Diffusion-Weighted Imaging Prior to Percutaneous Sclerotherapy of Venous Malformations—Proof of Concept Study for Prediction of Clinical Outcome
Abstract
:1. Introduction
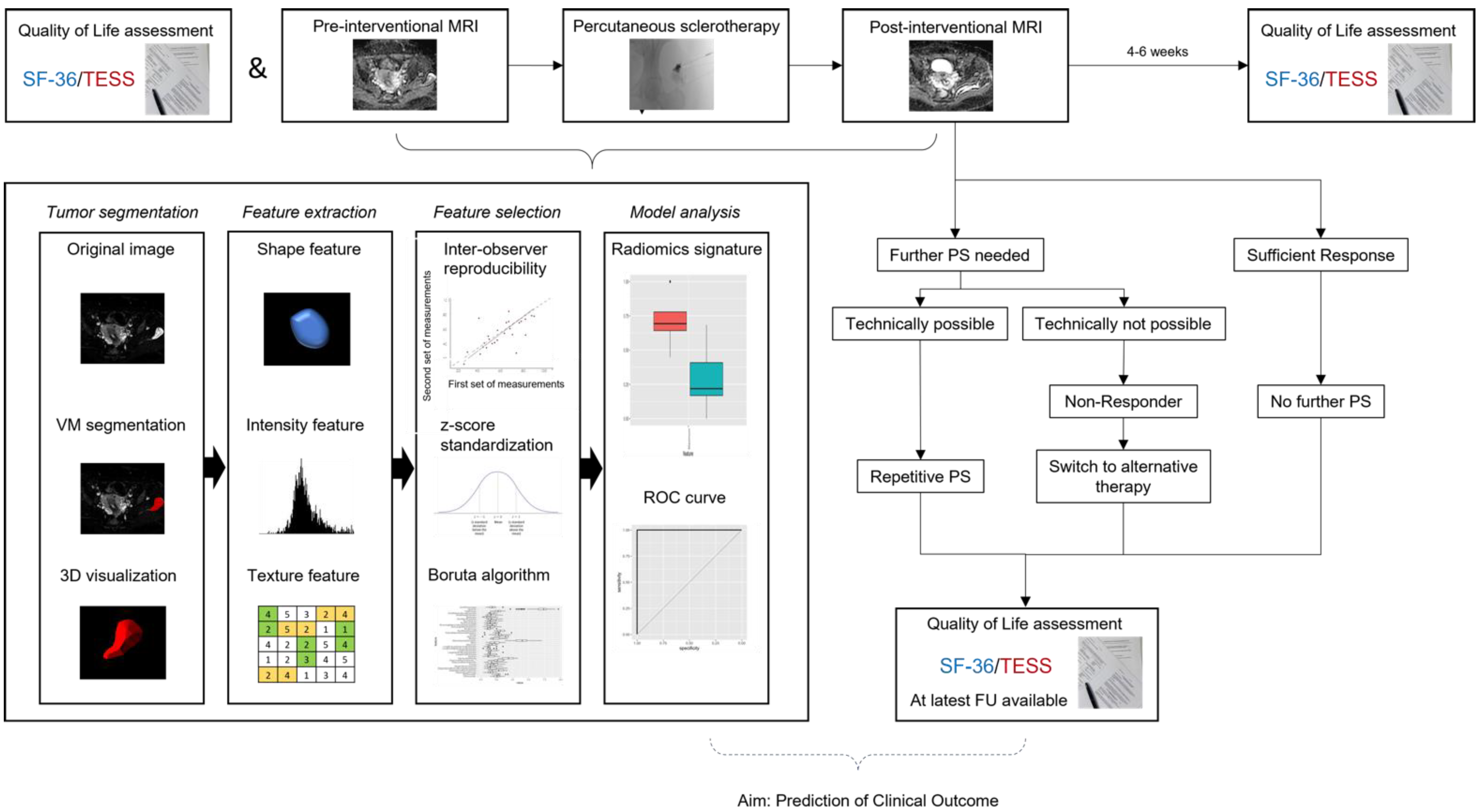
2. Materials and Methods
2.1. Study Design
2.2. MRI Examination
2.3. Interventional Therapy
2.4. Clinical Outcome and Quality-of-Life
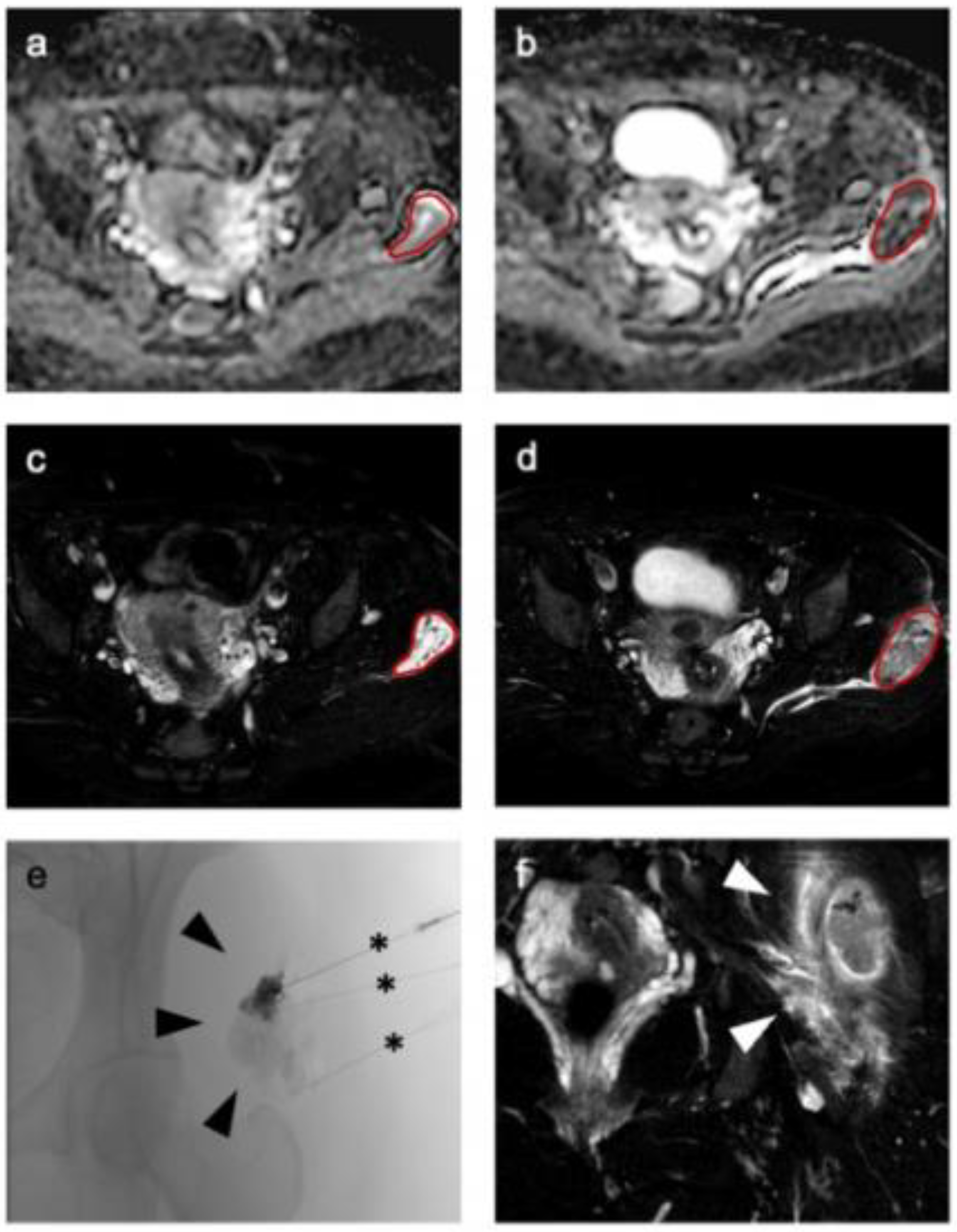
2.5. Image Analysis and Radiomic Feature Extraction
2.6. Radiomic Feature Selection and Dimension Reduction for Differentiation of Response to Interventional Therapy
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Interventional Therapy
3.3. Clinical Outcome—Quality-of-Life
3.4. Outcome Prediction–Binary Logistic Regression
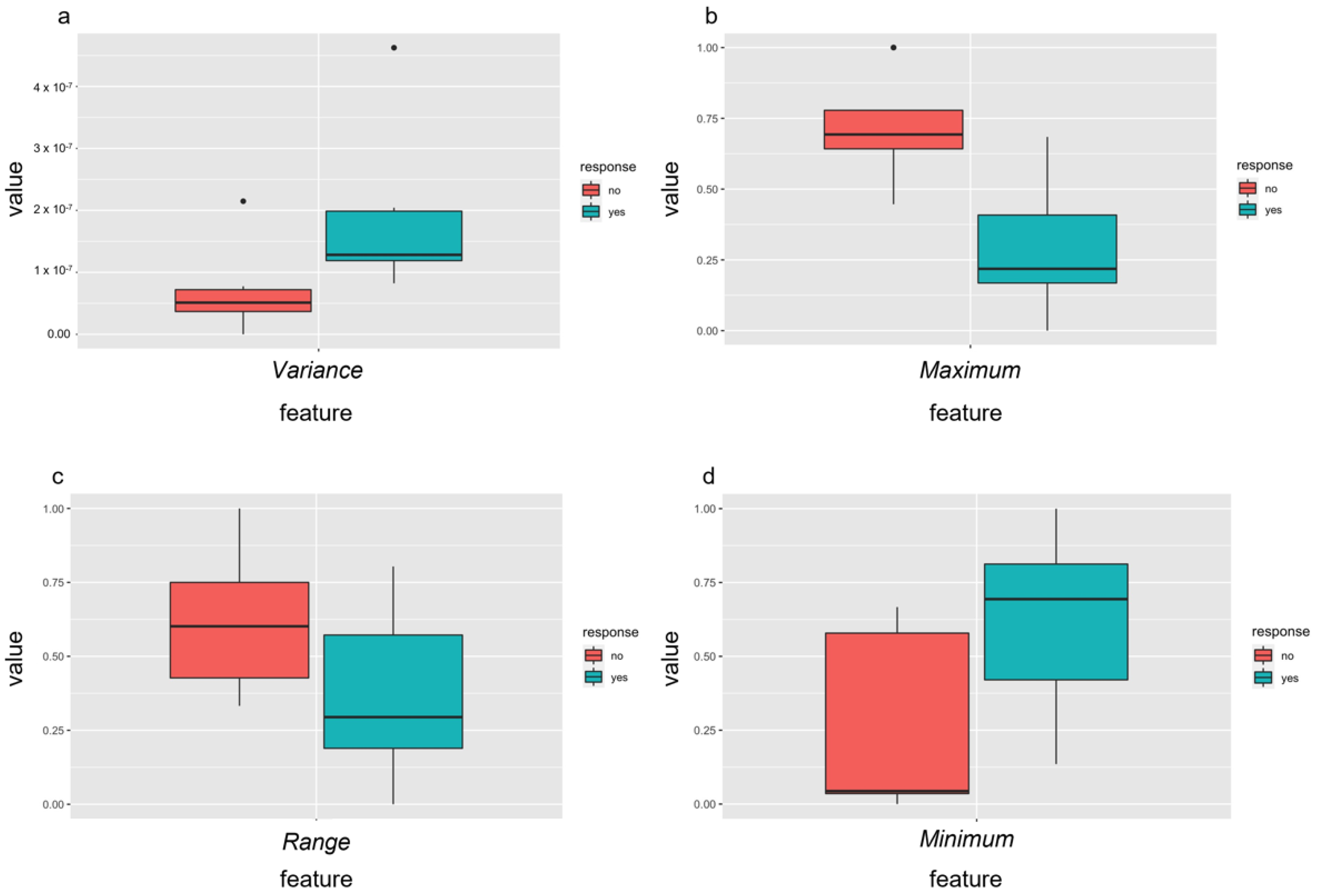
3.5. Outcome Prediction—Radiomics Features before Therapy
3.6. Outcome Prediction—Delta Radiomic Features before and after the First Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.C.; et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, V.F.; Masthoff, M.; Goldann, C.; Deniz, S.; Ocal, O.; Haberle, B.; Kohler, M.; Seidensticker, M.; Ricke, J.; Wohlgemuth, W.A.; et al. Percutaneous Sclerotherapy of Venous Malformations of the Hand: A Multicenter Analysis. Cardiovasc. Interv. Radiol. 2021, 44, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Bedoya, M.A.; Gaballah, M.; Low, D.W.; Cahill, A.M. Percutaneous sclerotherapy of foot venous malformations: Evaluation of clinical response. Clin. Radiol. 2014, 69, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Muller-Wille, R.; Wildgruber, M.; Sadick, M.; Wohlgemuth, W.A. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular Malformations. RoFo Fortschr. Geb. Rontgenstrahlen Nuklearmed. 2018, Online ahead of print. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, V.F.; Masthoff, M.; Czihal, M.; Cucuruz, B.; Haberle, B.; Brill, R.; Wohlgemuth, W.A.; Wildgruber, M. Imaging of peripheral vascular malformations—Current concepts and future perspectives. Mol. Cell Pediatr. 2021, 8, 19. [Google Scholar] [CrossRef]
- Favrole, P.; Guichard, J.P.; Crassard, I.; Bousser, M.G.; Chabriat, H. Diffusion-weighted imaging of intravascular clots in cerebral venous thrombosis. Stroke 2004, 35, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Reichel, T.; Fleckenstein, J.L. Diffusion findings in blood clot: The last word? AJNR Am. J. Neuroradiol. 2004, 25, 157. [Google Scholar]
- Lin, P.; Yang, P.F.; Chen, S.; Shao, Y.Y.; Xu, L.; Wu, Y.; Teng, W.; Zhou, X.Z.; Li, B.H.; Luo, C.; et al. A Delta-radiomics model for preoperative evaluation of Neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imag. 2020, 20, 7. [Google Scholar] [CrossRef] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Yang, L.; Gu, D.; Wei, J.; Yang, C.; Rao, S.; Wang, W.; Chen, C.; Ding, Y.; Tian, J.; Zeng, M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 373–386. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louthrenoo, W.; Kasitanon, N.; Morand, E.; Kandane-Rathnayake, R. Comparison of performance of specific (SLEQOL) and generic (SF36) health-related quality of life questionnaires and their associations with disease status of systemic lupus erythematosus: A longitudinal study. Arthritis Res. Ther. 2020, 22, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.M.; Wright, J.G.; Williams, J.I.; Bombardier, C.; Griffin, A.; Bell, R.S. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996, 5, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Clayer, M.; Doyle, S.; Sangha, N.; Grimer, R. The toronto extremity salvage score in unoperated controls: An age, gender, and country comparison. Sarcoma 2012, 2012, 717213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masthoff, M.; Gerwing, M.; Schneider, K.N.; Kohler, M.; Deventer, N.; Schindler, P.; Heindel, W.; Hardes, J.; Seidensticker, M.; Gosheger, G.; et al. Combined Transarterial Embolization and Percutaneous Sclerotherapy as Treatment for Refractory and Nonresectable Aneurysmal Bone Cysts. J. Vasc. Interv. Radiol. 2021, 32, 1425–1434.e1422. [Google Scholar] [CrossRef] [PubMed]
- Martin-Carreras, T.; Li, H.; Cooper, K.; Fan, Y.; Sebro, R. Radiomic features from MRI distinguish myxomas from myxofibrosarcomas. BMC Med. Imaging 2019, 19, 67. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imag. 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Baessler, B.; Mannil, M.; Maintz, D.; Alkadhi, H.; Manka, R. Texture analysis and machine learning of non-contrast T1-weighted MR images in patients with hypertrophic cardiomyopathy-Preliminary results. Eur. J. Radiol. 2018, 102, 61–67. [Google Scholar] [CrossRef]
- Xu, J.W.; Suzuki, K. Max-AUC feature selection in computer-aided detection of polyps in CT colonography. IEEE J. Biomed. Health Inform. 2014, 18, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Roll, W.; Schindler, P.; Masthoff, M.; Seifert, R.; Schlack, K.; Bogemann, M.; Stegger, L.; Weckesser, M.; Rahbar, K. Evaluation of (68)Ga-PSMA-11 PET-MRI in Patients with Advanced Prostate Cancer Receiving (177)Lu-PSMA-617 Therapy: A Radiomics Analysis. Cancers 2021, 13, 3849. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.N.; Singh, A.; Nazir, S.A.; Kanagala, P.; Gershlick, A.H.; McCann, G.P. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur. J. Radiol. 2015, 84, 840–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Baessler, B.; Mannil, M.; Oebel, S.; Maintz, D.; Alkadhi, H.; Manka, R. Subacute and chronic left ventricular myocardial scar: Accuracy of texture analysis on nonenhanced cine MR images. Radiology 2018, 286, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, J.S.; Park, H.; Kim, J.Y.; Huh, S.; Lee, J.M.; Lee, S.Y.; Lee, S.J.; Lee, J.S.; Lee, J.W.; et al. Venous malformations of the head and neck: A retrospective review of 82 cases. Arch. Plast. Surg. 2019, 46, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Behravesh, S.; Yakes, W.; Gupta, N.; Naidu, S.; Chong, B.W.; Khademhosseini, A.; Oklu, R. Venous malformations: Clinical diagnosis and treatment. Cardiovasc. Diagn. Ther. 2016, 6, 557–569. [Google Scholar] [CrossRef]
- Wohlgemuth, W.A.; Muller-Wille, R.; Teusch, V.; Hammer, S.; Wildgruber, M.; Uller, W. Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children—Results of a prospective study. Eur. Radiol. 2017, 27, 2482–2488. [Google Scholar] [CrossRef]
- Kask, G.; Barner-Rasmussen, I.; Repo, J.P.; Kjaldman, M.; Kilk, K.; Blomqvist, C.; Tukiainen, E.J. Functional Outcome Measurement in Patients with Lower-Extremity Soft Tissue Sarcoma: A Systematic Literature Review. Ann. Surg. Oncol. 2019, 26, 4707–4722. [Google Scholar] [CrossRef] [Green Version]
- Wohlgemuth, W.A.; Muller-Wille, R.; Meyer, L.; Wildgruber, M.; Guntau, M.; Heydt, S.V.; Pech, M.; Zanasi, A.; Flother, L.; Brill, R. Bleomycin electrosclerotherapy in therapy-resistant venous malformations of the body. J. Vasc Surg Venous Lymphat Disord 2021, 9, 731–739. [Google Scholar] [CrossRef]
- Kalin-Hajdu, E.; Colby, J.B.; Idowu, O.; Grumbine, F.L.; Kang, J.M.; Hirabayashi, K.S.; Glastonbury, C.M.; Vagefi, M.R.; Kersten, R.C. Diagnosing Distensible Venous Malformations of the Orbit With Diffusion-weighted Magnetic Resonance Imaging. Am. J. Ophthalmol. 2018, 189, 146–154. [Google Scholar] [CrossRef]
- Wu, G.; Morelli, J.; Xiong, Y.; Liu, X.; Li, X. Diffusion weighted cardiovascular magnetic resonance imaging for discriminating acute from non-acute deep venous Thrombus. J. Cardiovasc. Magn. Reson. 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwing, M.; Krahling, T.; Schliemann, C.; Harrach, S.; Schwoppe, C.; Berdel, A.F.; Klein, S.; Hartmann, W.; Wardelmann, E.; Heindel, W.L.; et al. Multiparametric Magnetic Resonance Imaging for Immediate Target Hit Assessment of CD13-Targeted Tissue Factor tTF-NGR in Advanced Malignant Disease. Cancers 2021, 13, 5880. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butova, X.; Shayakhmetov, S.; Fedin, M.; Zolotukhin, I.; Gianesini, S. Artificial Intelligence Evidence-Based Current Status and Potential for Lower Limb Vascular Management. J. Pers Med. 2021, 11, 1280. [Google Scholar] [CrossRef]
- Coroller, T.P.; Agrawal, V.; Huynh, E.; Narayan, V.; Lee, S.W.; Mak, R.H.; Aerts, H. Radiomic-Based Pathological Response Prediction from Primary Tumors and Lymph Nodes in NSCLC. J. Thorac Oncol. 2017, 12, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Sadick, M.; Overhoff, D.; Baessler, B.; von Spangenberg, N.; Krebs, L.; Wohlgemuth, W.A. Peripheral Vascular Anomalies—Essentials in Periinterventional Imaging. RoFo Fortschr. Geb. Rontgenstrahlen Nuklearmed. 2020, 192, 150–162. [Google Scholar] [CrossRef]
- Masthoff, M.; Helfen, A.; Claussen, J.; Karlas, A.; Markwardt, N.A.; Ntziachristos, V.; Eisenblatter, M.; Wildgruber, M. Use of Multispectral Optoacoustic Tomography to Diagnose Vascular Malformations. JAMA Dermatol. 2018, 154, 1457–1462. [Google Scholar] [CrossRef]


| ID | Age, y | Sex | Localization | Previous Therapy | No of Used Accesses | Sclerosing Agent | Quantity, mL | No of Performed Therapies | Follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | F | forearm | none | 4 | gelified ethanol/polidocanol | 2/2 | 2 | 19 |
| 2 | 24 | F | lower leg | none | 1 | gelified ethanol | 1 | 1 | 17 |
| 3 | 9 | M | elbow | none | 2 | polidocanol | 2 | 2 | 18 |
| 4 | 8 | F | lower leg | none | 3 | polidocanol | 2 | 2 | 17 |
| 5 | 46 | F | knee | none | 4 | gelified ethanol/polidocanol | 3/1 | 4 | 20 |
| 6 | 48 | M | forearm | resection | 3 | polidocanol | 2 | 3 | 16 |
| 7 | 25 | F | thigh | none | 1 | gelified ethanol | 2 | 4 | 15 |
| 8 | 17 | F | forearm | none | 2 | gelified ethanol | 2 | 1 | 12 |
| 9 | 21 | F | forearm | none | 1 | gelified ethanol | 2 | 1 | 9 |
| 10 | 25 | F | forearm | none | 1 | gelified ethanol | 1 | 3 | 12 |
| 11 | 26 | F | thigh | none | 4 | gelified ethanol | 4 | 2 | 10 |
| 12 | 34 | F | thigh | none | 3 | polidocanol | 4 | 3 | 8 |
| 13 | 15 | M | thigh/knee | none | 5 | polidocanol | 4 | 4 | 12 |
| 14 | 39 | F | forearm | resection/laser therapy | 1 | polidocanol | 4 | 3 | 5 |
| 15 | 18 | F | thoracic wall | resection | 4 | gelified ethanol/polidocanol | 2/2 | 3 | 8 |
| 16 | 49 | M | forearm | none | 4 | polidocanol | 4 | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerwing, M.; Schindler, P.; Schneider, K.N.; Sundermann, B.; Köhler, M.; Stamm, A.-C.; Schmidt, V.F.; Perkowski, S.; Deventer, N.; Heindel, W.L.; et al. Diffusion-Weighted Imaging Prior to Percutaneous Sclerotherapy of Venous Malformations—Proof of Concept Study for Prediction of Clinical Outcome. Diagnostics 2022, 12, 1430. https://doi.org/10.3390/diagnostics12061430
Gerwing M, Schindler P, Schneider KN, Sundermann B, Köhler M, Stamm A-C, Schmidt VF, Perkowski S, Deventer N, Heindel WL, et al. Diffusion-Weighted Imaging Prior to Percutaneous Sclerotherapy of Venous Malformations—Proof of Concept Study for Prediction of Clinical Outcome. Diagnostics. 2022; 12(6):1430. https://doi.org/10.3390/diagnostics12061430
Chicago/Turabian StyleGerwing, Mirjam, Philipp Schindler, Kristian Nikolaus Schneider, Benedikt Sundermann, Michael Köhler, Anna-Christina Stamm, Vanessa Franziska Schmidt, Sybille Perkowski, Niklas Deventer, Walter L. Heindel, and et al. 2022. "Diffusion-Weighted Imaging Prior to Percutaneous Sclerotherapy of Venous Malformations—Proof of Concept Study for Prediction of Clinical Outcome" Diagnostics 12, no. 6: 1430. https://doi.org/10.3390/diagnostics12061430









