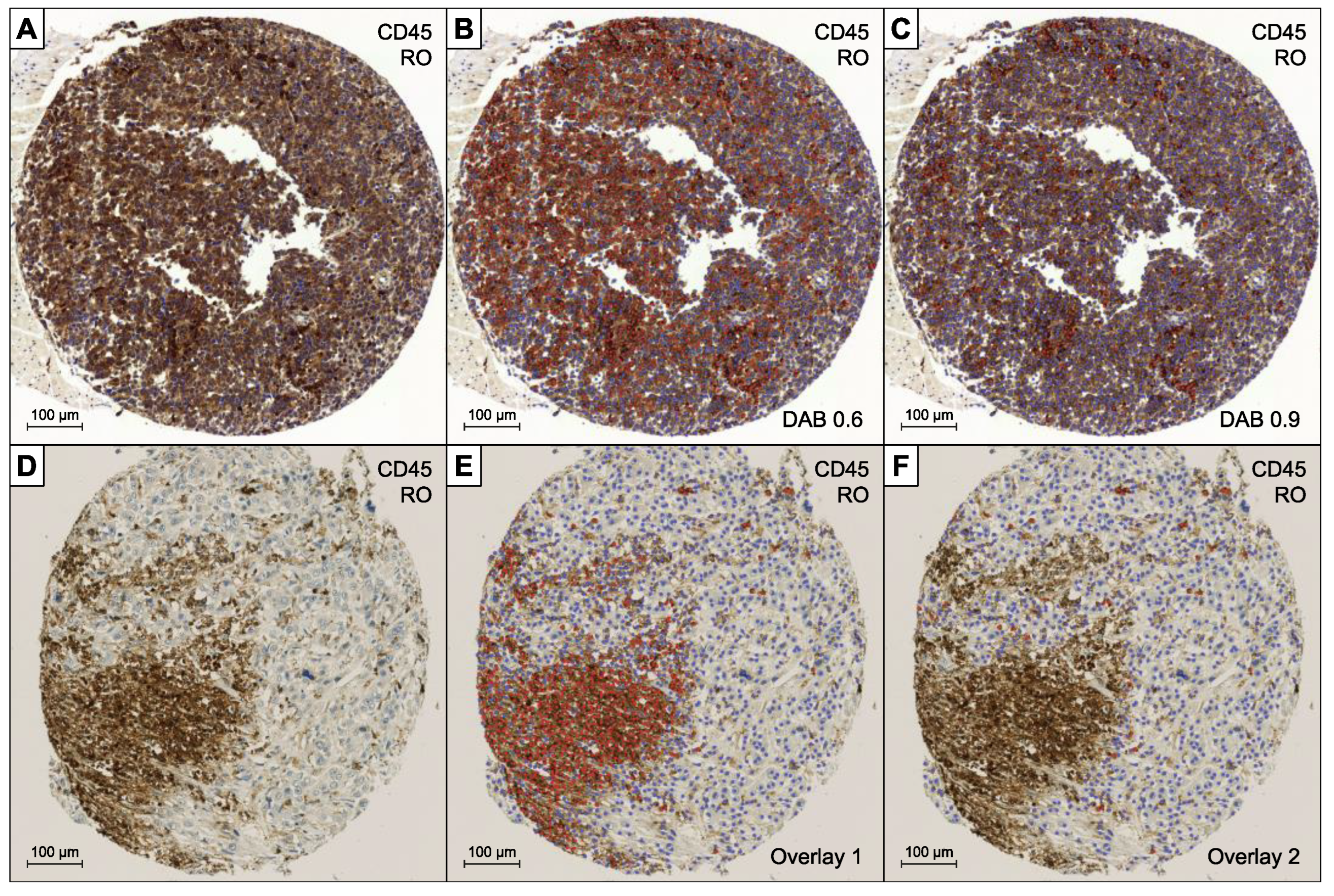
2.1. BC Tissue Microarray and Immunohistochemistry
- [1]
- QuPath’s automated “TMA dearrayer” was used to identify tissue cores. The resulting TMA grid was manually verified and amended where necessary.
- [2]
- Stain vector and background estimates were applied to improve stain separation using color deconvolution by QuPath’s “Estimate stain vectors” command.
- [3]
- QuPath’s built-in “Simple tissue detection” and “Fast cell counts” commands were applied. The measurements were visually controlled, and the parameters manually adjusted by a board-certified pathologist (LB) until convincing results could be achieved. In particular the “thresholdDAB value” determining the cut-off for positive cell count had to be adapted in dependence of the different staining intensities of the antibodies.
- [4]
- Output was cumulated, averaged, and reported as positive counts (pc), negative counts (nc), ratio (pc/pc + nc), and density (pc/mm2).
2.2. Construction of the Modified Immunoscore Prediction Model
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Immunhistochemistry
3.3. Univariable Analysis
3.4. Multivariable Analysis
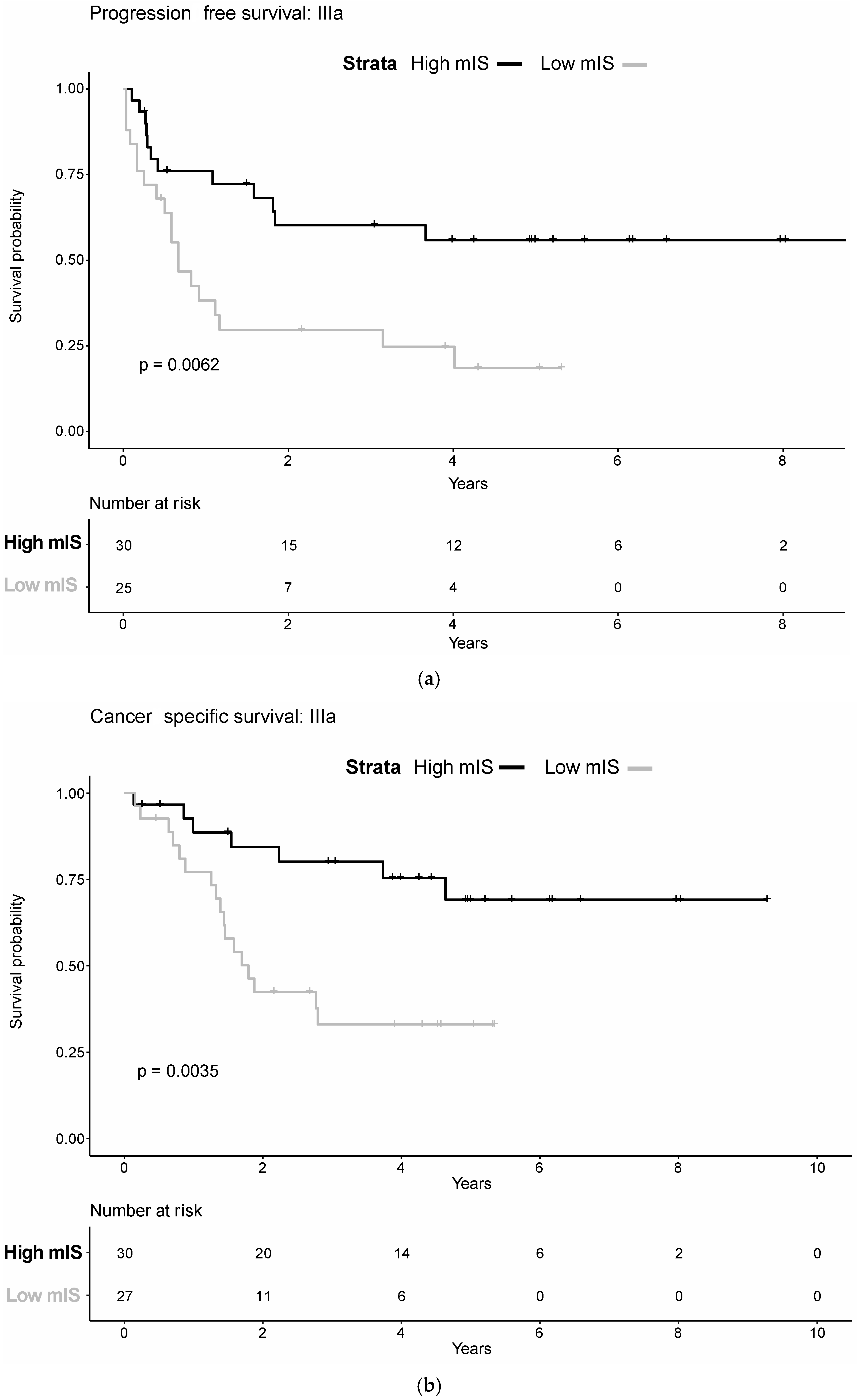
3.5. AJCC Sub-Stratification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Bochner, B.; Thangathurai, D.; Mikhail, M.; Raghavan, D.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Kamat, A.M.; Gee, J.R.; Dinney, C.P.N.; Grossman, H.B.; Swanson, D.A.; Millikan, R.E.; Detry, M.A.; Robinson, T.L.; Pisters, L.L. The Case for Early Cystectomy in the Treatment of Nonmuscle Invasive Micropapillary Bladder Carcinoma. J. Urol. 2006, 175, 881–885. [Google Scholar] [CrossRef]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; Gakis, G.; Hernández, V.; Lebret, T.; Lorch, A.; van der Heijden, A.G.; Ribal, M.J. Muscle-invasive and Metastatic Bladder Cancer-EAU Guidelines. EAU-Website 2016. Available online: http://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/ (accessed on 15 October 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, A.M.; Bellmunt, J.; Galsky, M.D.; Konety, B.R.; Lamm, D.L.; Langham, D.; Lee, C.T.; Milowsky, M.I.; O’Donnell, M.A.; O’Donnell, P.H.; et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma. J. Immunother. Cancer 2017, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C. Combining epigenetic and immune checkpoint inhibitors in bladder cancer. Nat. Rev. Urol. 2019, 16, 507. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer-A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hegarty, P.K.; Gee, J.R.; Clark, P.E.; Svatek, R.S.; Hegarty, N.; Shariat, S.F.; Xylinas, E.; Schmitz-Dräger, B.J.; Lotan, Y.; et al. ICUD-EAU international consultation on bladder cancer 2012: Screening, diagnosis, and molecular markers. Eur. Urol. 2013, 63, 4–15. [Google Scholar] [CrossRef]
- Pierconti, F.; Martini, M.; Cenci, T.; Fiorentino, V.; Di Gianfrancesco, L.; Ragonese, M.; Bientinesi, R.; Rossi, E.; Larocca, L.M.; Racioppi, M.; et al. The bladder epicheck test and cytology in the follow-up of patients with non-muscle-invasive high grade bladder carcinoma. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 108.e19–108.e25. [Google Scholar] [CrossRef]
- Pierconti, F.; Martini, M.; Cenci, T.; Fiorentino, V.; Sacco, E.; Bientinesi, R.; Pugliese, D.; Iacovelli, R.; Schinzari, G.; Larocca, L.M.; et al. Methylation study of the Paris system for reporting urinary (TPS) categories. J. Clin. Pathol. 2021, 74, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Pierconti, F.; Martini, M.; Fiorentino, V.; Cenci, T.; Capodimonti, S.; Straccia, P.; Sacco, E.; Pugliese, D.; Cindolo, L.; Larocca, L.M.; et al. The combination cytology/epichek test in non muscle invasive bladder carcinoma follow-up: Effective tool or useless expence? Urol. Oncol. Semin. Orig. Investig. 2021, 39, 131.e17–131.e21. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; American Joint Commission on Cancer: Cham, Switzerland, 2017. [Google Scholar]
- Mathieu, R.; Lucca, I.; Rouprêt, M.; Briganti, A.; Shariat, S.F. The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat. Rev. Urol. 2016, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ke, Z.B.; Lin, X.D.; Chen, Y.H.; Wu, Y.P.; Chen, Y.; Dong, R.N.; Chen, S.H.; Li, X.D.; Wei, Y.; et al. Development and validation of a molecular prognostic index of bladder cancer based on immunogenomic landscape analysis. Cancer Cell Int. 2020, 20, 302. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Angell, H.K.; Bedognetti, D.; Marincola, F.M. The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signatures|Elsevier Enhanced Reader. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Galon, J.; Pagès, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Du, L.; Zhou, Y.; Li, K. The prognostic value of Immunoscore in patients with cancer: A pooled analysis of 10,328 patients. Int. J. Biol. Markers 2020, 35, 3–35. [Google Scholar] [CrossRef]
- Yu, A.; Mansure, J.J.; Solanki, S.; Siemens, D.R.; Koti, M.; Dias, A.B.T.; Burnier, M.M.; Brimo, F.; Kassouf, W. Presence of lymphocytic infiltrate cytotoxic T lymphocyte CD3+, CD8+, and immunoscore as prognostic marker in patients after radical cystectomy. PLoS ONE 2018, 13, e0205746. [Google Scholar] [CrossRef]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [Green Version]
- Ingels, A.; Salas, R.E.S.; Ravery, V.; Fromont-Hankard, G.; Validire, P.; Patard, J.J.; Pignot, G.; Prapotnich, D.; Olivier, F.; Galiano, M.; et al. T-helper 1 immunoreaction influences survival in muscle-invasive bladder cancer: Proof of concept. Ecancermedicalscience 2014, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Horn, T.; Laus, J.; Seitz, A.K.; Maurer, T.; Schmid, S.C.; Wolf, P.; Haller, B.; Winkler, M.; Retz, M.; Nawroth, R.; et al. The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World J. Urol. 2016, 34, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Roudnicky, F.; Poyet, C.; Buser, L.; Saba, K.; Wild, P.; Otto, V.I.; Detmar, M. Characterization of Tumor Blood Vasculature Expression of Human Invasive Bladder Cancer by Laser Capture Microdissection and Transcriptional Profiling. Am. J. Pathol. 2020, 190, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Kononen, J.; Bubendorf, L.; Kallioniemi, A.; Bärlund, M.; Schraml, P.; Leighton, S.; Torhorst, J.; Mihatsch, M.J.; Sauter, G.; Kallionimeni, O.P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998, 4, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Roudnicky, F.; Poyet, C.; Wild, P.; Krampitz, S.; Negrini, F.; Huggenberger, R.; Rogler, A.; Stöhr, R.; Hartmann, A.; Provenzano, M.; et al. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Res. 2013, 73, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Loughrey, M.B.; Bankhead, P.; Coleman, H.G.; Hagan, R.S.; Craig, S.; McCorry, A.M.B.; Gray, R.T.; McQuaid, S.; Dunne, P.D.; Hamilton, P.W.; et al. Validation of the systematic scoring of immunohistochemically stained tumour tissue microarrays using QuPath digital image analysis. Histopathology 2018, 73, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Compérat, E.; Gontero, P.; Mostafid, A.H.; Palou, J.; Van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; Zigeuner, R. Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS) EAU Guidelines on; EAU Guidelines Office: Arnhem, The Netherlands, 2020. [Google Scholar]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. J. Immunol. 2017, 198, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Winerdal, M.E.; Marits, P.; Winerdal, M.; Hasan, M.; Rosenblatt, R.; Tolf, A.; Selling, K.; Sherif, A.; Winqvist, O. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011, 108, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.H.; Pagès, F.; et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef] [PubMed]
| Variable | Categorization | n Analyzable | Percent |
|---|---|---|---|
| Total (n = 158) † | |||
| Age at diagnosis Median Follow-up | Median, Range 68 (44–87) 36 months (Range 0.1–111.3) | ||
| Sex | Male | 122 | 77.2 |
| Female | 36 | 22.8 | |
| Tumor Stage | pTa | 8 | 5.1 |
| pTis | 1 | 0.6 | |
| pT1 | 26 | 16.5 | |
| pT2 | 40 | 25.3 | |
| pT3 | 55 | 34.8 | |
| pT4 | 28 | 17.7 | |
| Histological Grade ‡ | missing | 2 | 1.3 |
| G2 | 7 | 4.4 | |
| G3 | 149 | 94.3 | |
| Histological Grade § | missing | 2 | 1.3 |
| Low Grade | 7 | 4.5 | |
| High Grade | 149 | 94.3 | |
| Adjacent Carcinoma in situ | Yes | 68 | 43.1 |
| No | 90 | 57.0 | |
| Perineural Invasion | missing | 66 | 41.8 |
| Yes | 23 | 14.6 | |
| No | 69 | 43.7 | |
| Vascular Invasion | Yes | 32 | 20.3 |
| No | 126 | 79.7 | |
| Lymph-vascular Invasion | Yes | 56 | 35.4 |
| No | 102 | 64.6 | |
| Lymph Node metastasis | Yes | 53 | 33.6 |
| No | 105 | 66.5 | |
| AJCC Stage | 0a | 8 | 5.1 |
| 0is | 1 | 0.6 | |
| I | 22 | 13.9 | |
| II | 34 | 21.5 | |
| IIIa | 57 | 36.1 | |
| IIIb | 36 | 22.8 | |
| Neoadjuvant Chemotherapy | Yes | 7 | 4.4 |
| No | 151 | 95.6 | |
| Variable | PFS:HR | 95% CI | p Value | CSS:HR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Age (continuous per year) | 1.00 | 0.98–1.03 | 0.7669 | 1.00 | 0.97–1.03 | 0.9467 |
| Gender | ||||||
| Female | ref | ref | ||||
| Male | 1.16 | 0.69–1.95 | 0.5805 | 1.17 | 0.65–2.11 | 0.5909 |
| Pathological | ||||||
| Grade † (continuous G2/G3) | 1.23 | 0.39–3.91 | 0.7266 | 0.99 | 0.31–3.18 | 0.9929 |
| Cis | 0.99 | 0.62–1.5 | 0.546 | 1.21 | 0.73–2.02 | 0.4616 |
| Pn | 4.61 | 2.39–8.88 | <0.0001 | 4.58 | 2.27–9.21 | <0.0001 |
| V | 2.42 | 1.41–4.13 | 0.0013 | 2.71 | 1.55–4.74 | 0.0005 |
| LVI | 3.50 | 2.19–5.58 | <0.0001 | 4.65 | 2.72–7.92 | <0.0001 |
| AJCC-Stages | ||||||
| 0a/0is/I | ref | ref | ||||
| II | 1.59 | 0.64–3.95 | 0.3194 | 0.87 | 0.28–2.74 | 0.8092 |
| IIIa | 3.26 | 1.50–7.11 | 0.0029 | 2.60 | 1.12–6.05 | 0.0263 |
| IIIb | 6.69 | 2.96–15.14 | <0.0001 | 7.51 | 3.18–17.76 | <0.0001 |
| Immuno-histological markers | ||||||
| CD3 ‡ (continuous) | 0.89 | 0.79–1.01 | 0.690 | 0.82 | 0.72–0.95 | 0.0060 |
| CD8 ‡ (continuous) | 0.82 | 0.72–0.94 | 0.0054 | 0.75 | 0.64–0.88 | 0.005 |
| CD45RO ‡ (continuous) | 0.78 | 0.65–0.93 | 0.0063 | 0.77 | 0.63–0.93 | 0.0082 |
| FOXP3 ‡ (continuous) | 0.74 | 0.60–0.91 | 0.0036 | 0.65 | 0.52–0.81 | 0.0001 |
| Modfied Immunoscore (mIS) | ||||||
| Low mIS | ref | |||||
| High mIS | 0.43 | 0.27–0.70 | 0.0005 | 0.33 | 0.18–0.57 | <0.0001 |
| Variable | PFS.HR | 95% CI | p Value | CSS.HR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| AJCC-Stages | ||||||
| 0a/0is/I | ref | ref | ||||
| II | 2.24 | 0.87–5.76 | 0.0947 | 1.29 | 0.39–4.23 | 0.6754 |
| IIIa | 3.93 | 1.77–8.71 | 0.0008 | 3.21 | 1.36–7.58 | 0.0079 |
| IIIb | 7.53 | 3.24–17.5 | <0.0001 | 9.30 | 3.77–22.92 | <0.0001 |
| Immuno-histological markers | ||||||
| CD3 | 1.07 | 0.86–1.35 | 0.5422 | 0.91 | 0.71–1.16 | 0.4373 |
| CD8 | 1.03 | 0.82–1.28 | 0.8262 | 0.94 | 0.74–1.18 | 0.5732 |
| CD45RO | 0.86 | 0.60–1.23 | 0.3957 | 1.23 | 0.83–1.83 | 0.2926 |
| FOXP3 | 0.70 | 0.52–0.95 | 0.0208 | 0.64 | 0.46–0.89 | 0.0080 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieri, U.; Buser, L.; Wettstein, M.S.; Eberli, D.; Saba, K.; Moch, H.; Hermanns, T.; Poyet, C. Modified Immunoscore Improves Prediction of Survival Outcomes in Patients Undergoing Radical Cystectomy for Bladder Cancer—A Retrospective Digital Pathology Study. Diagnostics 2022, 12, 1360. https://doi.org/10.3390/diagnostics12061360
Bieri U, Buser L, Wettstein MS, Eberli D, Saba K, Moch H, Hermanns T, Poyet C. Modified Immunoscore Improves Prediction of Survival Outcomes in Patients Undergoing Radical Cystectomy for Bladder Cancer—A Retrospective Digital Pathology Study. Diagnostics. 2022; 12(6):1360. https://doi.org/10.3390/diagnostics12061360
Chicago/Turabian StyleBieri, Uwe, Lorenz Buser, Marian Severin Wettstein, Daniel Eberli, Karim Saba, Holger Moch, Thomas Hermanns, and Cédric Poyet. 2022. "Modified Immunoscore Improves Prediction of Survival Outcomes in Patients Undergoing Radical Cystectomy for Bladder Cancer—A Retrospective Digital Pathology Study" Diagnostics 12, no. 6: 1360. https://doi.org/10.3390/diagnostics12061360
APA StyleBieri, U., Buser, L., Wettstein, M. S., Eberli, D., Saba, K., Moch, H., Hermanns, T., & Poyet, C. (2022). Modified Immunoscore Improves Prediction of Survival Outcomes in Patients Undergoing Radical Cystectomy for Bladder Cancer—A Retrospective Digital Pathology Study. Diagnostics, 12(6), 1360. https://doi.org/10.3390/diagnostics12061360







