Altered Transmission of Cardiac Cycles to Ductus Venosus Blood Flow in Fetal Growth Restriction: Why Ductus Venosus Reflects Fetal Circulatory Changes More Precisely
Abstract
:1. Introduction
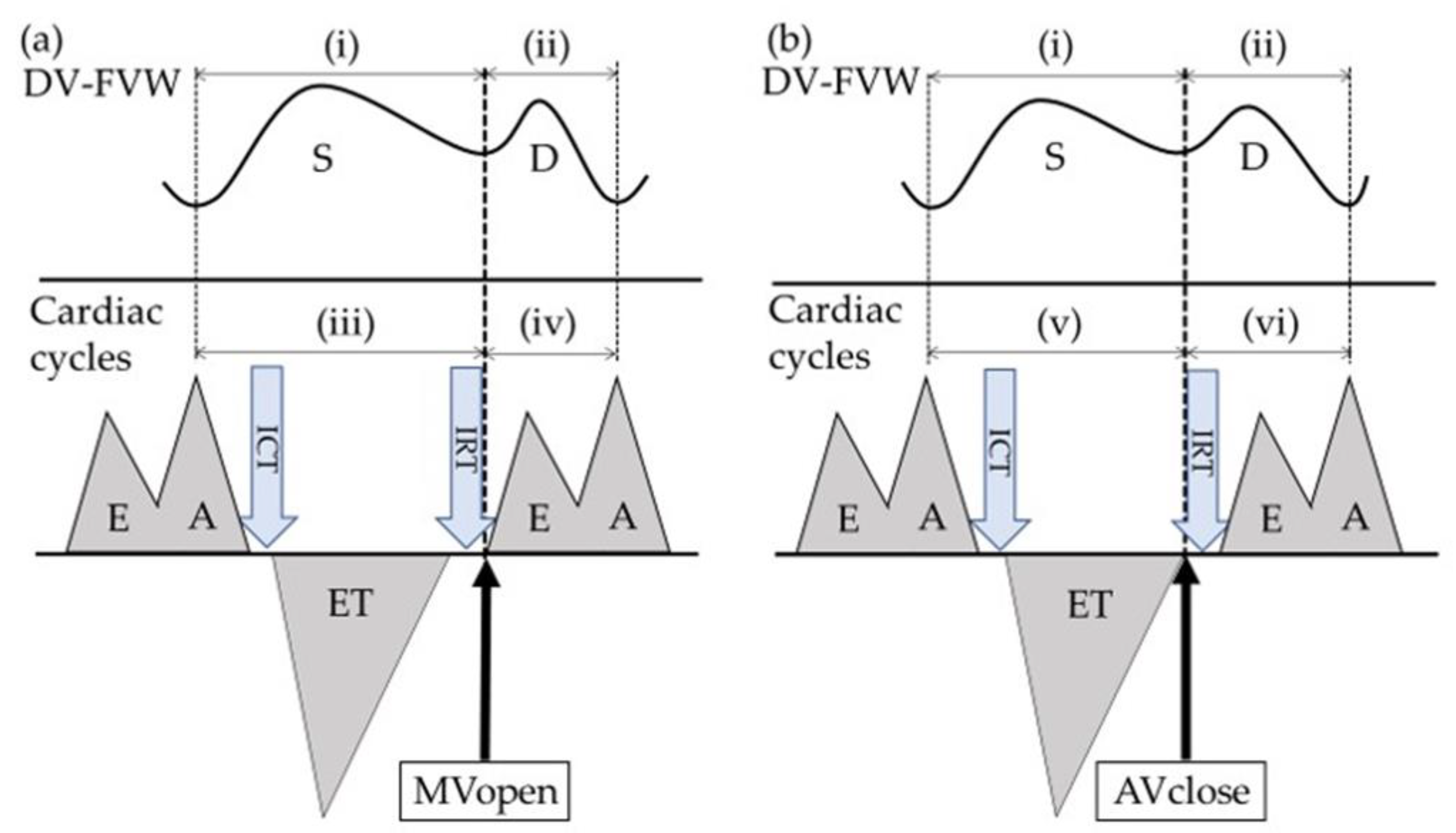
2. Materials and Methods
2.1. Study Design, Ethical Approval, and Study Population
2.2. Statistical Analysis
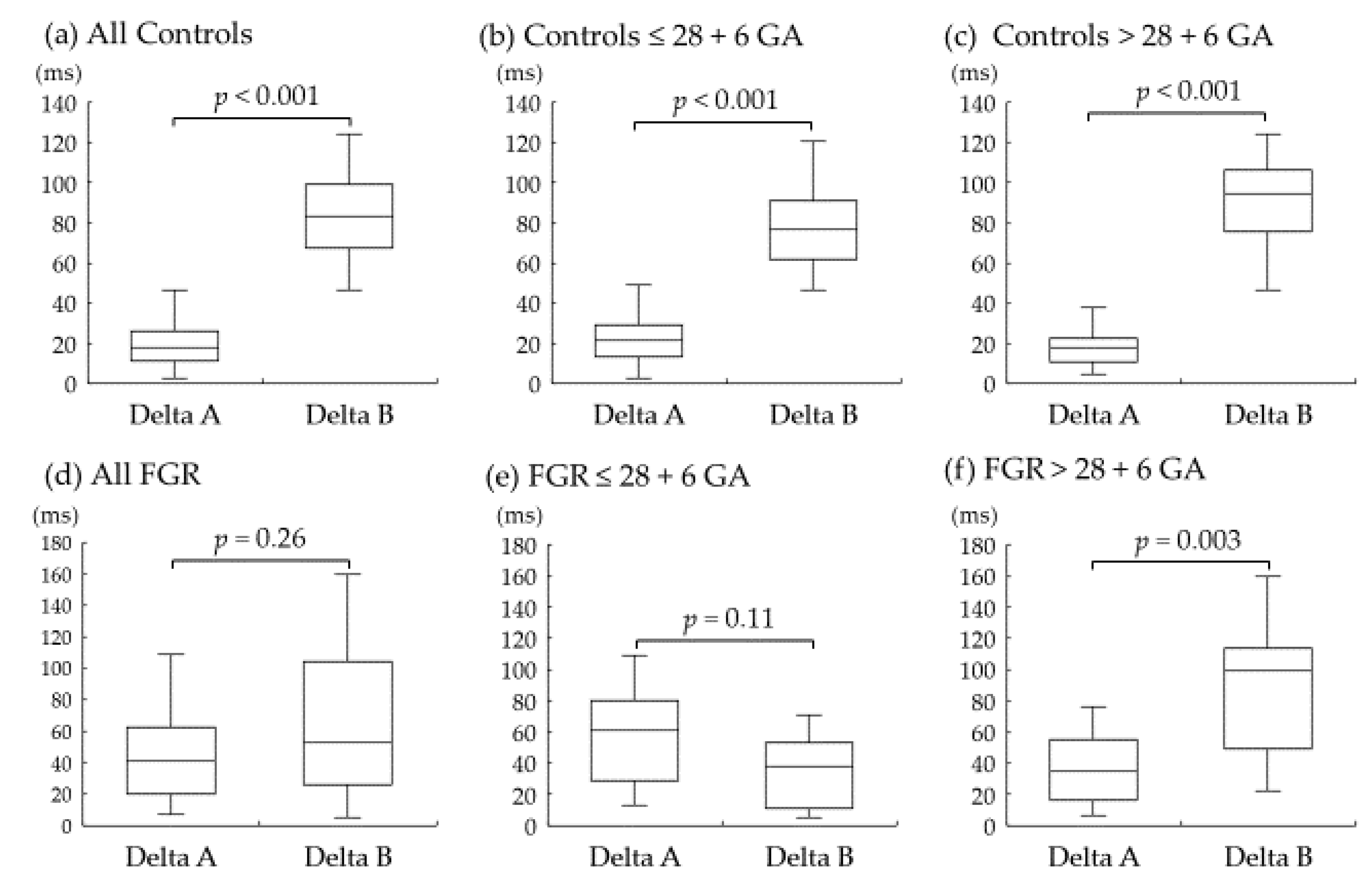
3. Results
4. Discussion
4.1. Main Findings and Importance
4.2. Representative Alterations in Doppler Measurements of Ductus Venosus in FGR
4.3. Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganzevoort, W.; Van Charante, N.M.; Thilaganathan, B.; Prefumo, F.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. How to monitor pregnancies complicated by fetal growth restriction and delivery before 32 weeks: Post-hoc analysis of TRUFFLE study. Ultrasound Obstet. Gynecol. 2017, 49, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, H.; Arabin, B.; Lees, C.C.; Oepkes, D.; Prefumo, F.; Thilaganathan, B.; Todros, T.; Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; et al. Longitudinal study of computerized cardiotocography in early fetal growth restriction: Computerized cardiotocography in early fetal growth restriction. Ultrasound Obstet. Gynecol. 2017, 50, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frusca, T.; Todros, T.; Lees, C.; Bilardo, C.M.; Hecher, K.; Visser, G.H.A.; Papageorghiou, A.T.; Marlow, N.; Thilaganathan, B.; van Wassenaer-Leemhuis, A.; et al. Outcome in early-onset fetal growth restriction is best combining computerized fetal heart rate analysis with ductus venosus Doppler: Insights from the Trial of Umbilical and Fetal Flow in Europe. Am. J. Obstet. Gynecol. 2018, 218, S783–S789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; Ferrazzi, E.; Fratelli, N.; Frusca, T.; Ganzevoort, W.; Lees, C.C.; Napolitano, R.; Todros, T.; et al. Fetal monitoring indications for delivery and 2-year outcome in 310 infants with fetal growth restriction delivered before 32 weeks’ gestation in the TRUFFLE study: TRUFFLE study sub-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hecher, K.; Bilardo, C.M.; Stigter, R.H.; Ville, Y.; Hackelöer, B.J.; Kok, H.J.; Senat, M.V.; Visser, G.H.A. Monitoring of fetuses with intrauterine growth restriction: A longitudinal study: Longitudinal fetal monitoring. Ultrasound Obstet. Gynecol. 2001, 18, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baschat, A.A.; Cosmi, E.; Bilardo, C.M.; Wolf, H.; Berg, C.; Rigano, S.; Germer, U.; Moyano, D.; Turan, S.; Hartung, J.; et al. Predictors of Neonatal Outcome in Early-Onset Placental Dysfunction. Obstet. Gynecol. 2007, 109, 253–261. [Google Scholar] [CrossRef]
- Nakagawa, K.; Tachibana, D.; Nobeyama, H.; Fukui, M.; Sumi, T.; Koyama, M.; Ishiko, O.; Hecher, K. Reference ranges for time-related analysis of ductus venosus flow velocity waveforms in singleton pregnancies. Prenat. Diagn. 2012, 32, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Tachibana, D.; Kurihar, Y.; Nakagawa, K.; Nakano, A.; Terada, H.; Tanaka, K.; Fukui, M.; Koyama, M.; Hecher, K. Alterations in time intervals of ductus venosus and atrioventricular flow velocity waveforms in growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2015, 46, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Sanapo, L.; Turan, O.M.; Turan, S.; Ton, J.; Atlas, M.; Baschat, A.A. Correlation analysis of ductus venosus velocity indices and fetal cardiac function: Ductus venosus waveform and fetal cardiac function. Ultrasound Obstet. Gynecol. 2014, 43, 515–519. [Google Scholar] [CrossRef]
- Shinozuka, N.; Okai, T.; Kohzuma, S.; Mukubo, M.; Shih, C.T.; Maeda, T.; Kuwabara, Y.; Mizuno, M. Formulas for fetal weight estimation by ultrasound measurements based on neonatal specific gravities and volumes. Am. J. Obstet. Gynecol. 1987, 157, 1140–1145. [Google Scholar] [CrossRef]
- Satoh, S.; Koyanagi, T.; Fukuhara, M.; Hara, K.; Nakano, H. Changes in vascular resistance in the umbilical and middle cerebral arteries in the human intrauterine growth-retarded fetus, measured with pulsed Doppler ultrasound. Early Hum. Dev. 1989, 20, 213–220. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Lopez-Tenorio, J.; Figueroa-Diesel, H.; Sanin-Blair, J.; Carreras, E.; Cabero, L.; Gratacos, E. A modified myocardial performance (Tei) index based on the use of valve clicks improves reproducibility of fetal left cardiac function assessment: Reproducibility of a modified fetal myocardial performance index. Ultrasound Obstet. Gynecol. 2005, 26, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Suekane, T.; Tachibana, D.; Kurihara, Y.; Yokoi, N.; Seo, N.; Kitada, K.; Tahara, M.; Hamuro, A.; Misugi, T.; Nakano, A.; et al. Time interval analysis of ductus venosus and cardiac cycles in relation with umbilical artery pH at birth in fetal growth restriction. BMC Pregnancy Childbirth 2021, 21, 671. [Google Scholar] [CrossRef] [PubMed]
- Seravalli, V.; Miller, J.L.; Block-Abraham, D.; Baschat, A.A. Ductus venosus Doppler in the assessment of fetal cardiovascular heal.th: An updated practical approach. Acta Obstet. Gynecol. Scand. 2016, 95, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Lees, C.C.; Marlow, N.; van Wassenaer-Leemhuis, A.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Calvert, S.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): A randomised trial. Lancet 2015, 385, 2162–2172. [Google Scholar] [CrossRef]
- Rizzo, G.; Capponi, A.; Rinaldo, D.; Arduini, D.; Romanini, C. Ventricular ejection force in growth-retarded fetuses. Ultrasound Obstet. Gynecol. 1995, 5, 247–255. [Google Scholar] [CrossRef]
- Richardson, B.S.; Bocking, A.D. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 717–723. [Google Scholar] [CrossRef]
- Patey, O.; Carvalho, J.S.; Thilaganathan, B. Perinatal changes in cardiac geometry and function in growth-restricted fetuses at term. Ultrasound Obstet. Gynecol. 2019, 53, 655–662. [Google Scholar] [CrossRef]
- Steel, S.A.; Pearce, J.M.; Nash, G.; Christopher, B.; Dormandy, J.; Bland, J.M. Correlation between Doppler flow velocity waveforms and cord blood viscosity. Br. J. Obstet. Gynaecol. 1989, 96, 1168–1172. [Google Scholar] [CrossRef]
- Jopling, J.; Henry, E.; Wiedmeier, S.E.; Christensen, R.D. Reference Ranges for Hematocrit and Blood Hemoglobin Concentration During the Neonatal Period: Data from a Multihospital Health Care System. Pediatrics 2009, 123, e333–e337. [Google Scholar] [CrossRef] [Green Version]
- Turan, O.M.; Turan, S.; Sanapo, L.; Rosenbloom, J.I.; Baschat, A.A. Semiquantitative classification of ductus venosus blood flow patterns: Ductus venosus waveform patterns. Ultrasound Obstet. Gynecol. 2014, 43, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Privitera, A.A.; Fiore, M.; Valenti, G.; Raniolo, S.; Schiattarella, A.; Riemma, G.; Gullo, G.; Sgalambro, F.; Garofalo, S.; D’Amico, S.; et al. The role of serum potassium and sodium levels in the development of postpartum hemorrhage. A retrospective study. Ital. J. Gynaecol. Obstet. 2020, 32, 126–135. [Google Scholar] [CrossRef]
- Margioula-Siarkou, G.; Margioula-Siarkou, C.; Petousis, S.; Margaritis, K.; Vavoulidis, E.; Gullo, G.; Alexandratou, M.; Dinas, K.; Sotiriadis, A.; Mavromatidis, G. The role of endoglin and its soluble form in pathogenesis of preeclampsia. Mol. Cell. Biochem. 2022, 477, 479–491. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Controls (n = 60) | FGR (n = 23) | p |
|---|---|---|---|
| Age (years) | 32.0 (18 to 44) | 33.0 (22 to 44) | 0.61 |
| Nulliparous/parous | 30/30 | 17/6 | 0.05 |
| GA (weeks) | 28.9 (25.0 to 33.6) | 29.0 (26.0 to 33.4) | 0.98 |
| GA (Weeks) at Examination: | |||
|---|---|---|---|
| Outcome | All FGR (n = 23) | ≤28 + 6 (n = 11) | >28 + 6 (n =12) |
| GA at delivery (weeks) | 29.1 (26.1 to 33.9) | 27.6 (26.1 to 29.1) | 30.6 (29.0 to 33.9) |
| Measurement before delivery (days) | 2.0 (0 to 5.0) | 1.0 (0 to 5.0) | 2.0 (0 to 4.0) |
| Birth weight (g) | 726 (328 to 1256) | 502 (328 to 760) | 859 (576 to 1256) |
| 1-min Apgar score | 5 (1 to 9) | 5 (1 to 7) | 6 (3 to 9) |
| 5-min Apgar score | 8 (3 to 9) | 6 (3 to 9) | 8 (6 to 9) |
| Umbilical artery pH | 7.25 (6.99 to 7.33) | 7.25 (6.99 to 7.32) | 7.24 (7.03 to 7.33) |
| Umbilical artery base excess | −4.1 (−14.5 to 2.3) | −3.4 (−11.0 to 2.3) | −4.8 (−14.5 to 0.9) |
| Neonatal hematocrit (%) | 49.7 (35.0 to 64.4) | 49.3 (35.0 to 55.2) | 55.4 (44.2 to 64.4) |
| Parameter | Controls (n = 60) | FGR (n = 23) | p |
|---|---|---|---|
| ICT (ms) | 31.1 (20.0 to 57.8) | 31.1 (20.0 to 42.2) | 0.68 |
| ET (ms) | 165.6 (144.4 to 188.9) | 166.7 (140.0 to 191.1) | 0.81 |
| IRT (ms) | 44.4 (31.1 to 55.6) | 46.7 (28.9 to 75.2) | 0.39 |
| MPI | 0.47 (0.29 to 0.66) | 0.49 (0.30 to 0.69) | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, N.; Kurihara, Y.; Suekane, T.; Yokoi, N.; Nakagawa, K.; Tahara, M.; Hamuro, A.; Misugi, T.; Nakano, A.; Koyama, M.; et al. Altered Transmission of Cardiac Cycles to Ductus Venosus Blood Flow in Fetal Growth Restriction: Why Ductus Venosus Reflects Fetal Circulatory Changes More Precisely. Diagnostics 2022, 12, 1393. https://doi.org/10.3390/diagnostics12061393
Seo N, Kurihara Y, Suekane T, Yokoi N, Nakagawa K, Tahara M, Hamuro A, Misugi T, Nakano A, Koyama M, et al. Altered Transmission of Cardiac Cycles to Ductus Venosus Blood Flow in Fetal Growth Restriction: Why Ductus Venosus Reflects Fetal Circulatory Changes More Precisely. Diagnostics. 2022; 12(6):1393. https://doi.org/10.3390/diagnostics12061393
Chicago/Turabian StyleSeo, Naomi, Yasushi Kurihara, Tomoki Suekane, Natsuko Yokoi, Kayoko Nakagawa, Mie Tahara, Akihiro Hamuro, Takuya Misugi, Akemi Nakano, Masayasu Koyama, and et al. 2022. "Altered Transmission of Cardiac Cycles to Ductus Venosus Blood Flow in Fetal Growth Restriction: Why Ductus Venosus Reflects Fetal Circulatory Changes More Precisely" Diagnostics 12, no. 6: 1393. https://doi.org/10.3390/diagnostics12061393
APA StyleSeo, N., Kurihara, Y., Suekane, T., Yokoi, N., Nakagawa, K., Tahara, M., Hamuro, A., Misugi, T., Nakano, A., Koyama, M., & Tachibana, D. (2022). Altered Transmission of Cardiac Cycles to Ductus Venosus Blood Flow in Fetal Growth Restriction: Why Ductus Venosus Reflects Fetal Circulatory Changes More Precisely. Diagnostics, 12(6), 1393. https://doi.org/10.3390/diagnostics12061393






