Different Lung Parenchyma Quantification Using Dissimilar Segmentation Software: A Multi-Center Study for COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Protocol
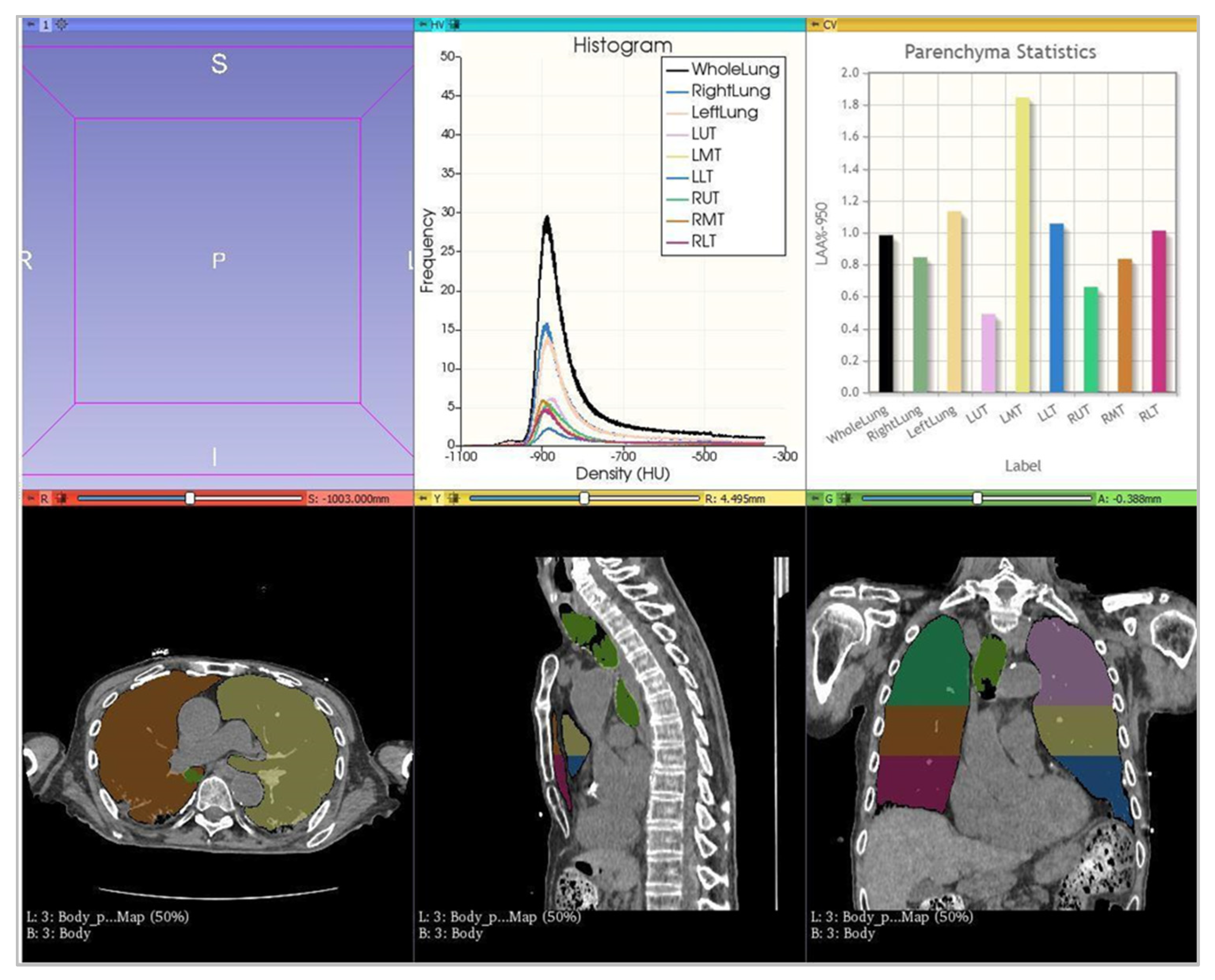
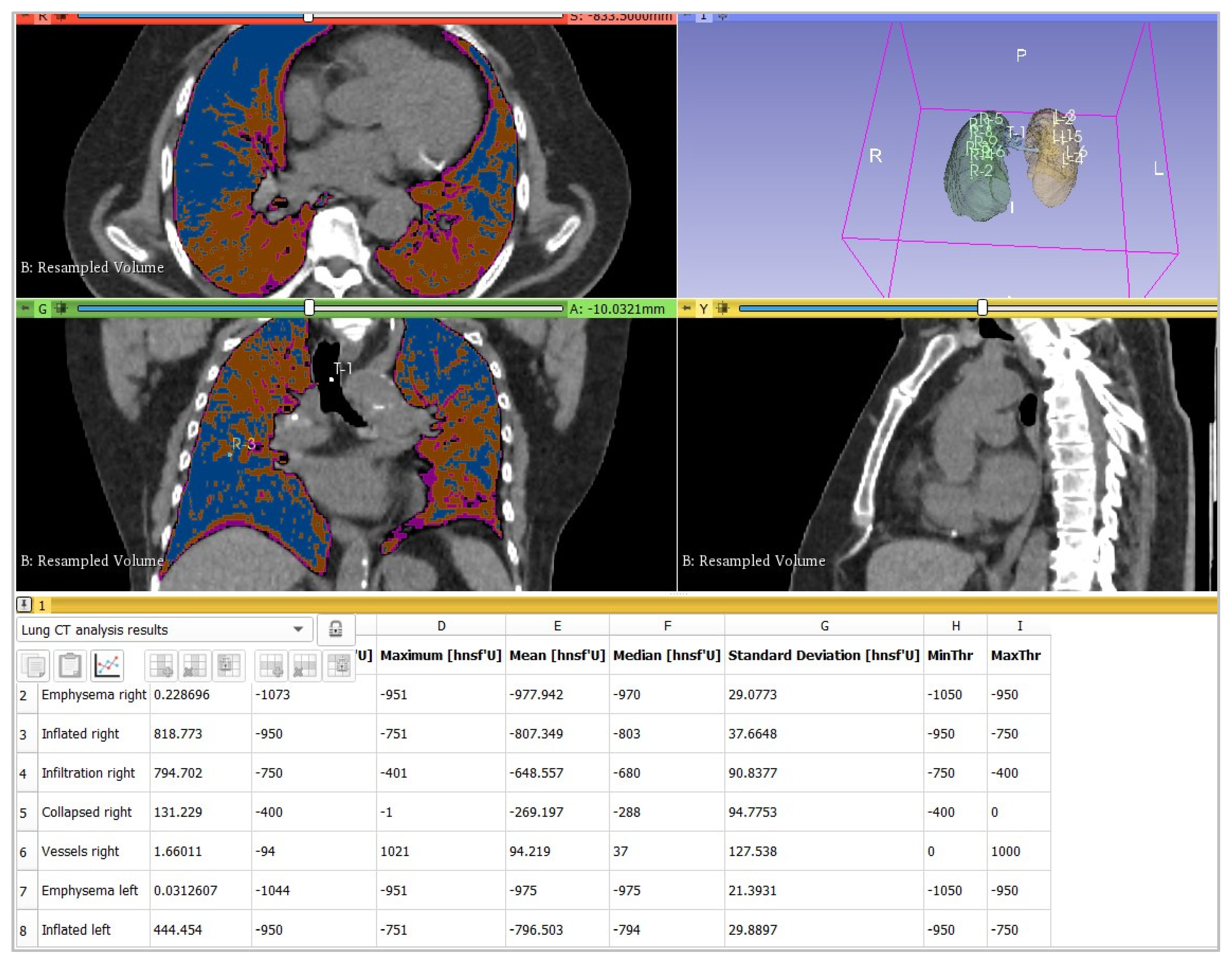
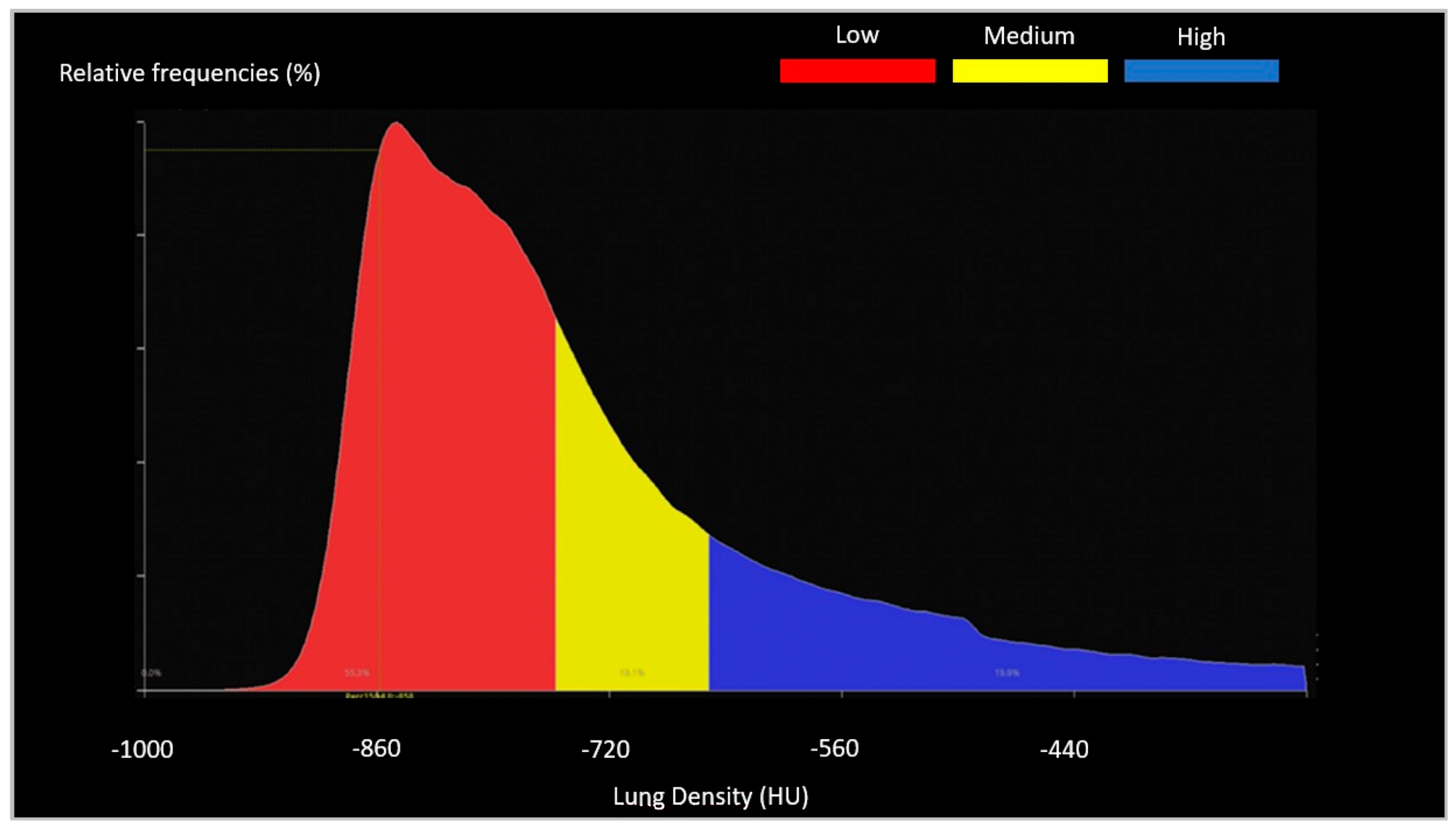
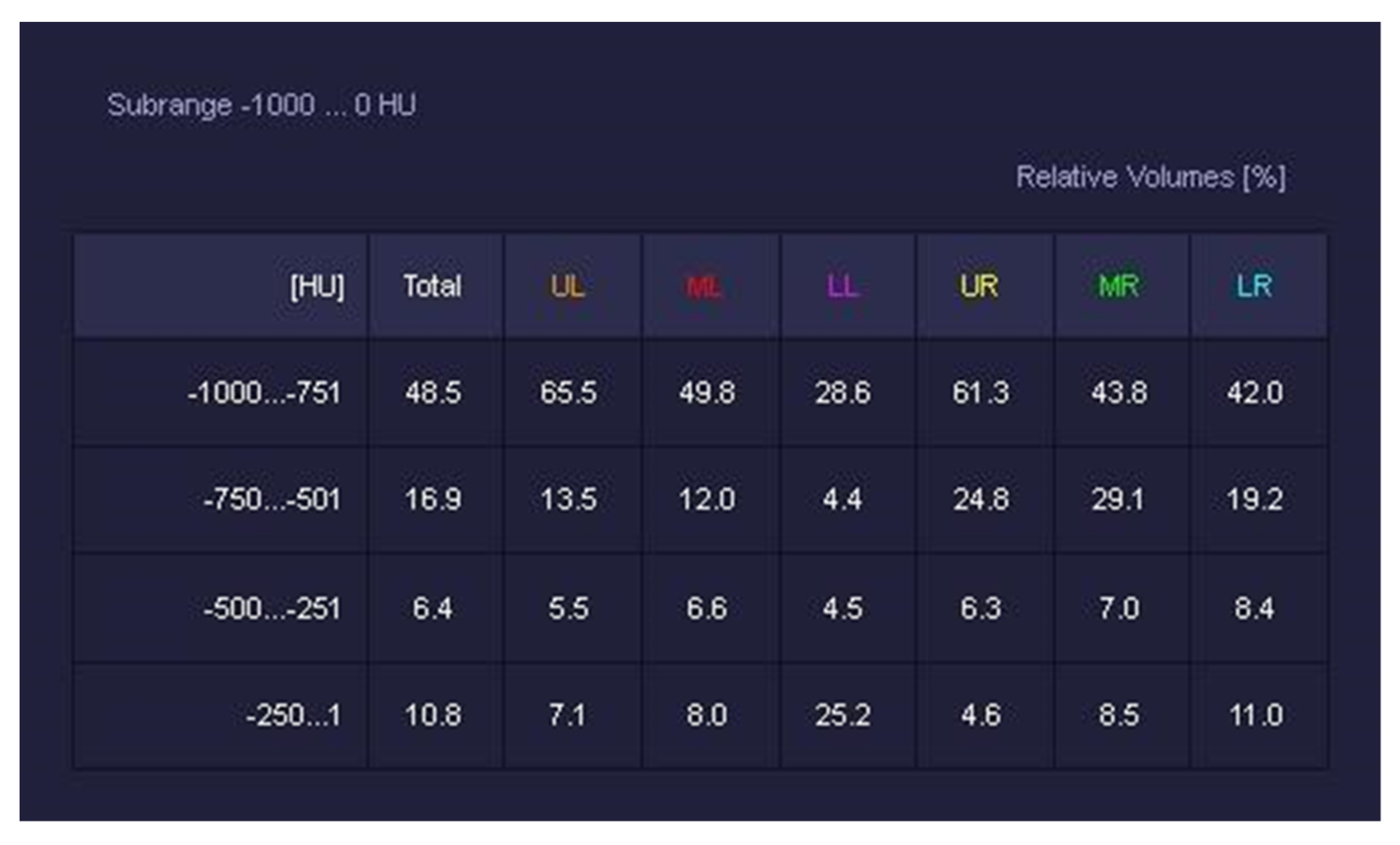
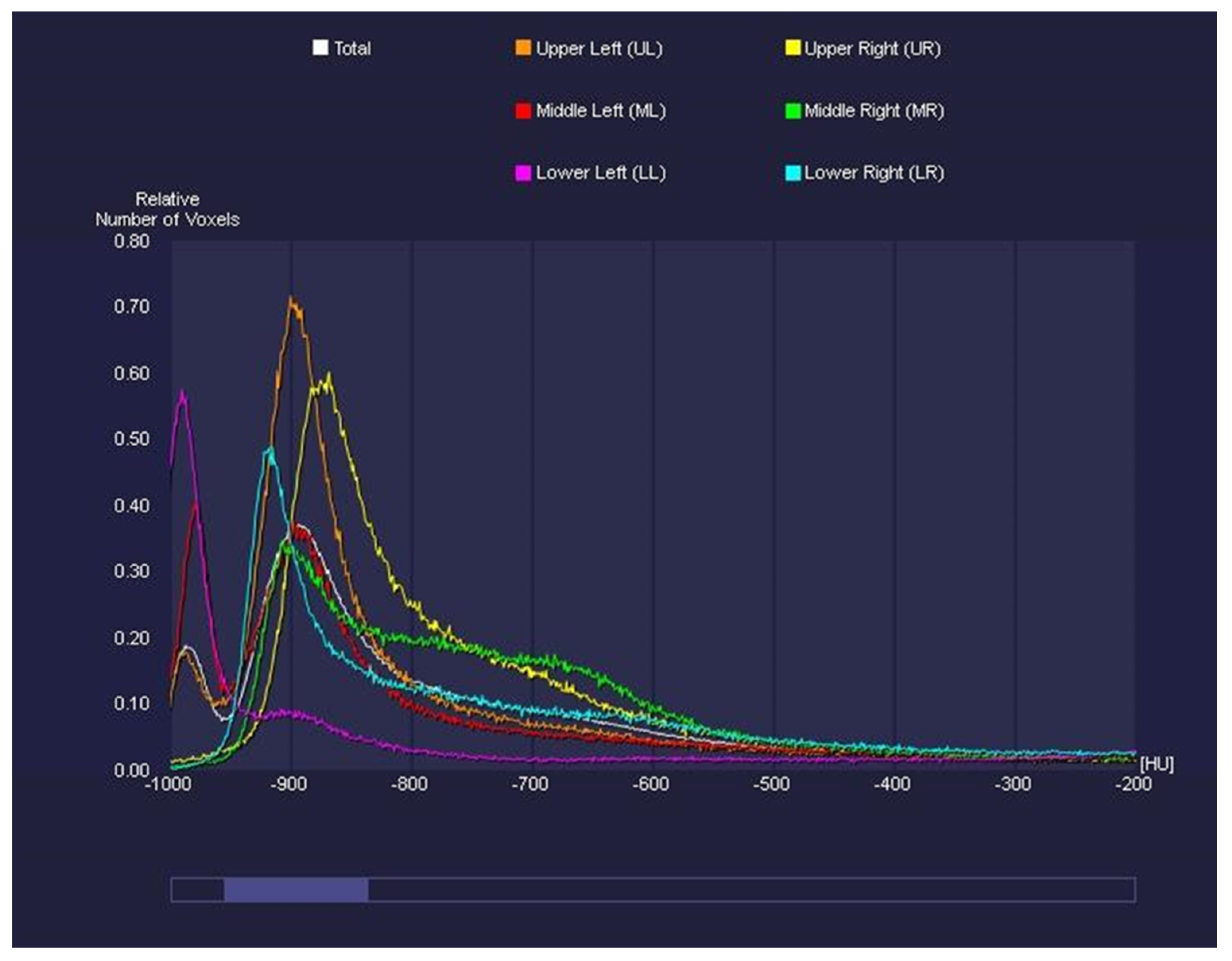
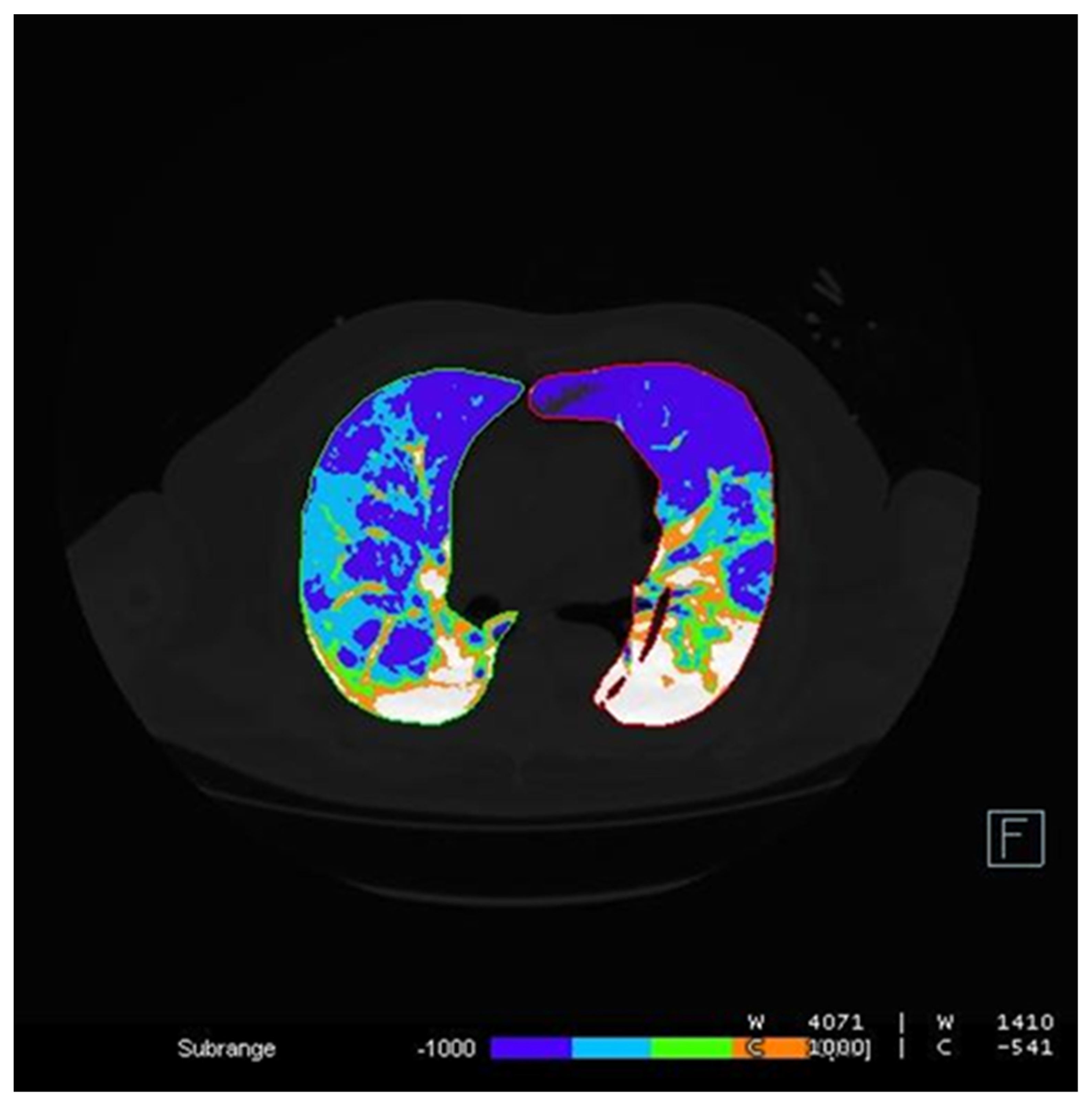
2.3. Image Analysis
- -
- -
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Relationships and Inter-Reader Agreement of Visual and Software-Based CT Assessment
4. Discussion
5. Conclusions
- -
- Semiautomatic segmentation software should be employed in daily work activities to make the reporting workflow smoother.
- -
- Different software have excellent concordance, so their employment is reliable and practicable in daily routines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Al World Health Organization. COVID-19—China. 2020. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233 (accessed on 5 December 2021).
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, M.C.S.; Pestana, D.V.S.; Gameiro, G.R.; da Silva, L.F.F.; Baron, Ė.; Rouby, J.-J.; Auler, J.O.C., Jr. SARS-CoV-2 pneumonia—Receptor binding and lung immunopathology: A narrative review. Crit. Care. 2021, 25, 53. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 5 December 2021).
- Tran, B.X.; Ha, G.H.; Nguyen, L.H.; Vu, G.T.; Hoang, M.T.; Le, H.T.; Latkin, C.A.; Ho, C.S.H.; Ho, R.C. Studies of Novel Coronavirus Disease 19 (COVID-19) Pandemic: A Global Analysis of Literature. Int. J. Environ. Res. Public Health 2020, 17, 4095. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.-J.; Martin, I.B.; et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Colombi, D.; Bodini, F.C.; Petrini, M.; Maffi, G.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology 2020, 296, E86–E96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, Q.; Huang, C.; Shi, C.; Wang, L.; Shi, N.; Fang, C.; Shan, F.; Mei, X.; Shi, J.; et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics 2020, 10, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Colombi, D.; Petrini, M.; Maffi, G.; Villani, G.D.; Bodini, F.C.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Comparison of admission chest computed tomography and lung ultrasound performance for diagnosis of COVID-19 pneumonia in populations with different disease prevalence. Eur. J. Radiol. 2020, 133, 109344. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Min, X.; Nan, Y.; Feng, Z.; Li, B.; Cai, W.; Xi, X.; Wang, L. Assessment of the Severity of Coronavirus Disease: Quantitative Computed Tomography Parameters versus Semiquantitative Visual Score. Korean J. Radiol. 2020, 21, 998–1006. [Google Scholar] [CrossRef]
- Colombi, D.; Villani, G.D.; Maffi, G.; Risoli, C.; Bodini, F.C.; Petrini, M.; Morelli, N.; Anselmi, P.; Milanese, G.; Silva, M.; et al. Qualitative and quantitative chest CT parameters as predictors of specific mortality in COVID-19 patients. Emerg. Radiol. 2020, 27, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef]
- 3D Slicer Image Computing Platform. Available online: https://www.slicer.org (accessed on 16 June 2022).
- CT Lung Density Analysis. Available online: https://global.medical.canon (accessed on 16 June 2022).
- Syngo CT Pulmo 3D. Available online: https://www.siemens-healthineers.com (accessed on 16 June 2022).
- Svahn, T.M.; Sjöberg, T.; Ast, J.C. Dose estimation of ultra-low-dose chest CT to different sized adult patients. Eur. Radiol. 2019, 29, 4315–4323. [Google Scholar] [CrossRef]
- Afadzi, M.; Fosså, K.; Andersen, H.K.; Aaløkken, T.M.; Martinsen, A.C.T. Image Quality Measured From Ultra-Low Dose Chest Computed Tomography Examination Protocols Using 6 Different Iterative Reconstructions From 4 Vendors, a Phantom Study. J. Comput. Assist. Tomogr. 2020, 44, 95–101. [Google Scholar] [CrossRef]
- Söderberg, M.; Gunnarsson, M. Automatic exposure control in computed tomography—An evaluation of systems from different manufacturers. Acta Radiol. 2010, 51, 625–634. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Sverzellati, N.; Milanese, G.; Milone, F.; Balbi, M.; Ledda, R.E.; Silva, M. Integrated Radiologic Algorithm for COVID-19 Pandemic. J. Thorac. Imaging 2020, 35, 228–233. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, Y.; Zhang, Y.; Zhang, N.; Zhao, S.; Zeng, H.; Deng, W.; Huang, Z.; Liu, S.; Song, B. A Quantitative and Radiomics approach to monitoring ARDS in COVID-19 patients based on chest CT: A retrospective cohort study. Int. J. Med. Sci. 2020, 17, 1773–1782. [Google Scholar] [CrossRef]
- LungCTAnalyzer Extension. Available online: https://github.com/rbumm/SlicerLungCTAnalyzer (accessed on 16 June 2022).
- Ippolito, D.; Ragusi, M.; Gandola, D.; Maino, C.; Pecorelli, A.; Terrani, S.; Peroni, M.; Giandola, T.; Porta, M.; Franzesi, C.T.; et al. Computed tomography semi-automated lung volume quantification in SARS-CoV-2-related pneumonia. Eur. Radiol. 2021, 31, 2726–2736. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Qu, C.; He, J.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China medical treatment expert group for COVID-19 2020. Clinical Characteristics of coronavirus disease in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Friedman Test in SPSS Statistics. Available online: https://statistics.laerd.com/spss-tutorials/friedman-test-using-spss-statistics.php (accessed on 23 February 2022).
- Ruch, Y.; Kaeuffer, C.; Ohana, M.; Labani, A.; Fabacher, T.; Bilbault, P.; Kepka, S.; Solis, M.; Greigert, V.; Lefebvre, N.; et al. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1417.e5–1417.e8. [Google Scholar] [CrossRef]
- Simpson, S.; Kay, F.U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; Henry, T.S.; Kanne, J.P.; Kligerman, S.; Ko, J.P.; et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA-Secondary Publication. J. Thorac. Imaging 2020, 35, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.M.; Ghonimy, M.B.I. Semi-quantitative CT imaging in improving visualization of faint ground glass opacities seen in early/mild coronavirus (COVID-19) cases. Egypt. J. Radiol. Nucl. Med. 2020, 51, 244. [Google Scholar] [CrossRef]
- Grassi, R.; Cappabianca, S.; Urraro, F.; Feragalli, B.; Montanelli, A.; Patelli, G.; Granata, V.; Giacobbe, G.; Russo, G.M.; Grillo, A.; et al. Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software. Int. J. Environ. Res. Public Health 2020, 17, 6914. [Google Scholar] [CrossRef]
- Huang, G.; Gong, T.; Wang, G.; Wang, J.; Guo, X.; Cai, E.; Li, S.; Li, X.; Yu, Y.; Lin, L. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores From Sequential Chest CT. Am. J. Roentgenol. 2020, 215, 367–373. [Google Scholar] [CrossRef]
- Shen, C.; Yu, N.; Cai, S.; Zhou, J.; Sheng, J.; Liu, K.; Zhou, H.; Guo, Y.; Niu, G. Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019. J. Pharm. Anal. 2020, 10, 123–129. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Jungmann, F.; Müller, L.; Hahn, F.; Weustenfeld, M.; Dapper, A.-K.; Mähringer-Kunz, A.; Graafen, D.; Düber, C.; Schafigh, D.; dos Santos, D.P.; et al. Commercial AI solutions in detecting COVID-19 pneumonia in chest CT: Not yet ready for clinical implementation? Eur. Radiol. 2022, 32, 3152–3160. Available online: https://link.springer.com/10.1007/s00330-021-08409-4 (accessed on 20 April 2022). [CrossRef]
- Galli, M.G.; Djuric, O.; Besutti, G.; Ottone, M.; Amidei, L.; Bitton, L.; Bonilauri, C.; Boracchia, L.; Campanale, S.; Curcio, V.; et al. Reggio Emilia COVID-19 Working Group. Clinical and imaging characteristics of patients with COVID-19 predicting hospital readmission after emergency department discharge: A single-centre cohort study in Italy. BMJ Open 2022, 12, e052665. [Google Scholar] [CrossRef]





| Rows | kV | mAs | Pitch | Collimation | Thickness | Increment | |
|---|---|---|---|---|---|---|---|
| Emotion 16 | 16 | 130 | fixed, adjusted on the patient body weight | >0.8 | 1.2 mm | 2 | 2 |
| Aquilion PrimeSP | 80 | 120 | AEC with SD 12.5 | >0.8 | 0.5 mm | 2 | 2 |
| Somatom Go.Top | 64 | 100-110-Sn110-Sn140 | AEC with Q.ref.mAs 10-60-100-110 | >0.8 | 0.625 mm | 2 | 2 |
| 25th Percentile | Median | 75th Percentile | |
|---|---|---|---|
| 1° radiologist (D.C.) | 15 | 25 | 35 |
| 2° radiologist (F.R.) | 10 | 20 | 35 |
| 3° radiologist (M.D.) | 10 | 20 | 30 |
| 4° radiologist (R.A.) | 10 | 20 | 32.5 |
| ICC = 0.79 | |||
| 25th Percentile | Median | 75th Percentile | |
|---|---|---|---|
| 3DSlicer | 27 | 35 | 46 |
| Canon | 22 | 32 | 43 |
| Siemens | 24 | 32 | 42 |
| ICC = 0.92 | |||
| Software Name | Vendor | Median Software Assessment | Median Visual Assessment | ICC |
|---|---|---|---|---|
| LungCTAnalyzer | 3DSlicer | 35 | 23 | 0.75 |
| CT Lung Density Analysis | Canon | 32 | 23 | 0.74 |
| CT Pulmo 3D | Siemens | 32 | 23 | 0.70 |
| Parenchyma Analysis | 3DSlicer | 25 | 23 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risoli, C.; Nicolò, M.; Colombi, D.; Moia, M.; Rapacioli, F.; Anselmi, P.; Michieletti, E.; Ambrosini, R.; Di Terlizzi, M.; Grazioli, L.; et al. Different Lung Parenchyma Quantification Using Dissimilar Segmentation Software: A Multi-Center Study for COVID-19 Patients. Diagnostics 2022, 12, 1501. https://doi.org/10.3390/diagnostics12061501
Risoli C, Nicolò M, Colombi D, Moia M, Rapacioli F, Anselmi P, Michieletti E, Ambrosini R, Di Terlizzi M, Grazioli L, et al. Different Lung Parenchyma Quantification Using Dissimilar Segmentation Software: A Multi-Center Study for COVID-19 Patients. Diagnostics. 2022; 12(6):1501. https://doi.org/10.3390/diagnostics12061501
Chicago/Turabian StyleRisoli, Camilla, Marco Nicolò, Davide Colombi, Marco Moia, Fausto Rapacioli, Pietro Anselmi, Emanuele Michieletti, Roberta Ambrosini, Marco Di Terlizzi, Luigi Grazioli, and et al. 2022. "Different Lung Parenchyma Quantification Using Dissimilar Segmentation Software: A Multi-Center Study for COVID-19 Patients" Diagnostics 12, no. 6: 1501. https://doi.org/10.3390/diagnostics12061501
APA StyleRisoli, C., Nicolò, M., Colombi, D., Moia, M., Rapacioli, F., Anselmi, P., Michieletti, E., Ambrosini, R., Di Terlizzi, M., Grazioli, L., Colmo, C., Di Naro, A., Natale, M. P., Tombolesi, A., Adraman, A., Tuttolomondo, D., Costantino, C., Vetti, E., & Martini, C. (2022). Different Lung Parenchyma Quantification Using Dissimilar Segmentation Software: A Multi-Center Study for COVID-19 Patients. Diagnostics, 12(6), 1501. https://doi.org/10.3390/diagnostics12061501









