The Effect of Face Masks during COVID-19 Pandemic on Ocular Surface Temperature—A Clinical Thermographic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
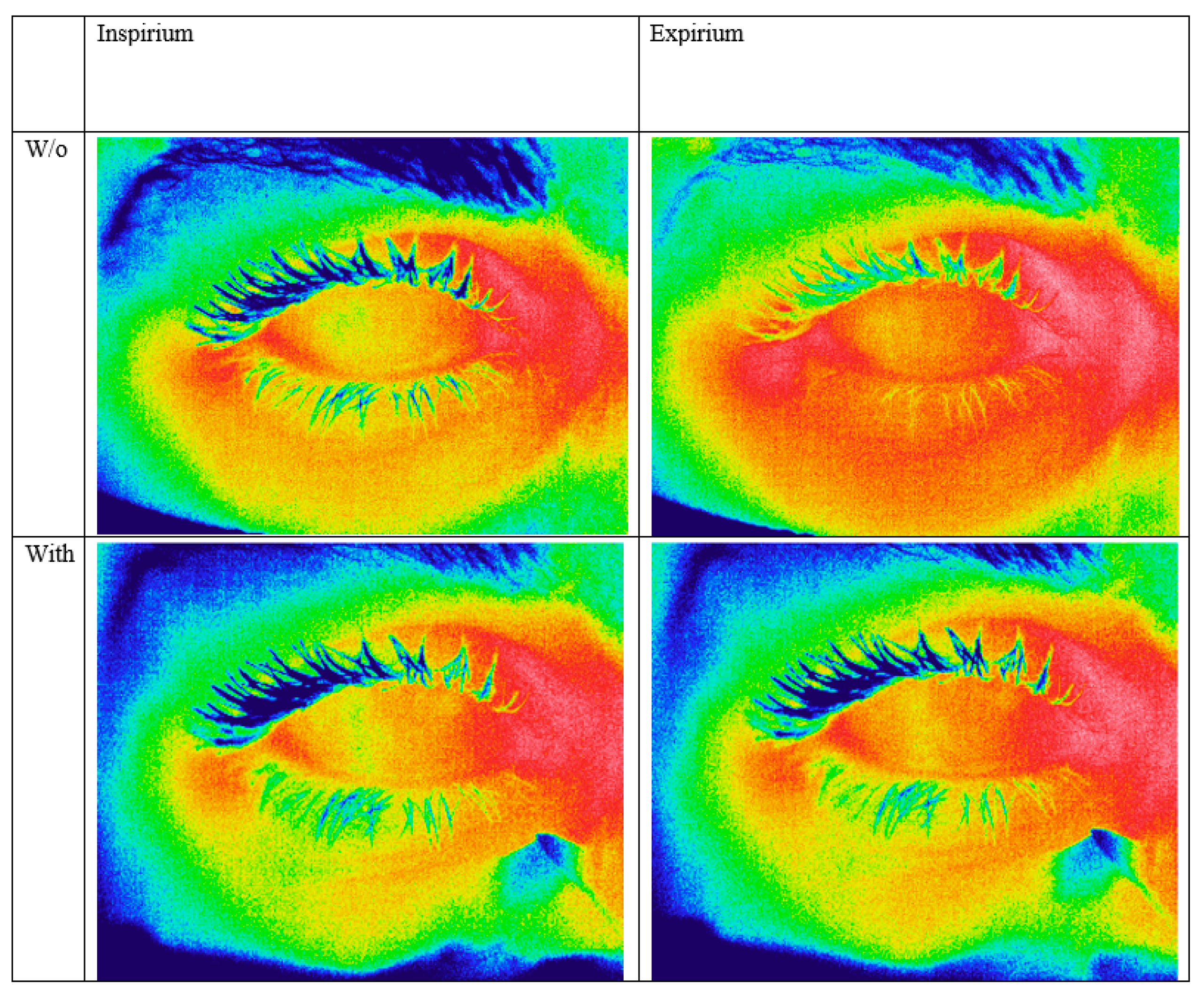
2.2. Thermographic Image Capture
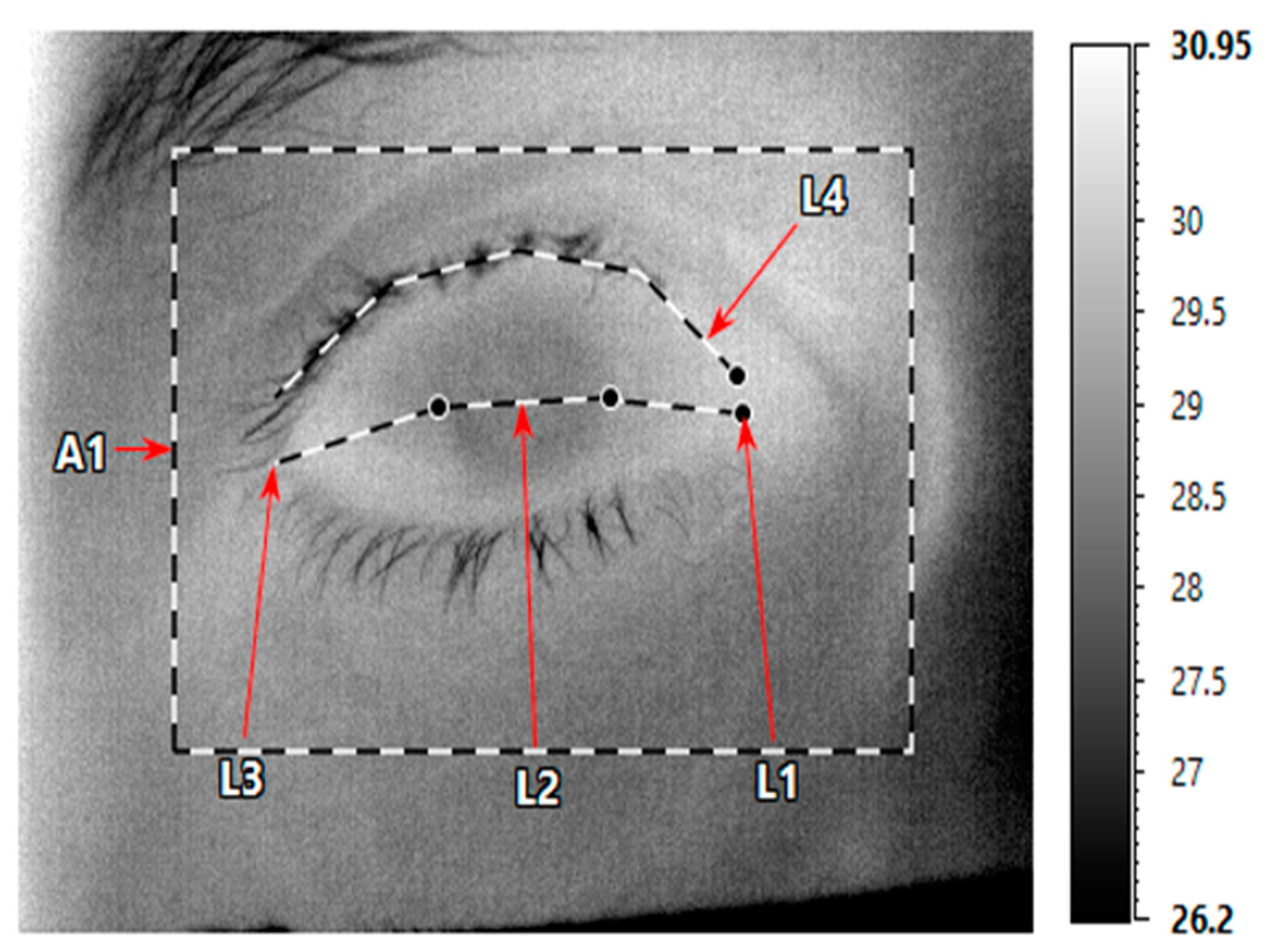
2.3. OST Measurements
2.4. Statistical Analysis
3. Results
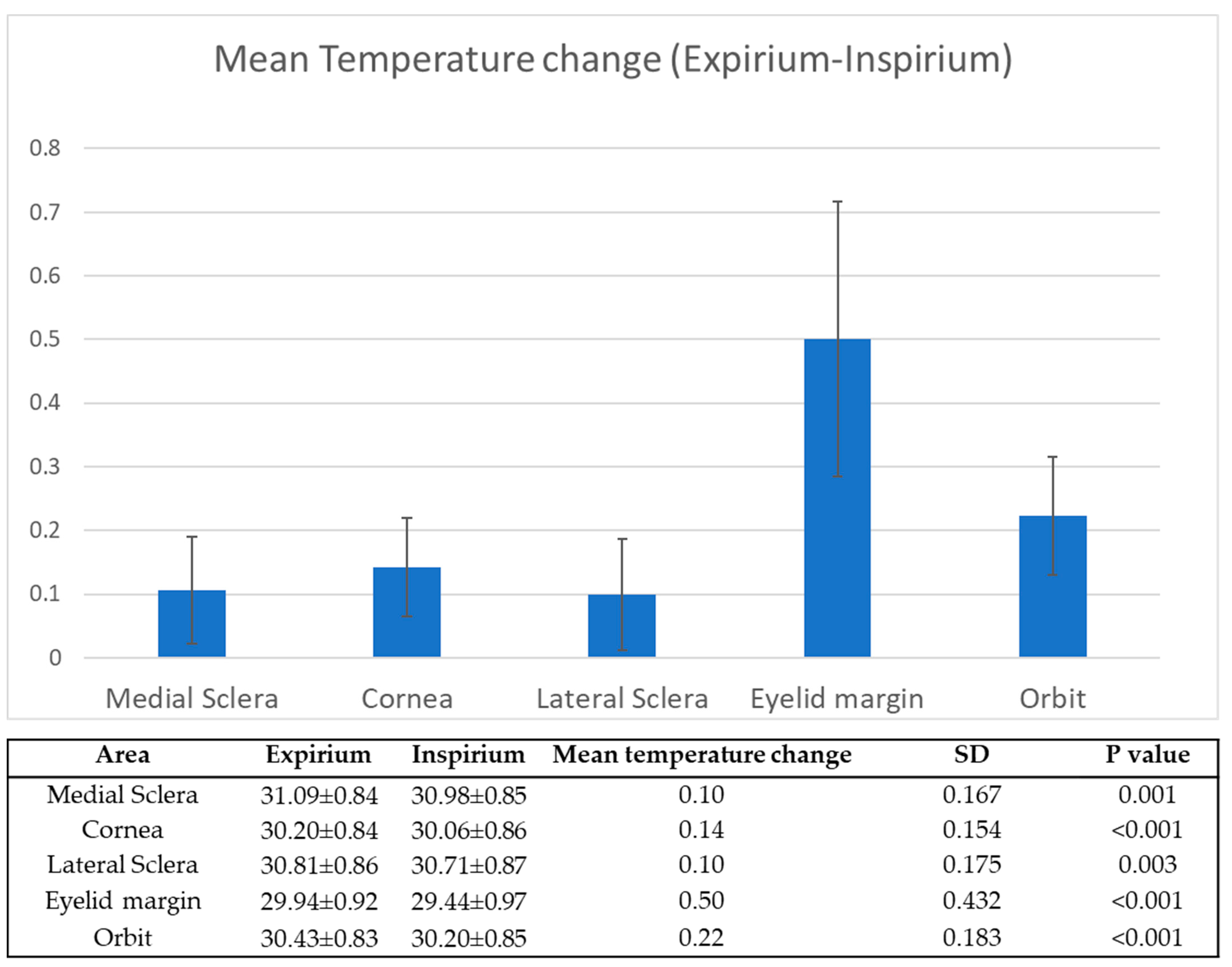
3.1. Expirium vs. Inspirium
3.2. The Effect of Taping
4. Discussion
4.1. Extrinsic Determinants of OST
4.2. Face-Mask-Induced OST Changes Might Affect the Tear Film
4.3. Use of Face-Mask and Ocular Pathology
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooks, J.T.; Beezhold, D.H.; Noti, J.D.; Coyle, J.P.; Derk, R.C.; Blachere, F.M.; Lindsley, W.G. Maximizing fit for cloth and medical procedure masks to improve performance and reduce SARS-CoV-2 transmission and exposure, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Butler, J.C. Effectiveness of mask wearing to control community spread of SARS-CoV-2. JAMA 2021, 325, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Logie, K.M.; Kusel, M.M.H.; Sly, P.D.; Hall, G.L. Exhaled breath temperature in healthy children is influenced by room temperature and lung volume. Pediatr. Pulmonol. 2011, 46, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, R. Measurement of corneal temperature. Exp. Eye Res. 1968, 7, 237–242. [Google Scholar] [CrossRef]
- Purslow, C.; Wolffsohn, J.S. Ocular surface temperature: A review. Eye Contact Lens. 2005, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.K.; Ooi, E.H. Ocular surface temperature: A 3D FEM prediction using bioheat equation. Comput. Biol. Med. 2007, 37, 829–835. [Google Scholar] [CrossRef]
- Efron, N.; Young, G.; Brennan, N.A. Ocular surface temperature. Curr. Eye Res. 1989, 8, 901–906. [Google Scholar]
- Tan, J.H.; Ng, E.Y.K.; Acharya, U.R.; Chee, C. Infrared thermography on ocular surface temperature: A review. Infrared Phys. Technol. 2009, 52, 97–108. [Google Scholar] [CrossRef]
- Regini, J.W.; Grossmann, J.G.; Burgio, M.R.; Malik, N.S.; Koretz, J.F.; Hodson, S.A.; Elliott, G.F. Structural changes in α-crystallin and whole eye lens during heating, observed by low-angle X-ray diffraction. J. Mol. Biol. 2004, 336, 1185–1194. [Google Scholar] [CrossRef]
- Heys, K.R.; Friedrich, M.G.; Truscott, R.J.W. Presbyopia and heat: Changes associated with aging of the human lens suggest a functional role for the small heat shock protein, α-crystallin, in maintaining lens flexibility. Aging Cell. 2007, 6, 807–815. [Google Scholar] [CrossRef]
- Kessel, L.; Johnson, L.; Arvidsson, H.; Larsen, M. The relationship between body and ambient temperature and corneal temperature. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6593–6597. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Fresina, M.; Campos, E.C. Subjective discomfort symptoms are related to low corneal temperature in patients with evaporative dry eye. Cornea 2015, 34, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Yoshida, A. The effect of ocular warming on ocular circulation in healthy humans. Arch. Ophthalmol. 2004, 122, 1477–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, G.Y.; Ben-David, G.; Singer, R.; Benyosef, S.; Shemesh, R.; Leshno, A.; Barkana, Y.; Skaat, A. Ocular surface temperature: Characterization in a large cohort of healthy human eyes and correlations to systemic cardiovascular risk factors. Diagnostics 2021, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Leshno, A.; Stern, O.; Barkana, Y.; Kapelushnik, N.; Singer, R.; Prat, D.L.; Cohen, G.; Ben-David, G.; Abrahami, D.; Huna-Baron, R.; et al. Ocular surface temperature differences in glaucoma. Eur. J. Ophthalmol. 2021, 11206721211023723. [Google Scholar] [CrossRef]
- Naidorf-Rosenblatt, H.; Landau-Part, D.; Moisseiev, J.; Alhalel, A.; Huna-Baron, R.; Skaat, A.; Pilus, S.; Levi, L.; Leshno, A. Ocular surface temperature differences in retinal vascular diseases. Retina 2022, 42, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Lu, C.W.; Liu, X.F.; Jia, Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet 2020, 395, e39. [Google Scholar] [CrossRef] [Green Version]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, A.; Rosani, U.; Brun, P. Ocular surface expression of SARS-CoV-2 receptors. Ocul. Immunol. Inflamm. 2020, 28, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Calonge, M.; Labetoulle, M.; Messmer, E.M.; Shah, S.; Akova, Y.A.; Boboridis, K.G.; Merayo-Lloves, J.; Aragona, P.; Benítez-Del-Castillo, J.; Geerling, G.; et al. Controlled adverse environment chambers in dry eye research. Curr. Eye Res. 2018, 43, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Abusharha, A.A.; Pearce, E.I.; Fagehi, R. Effect of ambient temperature on the human tear film. Eye Contact Lens 2016, 42, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Purslow, C.; Wolffsohn, J. The relation between physical properties of the anterior eye and ocular surface temperature. Optom. Vis. Sci. 2007, 84, 197–201. [Google Scholar] [CrossRef]
- Konieczka, K.; Schoetzau, A.; Koch, S.; Hauenstein, D.; Flammer, J. Cornea thermography: Optimal evaluation of the outcome and the resulting reproducibility. Trans. Vis. Sci Tech. 2018, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Statulator: An Online Statistical Calculator. Available online: http://statulator.com/SampleSize/ss2PM.html (accessed on 23 May 2022).
- Petznick, A.; Tan, J.H.; Boo, S.K.; Lee, S.Y.; Acharya, U.R.; Tong, L. Repeatability of a new method for measuring tear evaporation rates. Optom. Vis. Sci. 2013, 90, 366–371. [Google Scholar] [CrossRef]
- Mapstone, R. Determinants of corneal temperature. Br. J. Ophthalmol. 1968, 52, 729. [Google Scholar] [CrossRef] [Green Version]
- Abusharha, A.A.; Pearce, E.I. The effect of low humidity on the human tear film. Cornea 2013, 32, 429–434. [Google Scholar] [CrossRef]
- Shah, A.M.; Galor, A. Impact of ocular surface temperature on tear characteristics: Current insights. Clin. Optom. 2021, 13, 51. [Google Scholar] [CrossRef]
- Kamao, T.; Yamaguchi, M.; Kawasaki, S.; Mizoue, S.; Shiraishi, A.; Ohashi, Y. Screening for dry eye with newly developed ocular surface thermographer. Am. J. Ophthalmol. 2011, 151, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.B.; Tullo, A.B.; Efron, N. Infrared thermography of the tear film in dry eye. Eye 1995, 9, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, J.P.; Singh, I.; Tomlinson, A.; Morgan, P.B.; Efron, N. The role of tear physiology in ocular surface temperature. Eye 2000, 14, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. Tfos dews II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Invest Ophthalmol Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [Green Version]
- Mathers, W.D.; Lane, J.A. Meibomian Gland Lipids, Evaporation, and Tear Film Stability. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2; Sullivan, D.A., Dartt, D.A., Meneray, M.A., Eds.; Springer: Berlin, Germany, 1998; pp. 349–360. [Google Scholar] [CrossRef]
- Shimazaki, J. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 1995, 113, 1266. [Google Scholar] [CrossRef]
- Foulks, G.N. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 2007, 52, 369–374. [Google Scholar] [CrossRef]
- Craig, J.P.; Tomlinson, A. Importance of the lipid layer in human tear film stability and evaporation. Optom. Vis. Sci. 1997, 74, 8–13. [Google Scholar] [CrossRef]
- Goto, E.; Endo, K.; Suzuki, A.; Fujikura, Y.; Matsumoto, Y.; Tsubota, K. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest. Ophthalmol. Vis. Sci. 2003, 44, 533. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest. Ophthalmol. Vis. Sci. 2011, 52, 2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.T.M.; Liu, L.J.; McPherson, R.D.; Fuller, J.R.; Craig, J.P. Therapeutic profile of a latent heat eyelid warming device with temperature setting variation. Contact Lens Anterior Eye 2020, 43, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; West, W.B.; Marx, D.P. Face mask-associated ocular irritation and dryness. Ophthalmol. Ther. 2020, 9, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Schmid, M.B.; Czypionka, T.; Bassler, D.; Gruer, L. Face masks for the public during the COVID-19 crisis. BMJ 2020, 369, m1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, D.E. MADE: A New Coronavirus-Associated Eye Disease. Available online: https://www.healio.com (accessed on 23 May 2022).
- Krolo, I.; Blazeka, M.; Merdzo, I.; Vrtar, I.; Sabol, I.; Petric-Vickovic, I. Mask-Associated Dry Eye During COVID-19 Pandemic–How Face Masks Contribute to Dry Eye Disease Symptoms. Med. Arch. 2021, 75, 144. [Google Scholar] [CrossRef] [PubMed]
- Hadayer, A.; Zahavi, A.; Livny, E.; Gal-Or, O.; Gershoni, A.; Mimouni, K.; Ehrlich, R. Patients wearing face masks during intravitreal injections may be at a higher risk of endophthalmitis. Retina 2020, 40, 1651–1656. [Google Scholar] [CrossRef]
- Raevis, J.J.; Gjyzeli, G.; Mititelu, M.; Rogers, J.; Lasarev, M.; Chang, J.S. Face masks and bacterial dispersion toward the periocular area. Ophthalmology 2021, 128, 1236–1238. [Google Scholar] [CrossRef]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; García-Feijoó, J.; Sáenz-Francés, F.; Arriola-Villalobos, P.; Martinez-de-la-Casa, J.M.; Benítez-del-Castillo, J.M.; Herrera de la Muela, M. Conjunctivitis in COVID-19 patients: Frequency and clinical presentation. Graefe’s Arch. Clin. Exp. 2020, 258, 2501–2507. [Google Scholar] [CrossRef]
- Alcalde, C.F.; Fernández, M.G.; Moreno, M.N.; Rey, C.C.; Romero, I.F.; Martín, S.N. COVID-19 ocular findings in children: A case series. World J. Pediatr. 2021, 17, 329–334. [Google Scholar] [CrossRef]
- Ho, D.; Low, R.; Tong, L.; Gupta, V.; Veeraraghavan, A.; Agrawal, R. COVID-19 and the ocular surface: A review of transmission and manifestations. Ocul. Immunol. Inflamm. 2020, 28, 726–734. [Google Scholar] [CrossRef]
- Silkiss, R.Z.; Paap, M.K.; Ugradar, S. Increased incidence of chalazion associated with face mask wear during the COVID-19 pandemic. Am. J. Ophthalmol. Case Rep. 2021, 22, 101032. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Li Voti, R.; Rusciano, D.; Mutolo, M.G.; Pescosolido, N. Relationship between corneal temperature and intraocular pressure in healthy individuals: A clinical thermographic analysis. J. Ophthalmol. 2016, 2016, 3076031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.H.; Ng, E.Y.K.; Acharya, U.R. Evaluation of topographical variation in ocular surface temperature by functional infrared thermography. Infrared Phys. Technol. 2011, 54, 469–477. [Google Scholar] [CrossRef]
| Number of Patients (Eyes) | 31 (31) |
| Age (years), mean SD | 43.6 ± 14.8 |
| Female, n (%) | 21 (67.7%) |
| Body temp, mean SD | 36.8 ± 0.34 |
| Room temp, mean (SD) | 22.1 ± 1.2 |
| Area | Expirium No Tape | Taped Highest Measurements | Mean Temperature Change | SD | p Value |
|---|---|---|---|---|---|
| Medial sclera | 31.13 ± 0.83 | 30.99 ± 0.84 | 0.14 | 0.26 | 0.034 |
| Cornea | 30.28 ± 0.85 | 30.15 ± 0.79 | 0.13 | 0.26 | 0.037 |
| Lateral sclera | 30.86 ± 0.88 | 30.59 ± 1.03 | 0.27 | 0.43 | 0.013 |
| Eyelid margin | 29.97 ± 0.96 | 29.56 ± 1.06 | 0.41 | 0.60 | 0.006 |
| Orbit | 30.47 ± 0.85 | 30.26 ± 0.83 | 0.20 | 0.23 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapelushnik, N.; Benyosef, S.; Skaat, A.; Abdelkader, A.; Landau Prat, D.; Blum-Meirovitch, S.; Leshno, A. The Effect of Face Masks during COVID-19 Pandemic on Ocular Surface Temperature—A Clinical Thermographic Analysis. Diagnostics 2022, 12, 1431. https://doi.org/10.3390/diagnostics12061431
Kapelushnik N, Benyosef S, Skaat A, Abdelkader A, Landau Prat D, Blum-Meirovitch S, Leshno A. The Effect of Face Masks during COVID-19 Pandemic on Ocular Surface Temperature—A Clinical Thermographic Analysis. Diagnostics. 2022; 12(6):1431. https://doi.org/10.3390/diagnostics12061431
Chicago/Turabian StyleKapelushnik, Noa, Shahar Benyosef, Alon Skaat, Amir Abdelkader, Daphna Landau Prat, Sharon Blum-Meirovitch, and Ari Leshno. 2022. "The Effect of Face Masks during COVID-19 Pandemic on Ocular Surface Temperature—A Clinical Thermographic Analysis" Diagnostics 12, no. 6: 1431. https://doi.org/10.3390/diagnostics12061431






