Proton MR Spectroscopy of Pediatric Brain Disorders
Abstract
:1. Introduction
MR Spectroscopy in Pediatrics
2. Methods for Clinical Spectroscopy
3. Metabolic Maturation of the Human Brain
Prematurity
4. Clinical Applications of MR Spectroscopy
4.1. Pediatric Brain Tumors
4.2. Perinatal Hypoxic–Ischemic Encephalopathy
4.3. Inborn Errors of Metabolism
4.4. Trauma
4.5. Infections, Inflammation
4.6. Epilepsy
4.7. Neuropsychiatric Disorders
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüml, S.; Panigrahy, A. (Eds.) MR Spectroscopy of Pediatric Brain Disorders; Springer: New York, NY, USA, 2013. [Google Scholar]
- Liserre, R.; Pinelli, L.; Gasparotti, R. MR spectroscopy in pediatric neuroradiology. Transl. Pediatr. 2021, 10, 1169–1200. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.A. In Vivo NMR Spectroscopy Principles and Techniques, 3rd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- van der Knaap, M.S.; van der Grond, J.; van Rijen, P.C.; Faber, J.A.; Valk, J.; Willemse, K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology 1990, 176, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüppi, P.S.; Posse, S.; Lazeyras, F.; Burri, R.; Bossi, E.; Herschkowitz, N. Magnetic Resonance in Preterm and Term Newborns: 1H-Spectroscopy in Developing Human Brain. Pediatr. Res. 1991, 30, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Kreis, R.; Ernst, T.; Ross, B.D. Development of the human brain:In vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn. Reson. Med. 1993, 30, 424–437. [Google Scholar] [CrossRef]
- Toft, P.B.; Leth, H.; Lou, H.C.; Pryds, O.; Henriksen, O. Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J. Magn. Reson. Imaging 1994, 4, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Cady, E.B.; Penrice, J.; Amess, P.N.; Lorek, A.; Wylezinska, M.; Aldridge, R.F.; Franconi, F.; Wyatt, J.S.; Reynolds, E.O.R. Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn. Reson. Med. 1996, 36, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, P.J.W.; Brockmann, K.; Kruse, B.; Wilken, B.; Wick, M.; Hanefeld, F.; Frahm, J. Regional Age Dependence of Human Brain Metabolites from Infancy to Adulthood as Detected by Quantitative Localized Proton MRS. Pediatr. Res. 1999, 46, 474. [Google Scholar] [CrossRef] [Green Version]
- Kreis, R.; Hofmann, L.; Kuhlmann, B.; Boesch, C.; Bossi, E.; Hüppi, P. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 2002, 48, 949–958. [Google Scholar] [CrossRef]
- Blüml, S.; Wisnowski, J.L.; Nelson, M.D., Jr.; Paquette, L.; Gilles, F.H.; Kinney, H.C.; Panigrahy, A. Metabolic Maturation of the Human Brain from Birth through Adolescence: Insights from In Vivo Magnetic Resonance Spectroscopy. Cereb. Cortex 2012, 23, 2944–2955. [Google Scholar] [CrossRef] [Green Version]
- Degnan, A.J.; Ceschin, R.; Lee, V.; Schmithorst, V.J.; Blüml, S.; Panigrahy, A. Early Metabolic Development of Posteromedial cortex and Thalamus in Humans Using in vivo Quantitative MR Spectroscopy. J. Comp. Neurol. 2014, 522, 3717–3732. [Google Scholar] [CrossRef] [Green Version]
- Bjartmar, C.; Battistuta, J.; Terada, N.; DuPree, E.; Trapp, B.D. N-acetylaspartate is an axon-specific marker of mature white matter in vivo: A biochemical and immunohistochemical study on the rat optic nerve. Ann. Neurol. 2001, 51, 51–58. [Google Scholar] [CrossRef]
- Burri, R.; Steffen, C.; Herschkowitz, N. N-acetyl-l-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev. Neurosci. 1991, 13, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Baslow, M.H. Functions of N-acetyl-l-aspartate and N-acetyl-l-aspartylglutamate in the vertebrate brain: Role in glial cell-specific signaling. J. Neurochem. 2000, 75, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.B. Creatine: Biosynthesis, regulation, and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 50, 177–242. [Google Scholar] [PubMed]
- Erecinska, M.; Silver, I.A. Metabolism and role of glutamate in mammalian brain. Prog. Neurobiol. 1990, 35, 245–296. [Google Scholar] [CrossRef]
- Thurston, J.H.; Sherman, W.R.; Hauhart, R.E.; Kloepper, R.F. myo-inositol: A newly identified nonnitrogenous osmoregulatory molecule in mammalian brain. Pediatr. Res. 1989, 26, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Lien, Y.H.; Shapiro, J.I.; Chan, L. Effects of hypernatremia on organic brain osmoles. J. Clin. Investig. 1990, 85, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef]
- Isaacks, R.E.; Bender, A.S.; Kim, C.Y.; Prieto, N.M.; Norenberg, M.D. Osmotic regulation of myo-inositol uptake in primary astrocyte cultures. Neurochem. Res. 1994, 19, 331–338. [Google Scholar] [CrossRef]
- Berry, G.T. Is prenatal myo-inositol deficiency a mechanism of CNS injury in galactosemia? J. Inherit. Metab. Dis. 2011, 34, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.L.; Hansen, S.; Berry, K.; Mok, C.; Lesk, D. Free amino acids and related compounds in biopsies of human brain. J. Neurochem. 1971, 18, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kinney, H.C.; Karthigasan, J.; Borenshteyn, N.I.; Flax, J.D.; Kirschner, D.A. Myelination in the developing human brain: Biochemical correlates. Neurochem. Res. 1994, 19, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Ackerstaff, E.; Glunde, K.; Bhujwalla, Z.M. Choline phospholipid metabolism: A target in cancer cells? J. Cell. Biochem. 2003, 90, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Blüml, S.; Wisnowski, J.L.; Nelson, M.D.; Paquette, L.; Panigrahy, A. Metabolic maturation of white matter is altered in preterm infants. PLoS ONE 2014, 9, e85829. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. 4), iv1–iv62. [Google Scholar] [CrossRef]
- Gurney, J.; Smith, M.; Bunin, G. CNS and miscellaneous intracranial and intraspinal neoplasms. In Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; Ries, L., Smith, M., Gurney, J., Linet, M., Tamra, T., Young, J., Eds.; National Cancer Institute, SEER Program: Bethesda, MD, USA, 1999; NIH Pub. No. 99-4649. [Google Scholar]
- Wang, Z.; Sutton, L.N.; Cnaan, A.; Haselgrove, J.C.; Rorke, L.B.; Zhao, H.; Bilaniuk, L.T.; A Zimmerman, R. Proton MR spectroscopy of pediatric cerebellar tumors. AJNR Am. J. Neuroradiol. 1995, 16, 1821–1833. [Google Scholar]
- Sutton, L.N.; Wang, Z.J.; Wehrli, S.L.; Marwaha, S.; Molloy, P.; Phillips, P.C.; Zimmerman, R.A. Proton Spectroscopy of Suprasellar Tumors in Pediatric Patients. Neurosurgery 1997, 41, 388–395. [Google Scholar] [CrossRef]
- Arle, J.E.; Morriss, C.; Wang, Z.; Zimmerman, R.A.; Phillips, P.G.; Sutton, L.N. Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J. Neurosurg. 1997, 86, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Tzika, A.A.; Astrakas, L.G.; Zarifi, M.K.; Zurakowski, D.; Poussaint, T.Y.; Goumnerova, L.; Tarbell, N.J.; Black, P.M. Spectroscopic and perfusion magnetic resonance imaging predictors of progression in pediatric brain tumors. Cancer 2004, 100, 1246–1256. [Google Scholar] [CrossRef]
- A Lazareff, J.; Olmstead, C.; Bockhorst, K.H.; Alger, J.R. Proton magnetic resonance spectroscopic imaging of pediatric low-grade astrocytomas. Child’s Nerv. Syst. 1996, 12. [Google Scholar] [CrossRef] [PubMed]
- Peet, A.C.; Lateef, S.; MacPherson, L.; Natarajan, K.; Sgouros, S.; Grundy, R.G. Short echo time 1 H magnetic resonance spectroscopy of childhood brain tumours. Child’s Nerv. Syst. 2006, 23, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.P.; Wilson, M.; Harris, L.M.; Natarajan, K.; Lateef, S.; Macpherson, L.; Sgouros, S.; Grundy, R.G.; Arvanitis, T.N.; Peet, A.C. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed. 2008, 21, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, A.; Krieger, M.; Gonzalez-Gomez, I.; Liu, X.; McComb, J.; Finlay, J.; Nelson, M.; Gilles, F.; Blüml, S. Quantitative Short Echo Time 1H-MR Spectroscopy of Untreated Pediatric Brain Tumors: Preoperative Diagnosis and Characterization. Am. J. Neuroradiol. 2006, 27, 560–572. [Google Scholar] [PubMed]
- Shiroishi, M.S.; Panigrahy, A.; Moore, K.R.; Nelson, M.D.; Gilles, F.H.; González-Gómez, I.; Blüml, S. Combined MRI and MRS improves pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. Neuroradiology 2015, 57, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamrazi, B.; Nelson, M.D.; Blüml, S. MRS of pilocytic astrocytoma: The peak at 2 ppm may not be NAA. Magn. Reson. Med. 2016, 78, 452–456. [Google Scholar] [CrossRef]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.-J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncology 2016, 19, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Blüml, S.; Margol, A.S.; Sposto, R.; Kennedy, R.J.; Robison, N.J.; Vali, M.; Hung, L.T.; Muthugounder, S.; Finlay, J.L.; Erdreich-Epstein, A.; et al. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro Oncol. 2015, 18, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.M.; Davies, N.; MacPherson, L.; Lateef, S.; Natarajan, K.; Brundler, M.-A.; Sgouros, S.; English, M.W.; Arvanitis, T.; Grundy, R.G.; et al. Magnetic resonance spectroscopy in the assessment of pilocytic astrocytomas. Eur. J. Cancer 2008, 44, 2640–2647. [Google Scholar] [CrossRef]
- Opstad, K.; Provencher, S.; Bell, B.; Griffiths, J.; Howe, F. Detection of elevated glutathione in meningiomas by quantitative in vivo 1H MRS. Magn. Reson. Med. 2003, 49, 632–637. [Google Scholar] [CrossRef]
- Gill, S.S.; Thomas, D.G.; Van Bruggen, N.; Gadian, D.G.; Peden, C.J.; Bell, J.D.; Cox, I.J.; Menon, D.K.; Iles, R.A.; Bryant, D.J.; et al. Proton MR spectroscopy of intracranial tumours: In vivo and in vitro studies. J. Comput. Assist. Tomogr. 1990, 14, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Kumabe, T.; Shirane, R.; Yoshimoto, T. Correlation between Choline Level Measured by Proton MR Spectroscopy and Ki-67 Labeling Index in Gliomas. Am. J. Neuroradiol. 2000, 21, 659–665. [Google Scholar] [PubMed]
- Clymer, J.; Kieran, M.W. The Integration of Biology Into the Treatment of Diffuse Intrinsic Pontine Glioma: A Review of the North American Clinical Trial Perspective. Front. Oncol. 2018, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, E.; Prados, M. Pediatric CNS Tumors; Gupta, N., Haas-Kogen, D., Banerjee, A., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Volume 3, pp. 49–61.
- Yoshimura, J.; Onda, K.; Tanaka, R.; Takahashi, H. Clinicopathological Study of Diffuse Type Brainstem Gliomas: Analysis of 40 Autopsy Cases. Neurol. Med.-Chir. 2003, 43, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Panigrahy, A.; Nelson, M.D.; Finlay, J.L.; Sposto, R.; Krieger, M.D.; Gilles, F.H.; Blüml, S. Metabolism of diffuse intrinsic brainstem gliomas in children. Neuro-Oncology 2008, 10, 32–44. [Google Scholar] [CrossRef]
- Seymour, Z.A.; Panigrahy, A.; Finlay, J.L.; Nelson, M.D., Jr.; Bluml, S. Citrate in pediatric CNS tumors? AJNR Am. J. Neuroradiol. 2008, 29, 1006–1011. [Google Scholar] [CrossRef] [Green Version]
- Blüml, S.; Panigrahy, A.; Laskov, M.; Dhall, G.; Krieger, M.D.; Nelson, M.D.; Finlay, J.L.; Gilles, F.H. Elevated citrate in pediatric astrocytomas with malignant progression. Neuro-Oncology 2011, 13, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.P.; Wilson, M.; Natarajan, K.; Sun, Y.; MacPherson, L.; Brundler, M.A.; Arvanitis, T.N.; Grundy, R.G.; Peet, A.C. Non-invasive detection of glycine as a biomarker of malignancy in childhood brain tumours using in-vivo 1H MRS at 1.5 tesla confirmed by ex-vivo high-resolution magic-angle spinning NMR. NMR Biomed. 2010, 23, 80–87. [Google Scholar] [CrossRef]
- Carapella, C.M.; Carpinelli, G.; Knijn, A.; Raus, L.; Caroli, F.; Podo, F. Potential Role of in vitro 1H Magnetic Resonance Spectroscopy in the Definition of Malignancy Grading of Human Neuroepithelial Brain Tumours. Acta Neurochir. Suppl. 1997, 68, 127–132. [Google Scholar] [CrossRef]
- Tzika, A.A.; Righi, V.; Andronesi, O.C.; Mintzopoulos, D.; Black, P.M. High-resolution magic angle spinning magnetic resonance spectroscopy detects glycine as a biomarker in brain tumors. Int. J. Oncol. 2009, 36, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- A Northcott, P.; Dubuc, A.M.; Pfister, S.; Taylor, M.D. Molecular subgroups of medulloblastoma. Expert Rev. Neurother. 2012, 12, 871–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamrazi, B.; Venneti, S.; Margol, A.; Hawes, D.; Cen, S.; Nelson, M.; Judkins, A.; Biegel, J.; Blüml, S. Pediatric Atypical Teratoid/Rhabdoid Tumors of the Brain: Identification of Metabolic Subgroups Using In Vivo 1H-MR Spectroscopy. Am. J. Neuroradiol. 2019, 40, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Panwalkar, P.; Tamrazi, B.; Dang, D.; Chung, C.; Sweha, S.; Natarajan, S.K.; Pun, M.; Bayliss, J.; Ogrodzinski, M.P.; Pratt, D.; et al. Targeting integrated epigenetic and metabolic pathways in lethal childhood PFA ependymomas. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.; Shibuya, K.; Stein, C. No cry at birth: Global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull. World Health Organ. 2005, 83, 409–417. [Google Scholar] [PubMed]
- Barkovich, A.J.; Hajnal, B.L.; Vigneron, D.; Sola, A.; Partridge, J.C.; Allen, F.; Ferriero, D.M. Prediction of neuromotor outcome in perinatal asphyxia: Evaluation of MR scoring systems. AJNR Am. J. Neuroradiol. 1998, 19, 143–149. [Google Scholar]
- Miller, S.; Ramaswamy, V.; Michelson, D.; Barkovich, A.J.; Holshouser, B.; Wycliffe, N.; Glidden, D.; Deming, D.; Partridge, J.C.; Wu, Y.W.; et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 2005, 146, 453–460. [Google Scholar] [CrossRef]
- Groenendaal, F.; Veenhoven, R.H.; van der Grond, J.; Jansen, G.H.; Witkamp, T.D.; de Vries, L.S. Cerebral Lactate and N-Acetyl-Aspartate/Choline Ratios in Asphyxiated Full-Term Neonates Demonstrated In Vivo Using Proton Magnetic Resonance Spectroscopy. Pediatr. Res. 1994, 35, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Alderliesten, T.; De Vries, L.S.; Benders, M.J.N.L.; Koopman, C.; Groenendaal, F. MR Imaging and Outcome of Term Neonates with Perinatal Asphyxia: Value of Diffusion-weighted MR Imaging and H MR Spectroscopy. Radiology 2011, 261, 235–242. [Google Scholar] [CrossRef]
- Parmentier, C.E.J.; de Vries, L.S.; Groenendaal, F. Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics 2022, 12, 645. [Google Scholar] [CrossRef]
- Miller, S.P.; Newton, N.; Ferriero, D.M.; Partridge, J.C.; Glidden, D.V.; Barnwell, A.; A Chuang, N.; Vigneron, D.B.; Barkovich, A.J. Predictors of 30-Month Outcome after Perinatal Depression: Role of Proton MRS and Socioeconomic Factors. Pediatr. Res. 2002, 52, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzopardi, D.; Edwards, A.D. Magnetic resonance biomarkers of neuroprotective effects in infants with hypoxic ischemic encephalopathy. Semin. Fetal Neonatal Med. 2010, 15, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.; Cady, E.; Penrice, J.; Wyatt, J.; Cox, I.; Robertson, N. Proton MR Spectroscopy in Neonates with Perinatal Cerebral Hypoxic-Ischemic Injury: Metabolite Peak-Area Ratios, Relaxation Times, and Absolute Concentrations. Am. J. Neuroradiol. 2006, 27, 1546–1554. [Google Scholar] [PubMed]
- Barkovich, A.J.; Baranski, K.; Vigneron, D.; Partridge, J.C.; Hallam, D.K.; Hajnal, B.L.; Ferriero, D.M. Proton MR Spectroscopy for the Evaluation of Brain Injury in Asphyxiated, Term Neonates. Am. J. Neuroradiol. 1999, 20, 1399–1405. [Google Scholar] [PubMed]
- Mitra, S.; Kendall, G.S.; Bainbridge, A.; Sokolska, M.; Dinan, M.; Uria-Avellanal, C.; Price, D.; McKinnon, K.; Gunny, R.; Huertas-Ceballos, A.; et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 104, F424–F432. [Google Scholar] [CrossRef]
- Aida, N. 1H-MR Spectroscopy of the Early Developmental Brain, Neonatal Encephalopathies, and Neurometabolic Disorders. Magn. Reson. Med. Sci. 2022, 21, 9–28. [Google Scholar] [CrossRef]
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral Magnetic Resonance Biomarkers in Neonatal Encephalopathy: A Meta-analysis. Pediatrics 2010, 125, e382–e395. [Google Scholar] [CrossRef]
- Shanmugalingam, S.; Thornton, J.S.; Iwata, O.; Bainbridge, A.; O’Brien, F.E.; Priest, A.N.; Ordidge, R.J.; Cady, E.B.; Wyatt, J.S.; Robertson, N.J. Comparative Prognostic Utilities of Early Quantitative Magnetic Resonance Imaging Spin-Spin Relaxometry and Proton Magnetic Resonance Spectroscopy in Neonatal Encephalopathy. Pediatrics 2006, 118, 1467–1477. [Google Scholar] [CrossRef]
- Kreis, R.; Arcinue, E.; Ernst, T.; Shonk, T.K.; Flores, R.; Ross, B.D. Hypoxic encephalopathy after near-drowning studied by quantitative 1H-magnetic resonance spectroscopy. J. Clin. Investig. 1996, 97, 1142–1154. [Google Scholar] [CrossRef] [Green Version]
- Cady, E.B.; Lorek, A.; Penrice, J.; Reynolds, E.O.; Iles, R.A.; Burns, S.P.; Coutts, G.A.; Cowan, F.M. Detection of propan-1,2-diol in neonatal brain by in vivo proton magnetic resonance spectroscopy. Magn. Reson. Med. 1994, 32, 764–767. [Google Scholar] [CrossRef]
- Whitehead, M.T.; Lai, L.M.; Blüml, S. Clinical 1H MRS in childhood neurometabolic diseases—Part 1: Technique and age-related normal spectra. Neuroradiology 2022, 64, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; Lindquist, D.M. Leukodystrophies. In MR Spectroscopy of Pediatric Brain Disorders; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2013; pp. 105–122. [Google Scholar]
- Cecil, K.M.; Lindquist, D.M. Metabolic Disorders. In MR Spectroscopy of Pediatric Brain Disorders; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2013; pp. 123–148. [Google Scholar]
- Wilken, B.; Dechent, P.; Hanefeld, F.; Frahm, J. Proton MRS of a child with Sandhoff disease reveals elevated brain hexosamine. Eur. J. Paediatr. Neurol. 2008, 12, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.R.; Adamo, M.A. Non-accidental trauma in pediatric patients: A review of epidemiology, pathophysiology, diagnosis and treatment. Transl. Pediatr. 2014, 3, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Keenan, H.T.; Runyan, D.K.; Marshall, S.W.; Nocera, M.A.; Merten, D.F.; Sinal, S.H. A population-based study of inflicted traumatic brain injury in young children. JAMA 2003, 290, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodore, A.D.; Chang, J.J.; Runyan, D.K.; Hunter, W.M.; Bangdiwala, S.I.; Agans, R. Epidemiologic Features of the Physical and Sexual Maltreatment of Children in the Carolinas. Pediatrics 2005, 115, e331–e337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, T.; Harrington, D.E.R.A.; Anderson, T.; Berrol, S.; Cicerone, K. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993, 8, 86–87. [Google Scholar] [CrossRef]
- Arbogast, K.B.; Curry, A.; Pfeiffer, M.R.; Zonfrillo, M.; Haarbauer-Krupa, J.; Breiding, M.J.; Coronado, V.G.; Master, C. Point of Health Care Entry for Youth With Concussion Within a Large Pediatric Care Network. JAMA Pediatr. 2016, 170, e160294. [Google Scholar] [CrossRef]
- Meehan, W.P., 3rd; Bachur, R.G. Sport-related concussion. Pediatrics 2009, 123, 114–123. [Google Scholar] [CrossRef]
- Field, M.; Collins, M.W.; Lovell, M.R.; Maroon, J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J. Pediatr. 2003, 142, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Makoroff, K.L.; Cecil, K.M.; Caré, M.; Ball, W.S. Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr. Radiol. 2005, 35, 668–676. [Google Scholar] [CrossRef]
- Ashwal, S.; A Holshouser, B.; Shu, S.K.; Simmons, P.L.; Perkin, R.M.; Tomasi, L.G.; Knierim, D.S.; Sheridan, C.; Craig, K.; Andrews, G.H.; et al. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr. Neurol. 2000, 23, 114–125. [Google Scholar] [CrossRef]
- Aaen, G.S.; Holshouser, B.A.; Sheridan, C.; Colbert, C.; McKenney, M.; Kido, D.; Ashwal, S. Magnetic Resonance Spectroscopy Predicts Outcomes for Children With Nonaccidental Trauma. Pediatrics 2010, 125, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Haseler, L.J.; Arcinue, E.; Danielsen, E.R.; Bluml, S.; Ross, B.D. Evidence From Proton Magnetic Resonance Spectroscopy for a Metabolic Cascade of Neuronal Damage in Shaken Baby Syndrome. Pediatrics 1997, 99, 4–14. [Google Scholar] [CrossRef] [PubMed]
- A Holshouser, B.; Ashwal, S.; Luh, G.Y.; Shu, S.; Kahlon, S.; Auld, K.L.; Tomasi, L.G.; Perkin, R.M.; Hinshaw, D.B. Proton MR spectroscopy after acute central nervous system injury: Outcome prediction in neonates, infants, and children. Radiology 1997, 202, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.D.; Ernst, T.; Kreis, R.; Haseler, L.J.; Bayer, S.; Danielsen, E.; Bluml, S.; Shonk, T.; Mandigo, J.C.; Caton, W.; et al. 1H MRS in acute traumatic brain injury. J. Magn. Reson. Imaging 1998, 8, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Holshouser, B.A.; Ashwal, S.; Shu, S.; Hinshaw, D.B., Jr. Proton MR spectroscopy in children with acute brain injury: Comparison of short and long echo time acquisitions. J. Magn. Reson. Imaging 2000, 11, 9–19. [Google Scholar] [CrossRef]
- Friedman, S.; Brooks, W.; Jung, R.; Chiulli, S.; Sloan, J.; Montoya, B.; Hart, B.; Yeo, R. Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology 1999, 52, 1384. [Google Scholar] [CrossRef] [PubMed]
- Holshouser, B.A.; Tong, K.A.; Ashwal, S. Proton MR Spectroscopic Imaging Depicts Diffuse Axonal Injury in Children with Traumatic Brain Injury. Am. J. Neuroradiol. 2005, 26, 1276–1285. [Google Scholar]
- Govindaraju, V.; Gauger, G.E.; Manley, G.T.; Ebel, A.; Meeker, M.; Maudsley, A.A. Volumetric Proton Spectroscopic Imaging of Mild Traumatic Brain Injury. Am. J. Neuroradiol. 2004, 25, 730–737. [Google Scholar]
- Brooks, W.M.; Stidley, C.A.; Petropoulos, H.; Jung, R.E.; Weers, D.C.; Friedman, S.; Barlow, M.A.; Sibbitt, W.; Yeo, R.A. Metabolic and Cognitive Response to Human Traumatic Brain Injury: A Quantitative Proton Magnetic Resonance Study. J. Neurotrauma 2000, 17, 629–640. [Google Scholar] [CrossRef]
- Gasparovic, C.; Arfai, N.; Smid, N.; Feeney, D.M. Decrease and Recovery of N-Acetylaspartate/Creatine in Rat Brain Remote from Focal Injury. J. Neurotrauma 2001, 18, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, M.U.; Stiller, D.; Skardelly, M.; Thomas, S.; Samii, M.; Brinker, T. Long-Time in-Vivo Metabolic Monitoring Following Experimental Brain Contusion Using Proton Magnetic Resonance Spectroscopy. Acta Neurochir. Suppl. 2002, 81, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; Hills, E.C.; Sandel, M.E.; Smith, D.H.; McIntosh, T.K.; Mannon, L.J.; Sinson, G.P.; Bagley, L.J.; Grossman, R.I.; Lenkinski, R.E. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J. Neurosurg. 1998, 88, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, D.; Bhattacharyya, A.; Husain, M.; Prasad, K.; Pandey, C.; Gupta, R. In Vivo Proton MR Spectroscopy Evaluation of Pyogenic Brain Abscesses: A Report of 194 Cases. Am. J. Neuroradiol. 2009, 31, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, P.-H.; Hsu, S.-S.; Ding, S.-W.; Ko, C.-W.; Fu, J.-H.; Weng, M.-J.; Yeh, L.-R.; Wu, M.-T.; Liang, H.-L.; Chen, C.-K.; et al. Proton magnetic resonance spectroscopy and diffusion-weighted imaging in intracranial cystic mass lesions. Surg. Neurol. 2007, 68, S25–S36. [Google Scholar] [CrossRef] [PubMed]
- Luthra, G.; Parihar, A.; Nath, K.; Jaiswal, S.; Prasad, K.; Husain, N.; Husain, M.; Singh, S.; Behari, S.; Gupta, R. Comparative Evaluation of Fungal, Tubercular, and Pyogenic Brain Abscesses with Conventional and Diffusion MR Imaging and Proton MR Spectroscopy. Am. J. Neuroradiol. 2007, 28, 1332–1338. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Jain, K.K.; Mittal, S.K.; Kumar, S. Imaging features of central nervous system fungal infections. Neurol. India 2007, 55, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Ferraz-Filho, J.R.; Santana-Netto, P.V.; Rocha-Filho, J.A.; Sgnolf, A.; Mauad, F.; Sanches, R.A. Application of magnetic resonance spectroscopy in the differentiation of high-grade brain neoplasm and inflammatory brain lesions. Arq. Neuro-Psiquiatr. 2009, 67, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.A.; Venkatraman, T.N.; Thomas, A.; Deveikis, A.; LoPresti, C.; Hayes, J.; Berman, N.; Walot, I.; Padilla, S.; Johnston-Jones, J.; et al. Altered neurometabolite development in HIV-infected children: Correlation with neuropsychological tests. Neurology 2004, 62, 1810–1817. [Google Scholar] [CrossRef]
- van der Voorn, J.P.; Pouwels, P.J.; Vermeulen, R.J.; Barkhof, F.; van der Knaap, M.S. Quantitative MR imaging and spectroscopy in congenital cytomegalovirus infection and periventricular leukomalacia suggests a comparable neuropathological substrate of the cerebral white matter lesions. Neuropediatrics 2009, 40, 168–173. [Google Scholar]
- Takanashi, J.-I.; Sugita, K.; Ishii, M.; Aoyagi, M.; Niimi, H. Longitudinal MR imaging and proton MR spectroscopy in herpes simplex encephalitis. J. Neurol. Sci. 1997, 149, 99–102. [Google Scholar] [CrossRef]
- Cecil, K.M.; Jones, B.V.; Williams, S.; Hedlund, G.L. CT, MRI and MRS of Epstein-Barr virus infection: Case report. Neuroradiology 2000, 42, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; Lindquist, D.M. Infection and Encephalitis. In MR Spectroscopy of Pediatric Brain Disorders; Springer: New York, NY, USA, 2013; pp. 155–166. [Google Scholar]
- Mader, I.; Wolff, M.; Nägele, T.; Niemann, G.; Grodd, W.; Küker, W. MRI and proton MR spectroscopy in acute disseminated encephalomyelitis. Child’s Nerv. Syst. 2005, 21, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-E.; Hwang, S.-K.; Choe, B.H.; Cho, M.-H.; Park, S.-P.; Kwon, S. Clinical Spectrum and Prognostic Factors of Acute Necrotizing Encephalopathy in Children. J. Korean Med. Sci. 2010, 25, 449–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K.; Roy, R.; Dev, R.; Husain, M.; Poptani, H.; Pandey, R.; Kishore, J.; Bhaduri, A.P. Finger printing of Mycobacterium tuberculosis in patients with intracranial tuberculomas by using in vivo, ex vivo, and in vitro magnetic resonance spectroscopy. Magn. Reson. Med. 1996, 36, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Husain, M.; Vatsal, D.K.; Kumar, R.; Chawla, S.; Husain, N. Comparative evaluation of magnetization transfer MR imaging and in-vivo proton MR spectroscopy in brain tuberculomas. Magn. Reson. Imaging 2002, 20, 375–381. [Google Scholar] [CrossRef]
- Malhotra, H.; Jain, K.; Agarwal, A.; Singh, M.; Yadav, S.; Husain, M.; Krishnani, N.; Gupta, R. Characterization of tumefactive demyelinating lesions using MR imaging and in-vivo proton MR spectroscopy. Mult. Scler. J. 2008, 15, 193–203. [Google Scholar] [CrossRef]
- Cianfoni, A.; Niku, S.; Imbesi, S. Metabolite Findings in Tumefactive Demyelinating Lesions Utilizing Short Echo Time Proton Magnetic Resonance Spectroscopy. Am. J. Neuroradiol. 2007, 28, 272–277. [Google Scholar]
- Saindane, A.M.; Cha, S.; Law, M.; Xue, X.; Knopp, E.A.; Zagzag, D. Proton MR Spectroscopy of Tumefactive Demyelinating Lesions. Am. J. Neuroradiol. 2002, 23, 1378–1386. [Google Scholar]
- Urenjak, J.; Williams, S.R.; Gadian, D.G.; Noble, M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J. Neurochem. 1992, 59, 55–61. [Google Scholar] [CrossRef]
- Signoretti, S.; Marmarou, A.; Tavazzi, B.; Lazzarino, G.; Beaumont, A.; Vagnozzi, R. N-Acetylaspartate Reduction as a Measure of Injury Severity and Mitochondrial Dysfunction Following Diffuse Traumatic Brain Injury. J. Neurotrauma 2001, 18, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Varho, T.; Komu, M.; Sonninen, P.; Lähdetie, J.; Holopainen, I.E. Quantitative 1H MRS and MRI Volumetry Indicate Neuronal Damage in the Hippocampus of Children with Focal Epilepsy and Infrequent Seizures. Epilepsia 2005, 46, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Widjaja, E. Magnetic Resonance Spectroscopy in Epilepsy. In MR Spectroscopy of Pediatric Brain Disorders; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2013; pp. 175–192. [Google Scholar]
- Najm, I.M.; Wang, Y.; Hong, S.C.; Luders, H.O.; Ng, T.C.; Comair, Y.G. Temporal Changes in Proton MRS Metabolites After Kainic Acid-Induced Seizures in Rat Brain. Epilepsia 1997, 38, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Baslow, M.H. Evidence supporting a role for N-acetyl-l-aspartate as a molecular water pump in myelinated neurons in the central nervous system: An analytical review. Neurochem. Int. 2002, 40, 295–300. [Google Scholar] [CrossRef]
- Najm, I.M.; Wang, Y.; Shedid, D.; Luders, H.O.; Ng, T.C.; Comair, Y.G. MRS metabolic markers of seizures and seizure-induced neuronal damage. Epilepsia 1998, 39, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Woermann, F.G.; McLean, M.A.; Bartlett, P.A.; Parker, G.J.; Barker, G.J.; Duncan, J.S. Short echo time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging-negative temporal lobe epilepsy: Different biochemical profile compared with hippocampal sclerosis. Ann. Neurol. 1999, 45, 369–376. [Google Scholar] [CrossRef]
- Simister, R.J.; McLean, M.A.; Barker, G.J.; Duncan, J.S. A Proton Magnetic Resonance Spectroscopy Study of Metabolites in the Occipital Lobes in Epilepsy. Epilepsia 2003, 44, 550–558. [Google Scholar] [CrossRef]
- Sherwin, A.; Robitaille, Y.; Quesney, F.; Olivier, A.; Villemure, J.; Leblanc, R.; Feindel, W.; Andermann, E.; Gotman, J.; Ethier, R.; et al. Excitatory amino acids are elevated in human epileptic cerebral cortex. Neurology 1988, 38, 920. [Google Scholar] [CrossRef]
- Petroff, O.A.; Pleban, L.A.; Spencer, D.D. Symbiosis between in vivo and in vitro NMR spectroscopy: The creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magn. Reson. Imaging 1995, 13, 1197–1211. [Google Scholar] [CrossRef]
- Pfund, Z.; Chugani, D.C.; Juhász, C.; Muzik, O.; Chugani, H.T.; Wilds, I.B.; Seraji-Bozorgzad, N.; Moore, G.J. Evidence for Coupling between Glucose Metabolism and Glutamate Cycling Using FDG PET and 1H Magnetic Resonance Spectroscopy in Patients with Epilepsy. J. Cereb. Blood Flow Metab. 2000, 20, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Seymour, K.J.; Bluml, S.; Sutherling, J.; Sutherling, W.; Ross, B.D. Identification of cerebral acetone by 1H-MRS in patients with epilepsy controlled by ketogenic diet. Magma 1999, 8, 33–42. [Google Scholar] [PubMed]
- Horska, A.; Mahone, E.M. 1H Magnetic Resonance Spectroscopy of the Brain During Adolescence: Normal Brain Development and Neuropsychiatric Disorders. In MR Spectroscopy of Pediatric Brain Disorders; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2013; pp. 193–212. [Google Scholar]
- Levitt, J.G.; O’Neill, J.; Alger, J.R. Magnetic Resonance Spectroscopy Studies of Autistic Spectrum Disorders. In MR Spectroscopy of Pediatric Brain Disorders; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2013; pp. 213–227. [Google Scholar]
- O’Neill, J.; Levitt, J.G.; Alger, J.R. Magnetic Resonance Spectroscopy Studies of Attention Deficit Hyperactivity Disorder. In MR Spectroscopy of Pediatric Brain Diseases; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2013; pp. 229–275. [Google Scholar]
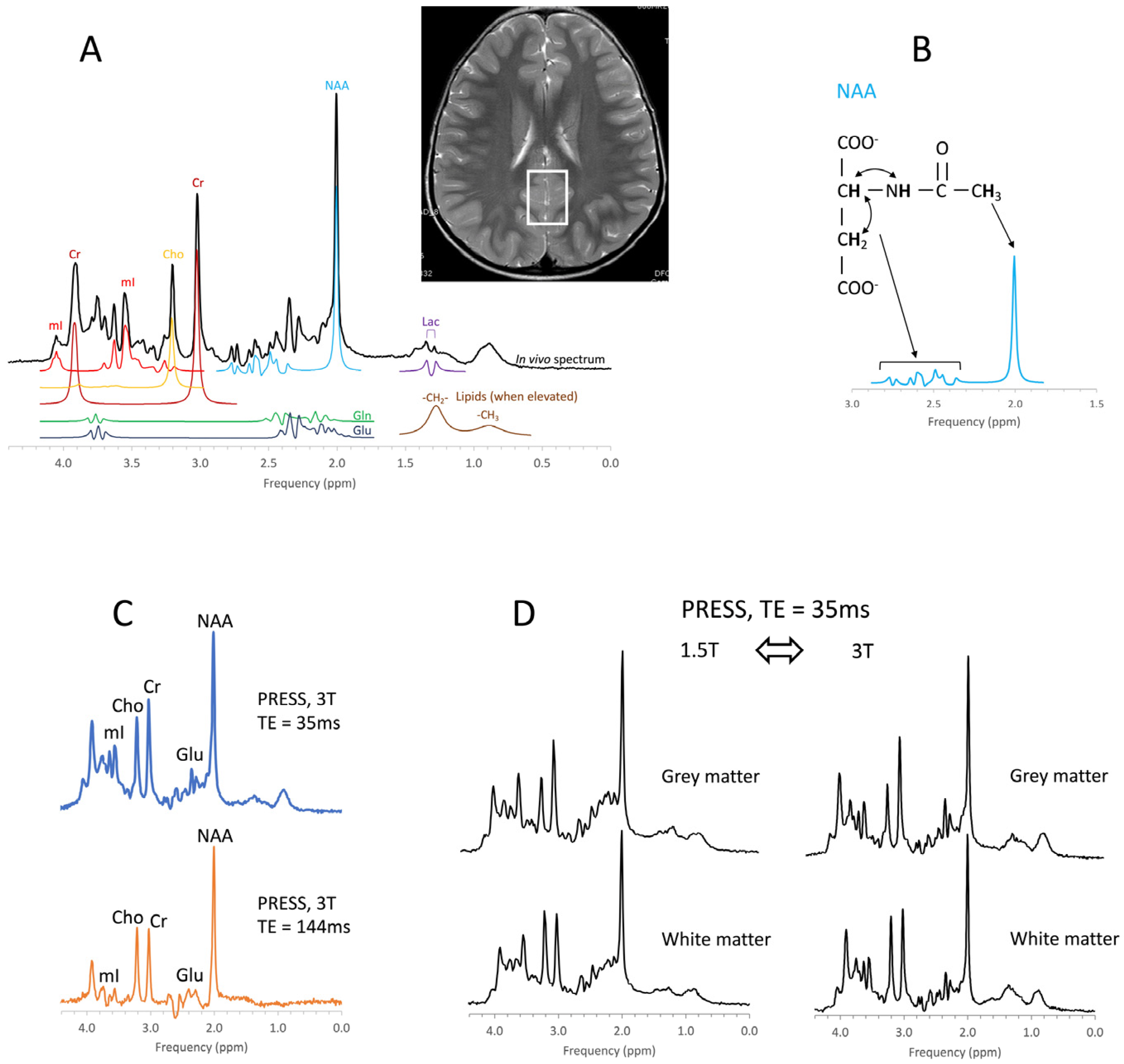








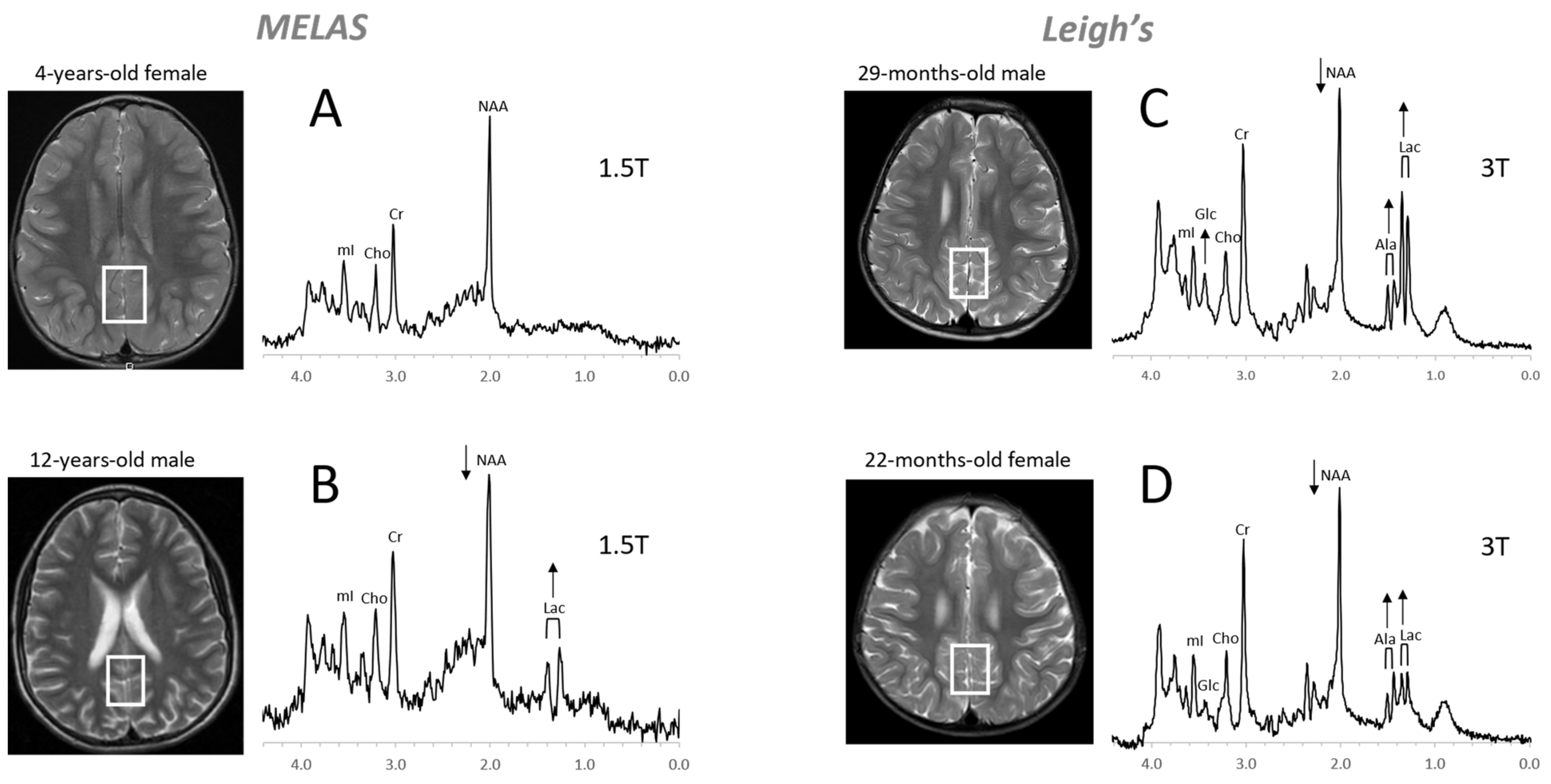

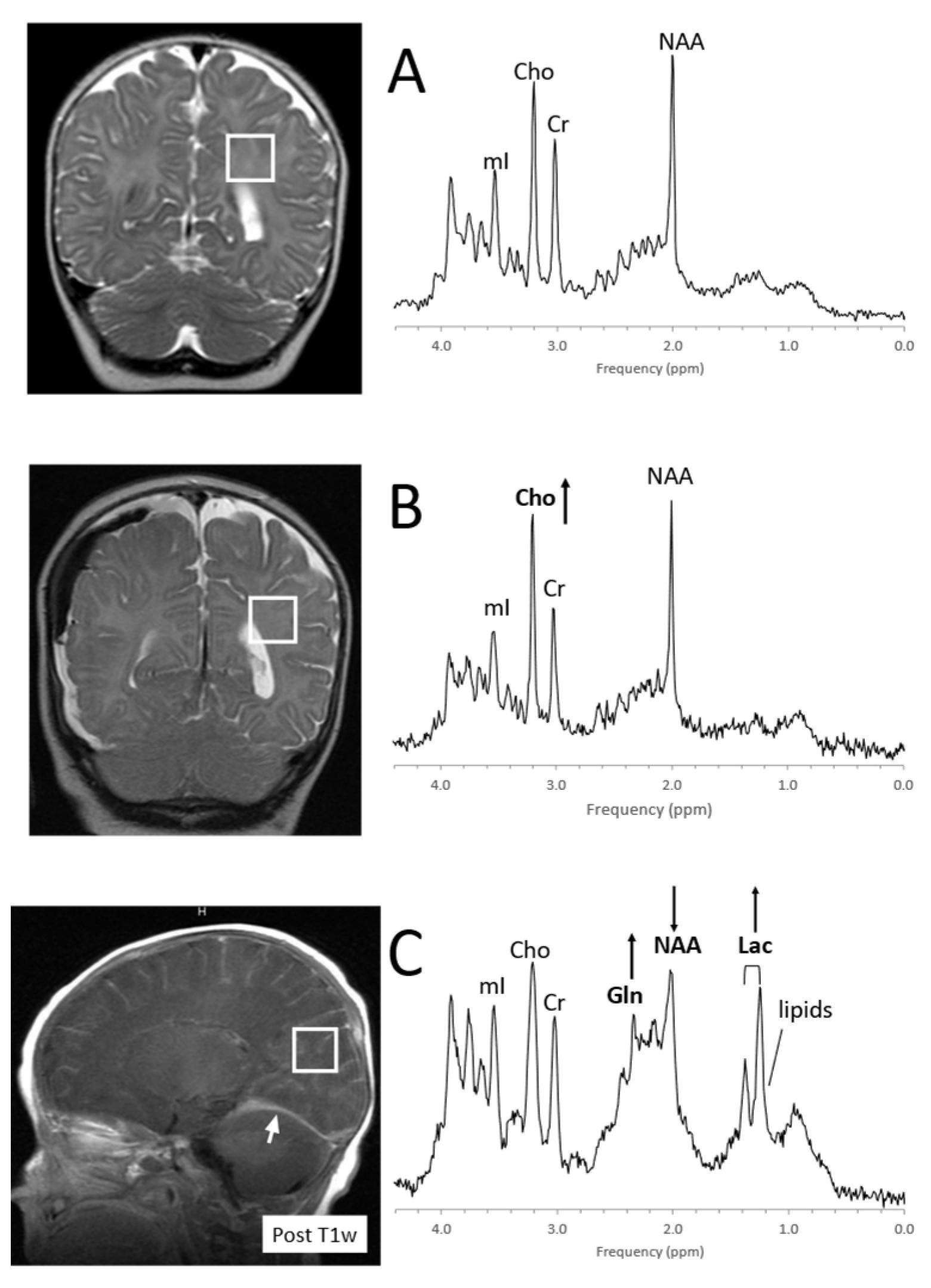


| Metabolite (Abbr.) | Functional Role and Remarks | Decreased a | Increased a |
|---|---|---|---|
| Acetate (Act) | Energy source, precursor of acetyl-CoA, common building block for biosynthesis | Disease correlate unknown | Infection/abscesses, brain death |
| Acetoacetate (AcAc) | Energy source, produced in the mitochondria of liver cells from acetoacetyl coenzyme A (CoA) | Disease correlate unknown | Ketosis |
| Acetone (Acn) | Produced by decarboxylation of acetoacetate, singlet at 2.22 ppm more readily detectable than βHB (see below) | Disease correlate unknown | Ketosis |
| Alanine (Ala) | Amino acid, protein constituent, glucose–alanine cycle | Disease correlate unknown | Inborn errors; meningioma and subgroups of other tumors |
| Aspartate (Asp) | Excitatory neurotransmitter NAA and Glu precursor | Disease correlate unknown | Challenging to recognize due to complex signal and signal overlap with NAA and other chemicals |
| β-Hydroxybutyrate (βHB) | Produced by the decarboxylation of acetoacetate, doublet similar to lactate but at 1.19 ppm | Disease correlate unknown | Ketosis |
| Choline (Cho) = glycerophosphocholine + phosphocholine + free choline | Membrane/myelin synthesis/degradation, acetylcholine precursor, osmolyte | Liver disease; hypo-osmotic state; during cooling (hypometabolic?) | De novo synthesis of biomass, including tumors, brain growth, tissue repair; hyper-osmotic state |
| Citrate (Cit) | TCA cycle intermediate, produced when the glycolytic rate exceeds TCA activity, fatty acid synthesis | Disease correlate unknown | Newborns, subgroups of tumors, most common in diffuse intrinsic brainstem gliomas |
| Creatine (Cr) = free creatine (fCR) + phosphocreatine (PCr) | Energy metabolism, energy storage PCr <-> fCr + ATP | Cells without creatine kinase, creatine deficiencies, some tumors | Subgroups of gliomas, gliosis? |
| γ-Aminobutyric acid (GABA) | Inhibitory neurotransmitter | Disease correlate unknown | Challenging to recognize due to complex signal and signal overlap with other chemicals |
| Glucose (Glc) (α and β isomers) | Principal fuel for cells | Hypoglycemia, detection challenging | Uncontrolled diabetes; hyperglycemia |
| Glutamate (Glu) | Excitatory neurotransmitter | Most tumors, hepatic encephalopathy, acute hypoxic/ischemic injury | Subgroup of seizures |
| Glutamine (Gln) | Part of the Glu–Gln neurotransmitter cycle; hyper ammonia detoxifier, fuel, osmolyte | Disease correlate unknown | Most tumors, edema (relative increase), demyelinating lesions, hepatic encephalopathy, acute hypoxic/ischemic injury |
| Glutathione (GSH) | Consists of glycine, cysteine, and glutamate. Present in reduced (predominant) and oxidized form. Marker of oxidative stress | Disease correlate unknown | Meningioma |
| Glycine (Glyc) | Neurotransmitter inhibitory and excitatory, cellular migration and circuit formation, antioxidant | Disease correlate unknown | Medulloblastoma and other tumors; hyperglycinemia |
| Lactate (Lac) | Endpoint of anaerobic glycolysis, in normal brain present in cerebrospinal fluid at higher concentrations than in tissue | Disease correlate unknown | Inborn errors of energy metabolism, hypoxic/ischemic injury; tumors, cystic lesions, normal newborn |
| Lipids (Lip) with contributions from macromolecules (MM) | Indicators for cell membrane breakdown when elevated | Disease correlate unknown | Injury/cell death and tumor subgroups |
| Leucine (Leu), iso-leucine (ILeu), valine (Val) | Branched-chain amino acids (BCAA) | Disease correlate unknown | Elevated in inborn error of BCAA metabolism, acute abscesses |
| Myo-inositol (mI) | Glial marker, involved in phospholipid membrane metabolism, osmolyte | Liver disease, hepatic encephalopathy, osmotic imbalance | Normal newborns, astrocytes, subgroups of tumors (e.g., astrocytoma, ependymoma, choroid plexus papilloma), osmotic imbalance |
| N-acetylaspartate (NAA) | Marker for mature neurons and axons | Pathologies associated with neuronal/axonal damage/loss, mitochondrial function? | Canavan disease |
| N-acetylaspartate glutamate (NAAG) | Neurotransmitter release modulator, small shoulder next to NAA, detectable in high-quality spectra | Disease correlate unknown | unknown |
| Phenylalanine | Essential amino acid | Disease correlate unknown | Uncontrolled phenylketonuria (PKU, phenylalanine hydroxylase deficiency) |
| Propylene glycol (Pgc) | Medication solvent (e.g., anticonvulsants), metabolizes to lactate, doublet similar to lactate but at 1.14 ppm | Disease correlate unknown | Frequently seen in newborns on medications, possibly because of underdeveloped blood–brain barrier |
| Scyllo-inositol (sI) | Symmetric sugar–alcohol isomer, osmolyte, inhibits amyloid-beta aggregation? | Disease correlate unknown in majority of population | Detectable under normal conditions in a subgroup of the population; glial tumors |
| Succinate (Suc) | TCA cycle intermediate | Disease correlate unknown | Abscesses, infection |
| Taurine (Tau) | Osmolyte, modulator of neurotransmission | Decreasing with normal brain maturation | Newborns; medulloblastoma (group 3, group 4), germinoma, pineoblastoma, and possibly others |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blüml, S.; Saunders, A.; Tamrazi, B. Proton MR Spectroscopy of Pediatric Brain Disorders. Diagnostics 2022, 12, 1462. https://doi.org/10.3390/diagnostics12061462
Blüml S, Saunders A, Tamrazi B. Proton MR Spectroscopy of Pediatric Brain Disorders. Diagnostics. 2022; 12(6):1462. https://doi.org/10.3390/diagnostics12061462
Chicago/Turabian StyleBlüml, Stefan, Alexander Saunders, and Benita Tamrazi. 2022. "Proton MR Spectroscopy of Pediatric Brain Disorders" Diagnostics 12, no. 6: 1462. https://doi.org/10.3390/diagnostics12061462
APA StyleBlüml, S., Saunders, A., & Tamrazi, B. (2022). Proton MR Spectroscopy of Pediatric Brain Disorders. Diagnostics, 12(6), 1462. https://doi.org/10.3390/diagnostics12061462






