Hepatocellular Carcinoma Presenting Simultaneously with Echinococcal Cyst Mimicking a Single Liver Lesion in a Non-Cirrhotic Patient: A Case Report and Review of the Literature
Abstract
1. Introduction
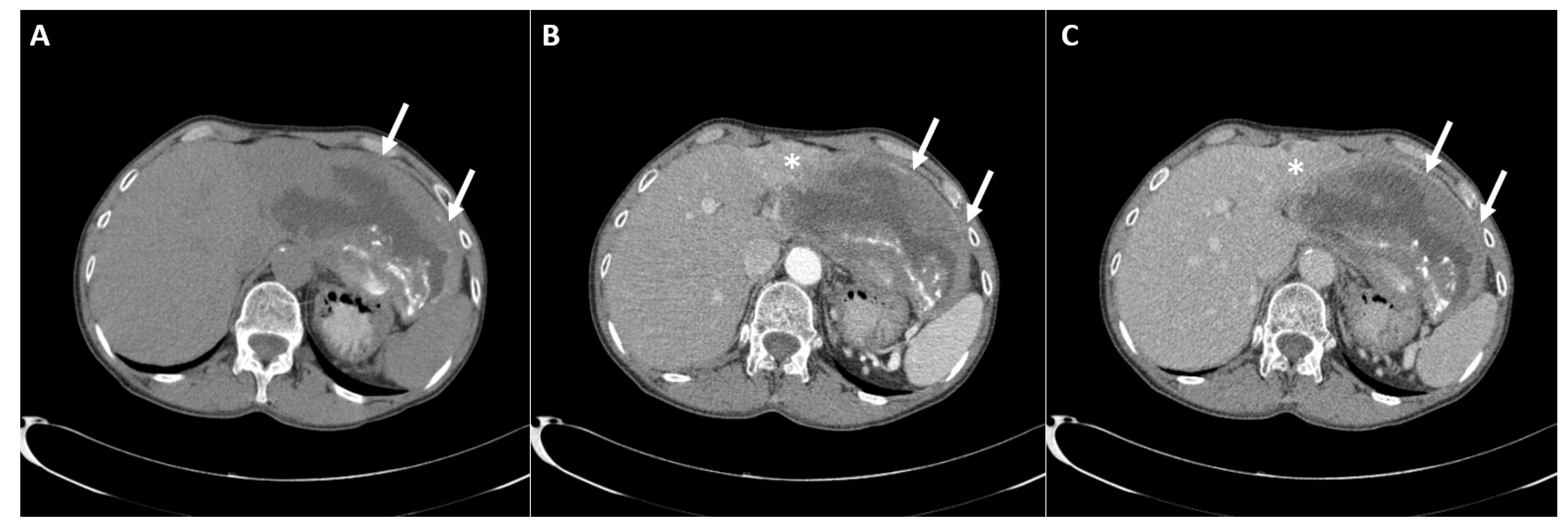
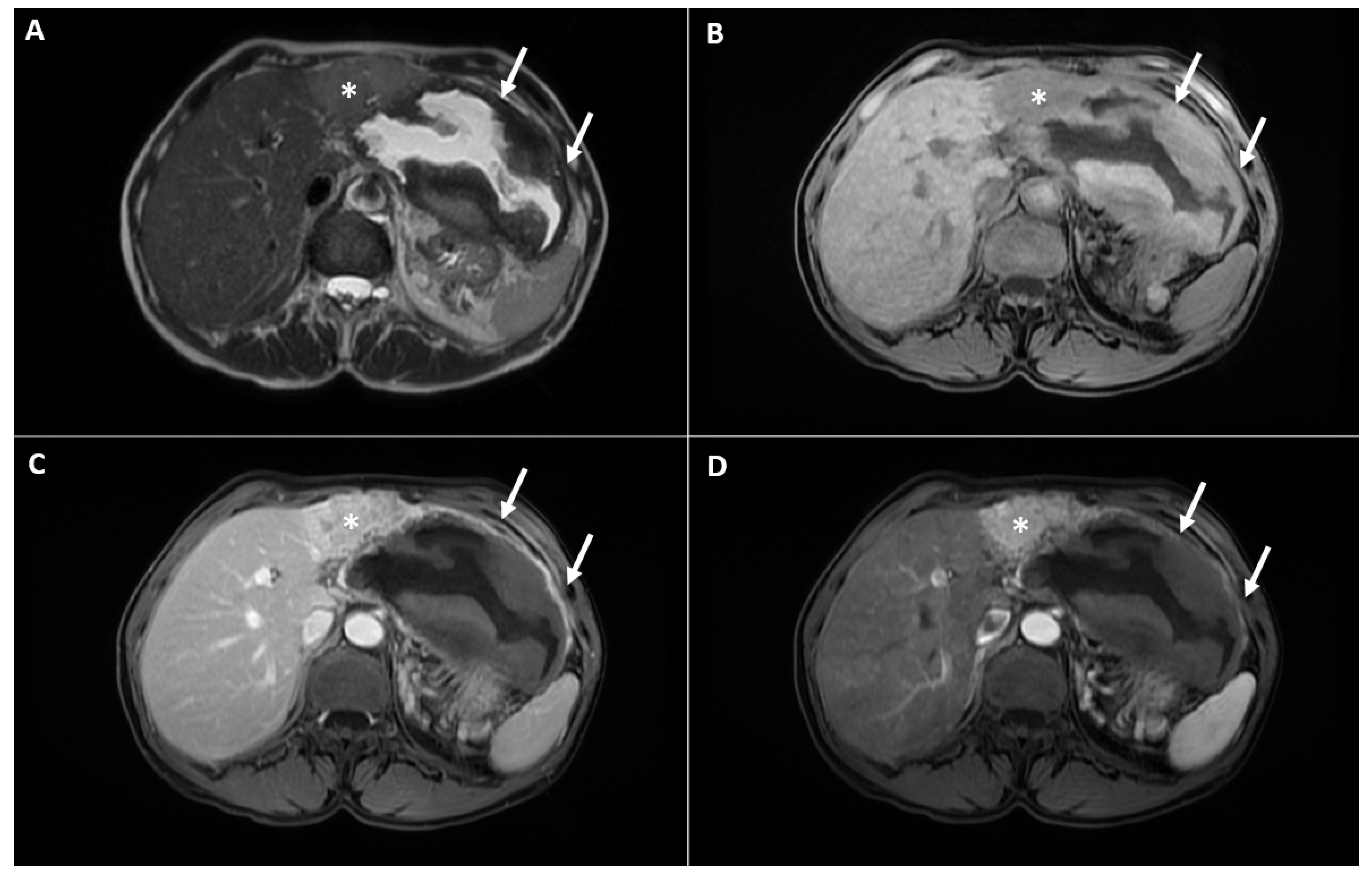
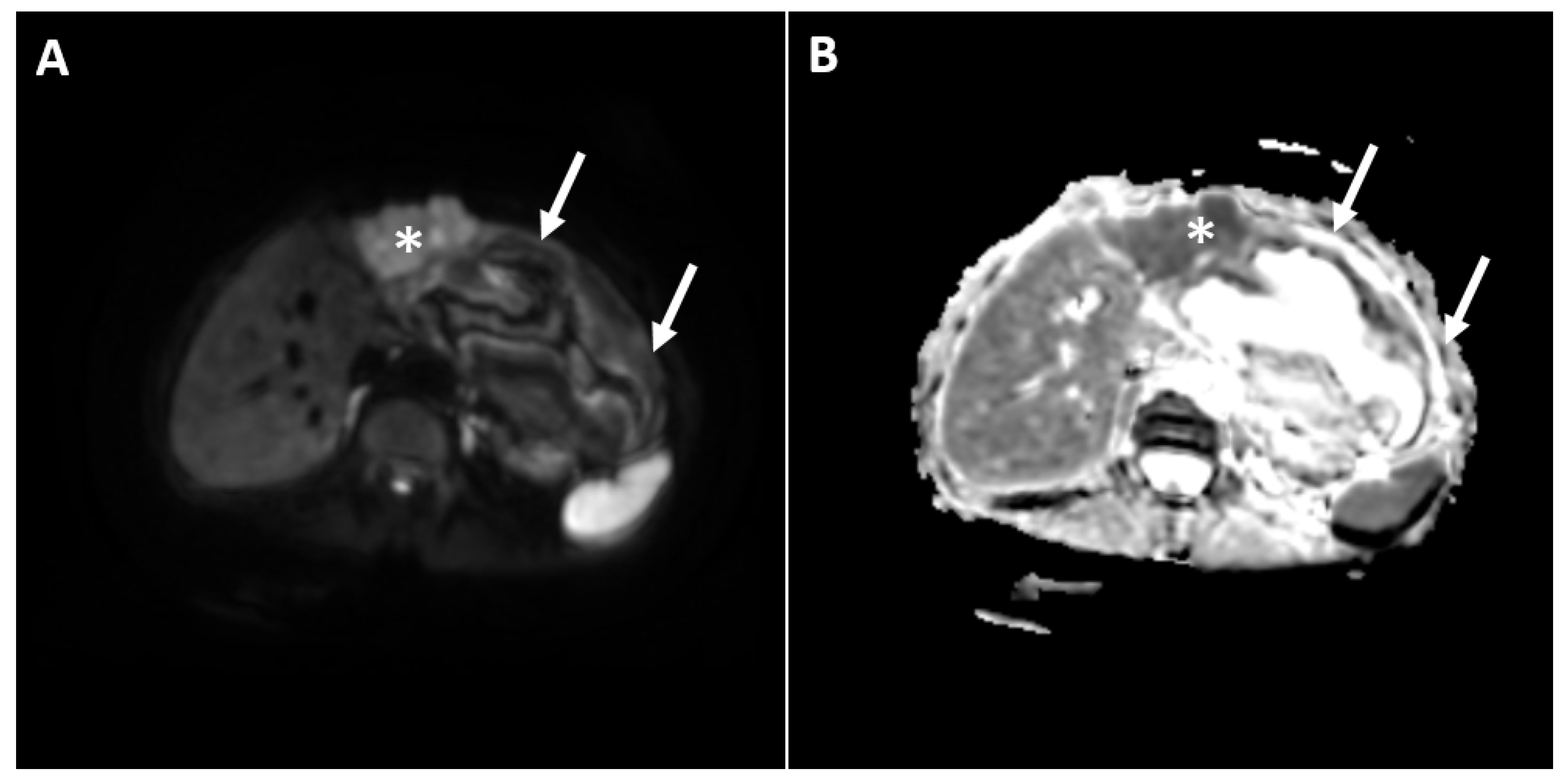
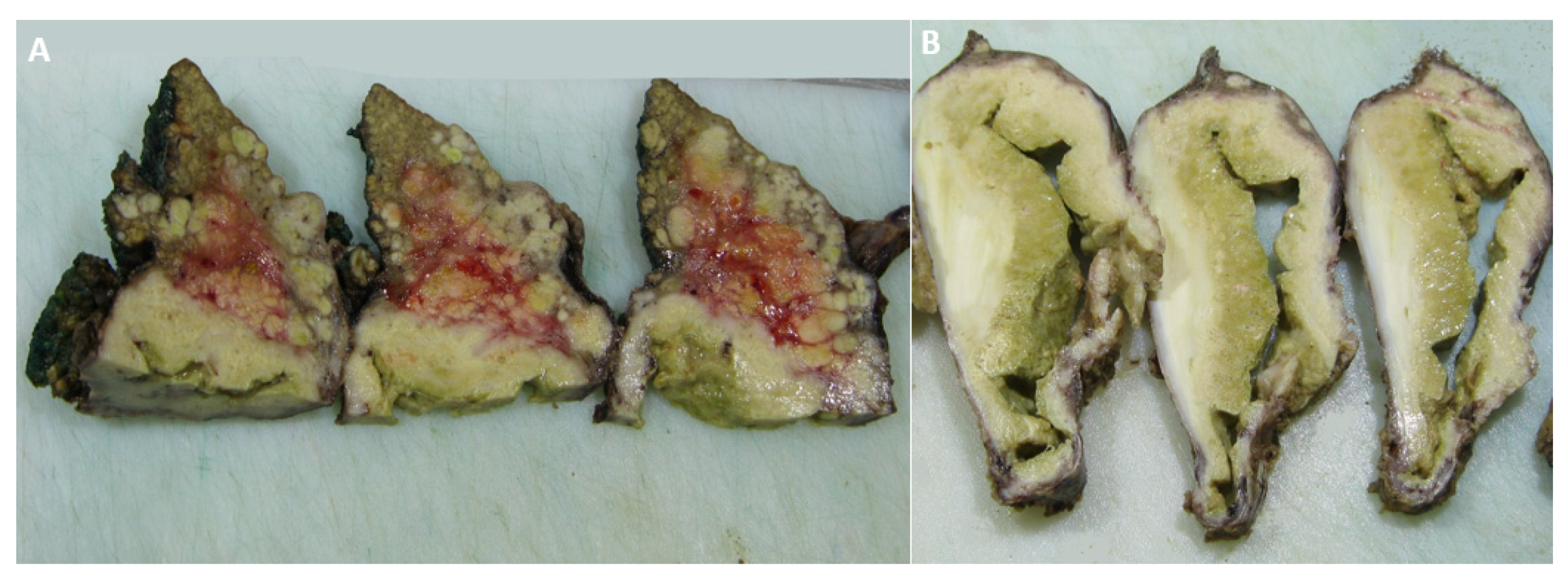
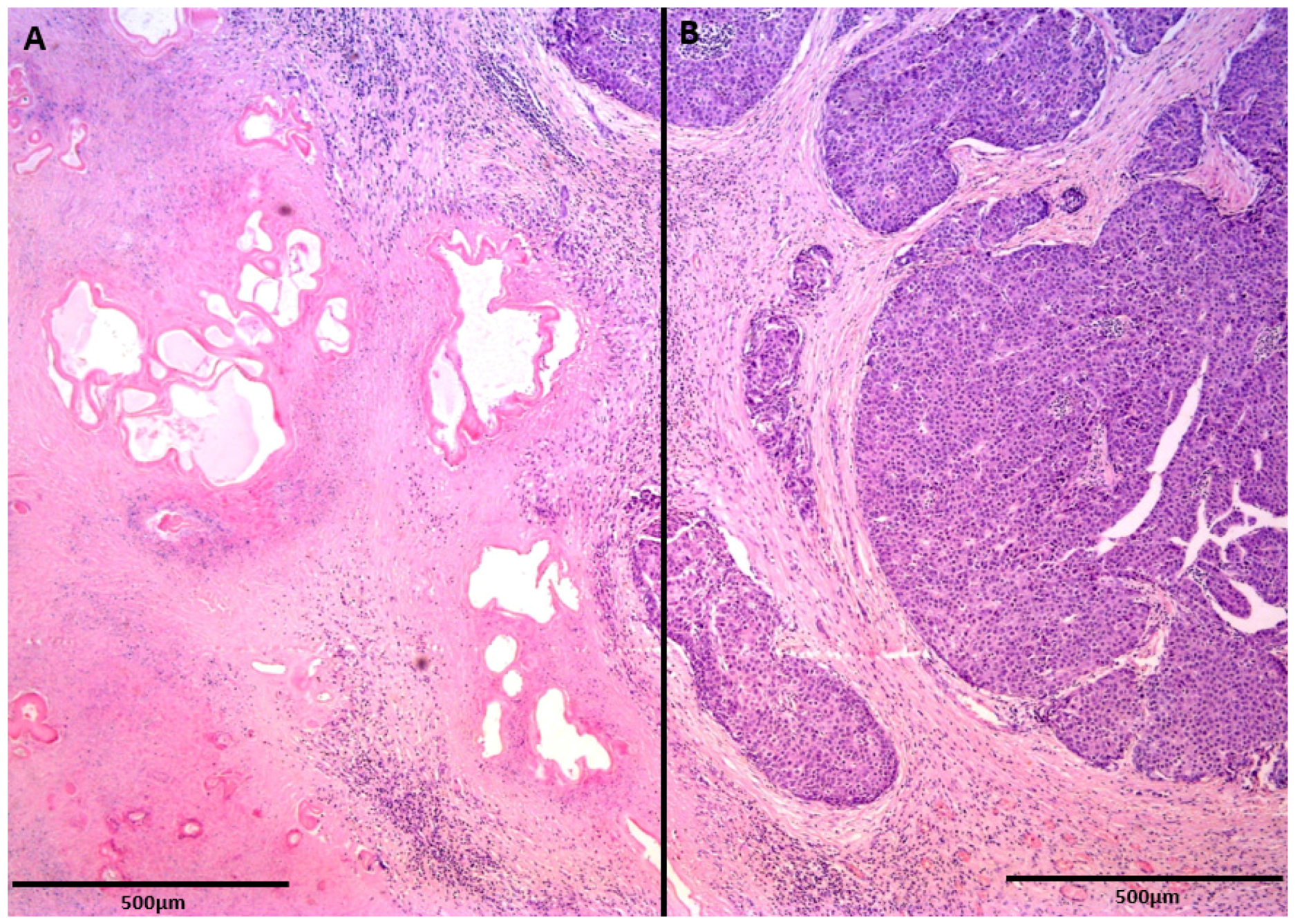
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Hennedige, T.; Venkatesh, S.K. Imaging of hepatocellular carcinoma: Diagnosis, staging and treatment monitoring. Cancer Imaging 2013, 12, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.D.; Milovanovic, T.; Dugalic, V.; Dumic, I. Pearls and pitfalls in magnetic resonance imaging of hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 2012–2029. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef]
- Czermak, B.V.; Unsinn, K.M.; Gotwald, T.; Niehoff, A.A.; Freund, M.C.; Waldenberger, P.; Vogel, W.; Jaschke, W.R. Echinococcus granulosus revisited: Radiologic patterns seen in pediatric and adult patients. Am. J. Roentgenol. 2001, 177, 1051–1056. [Google Scholar] [CrossRef]
- Kalifu, B.; Meng, Y.; Maimaitinijiati, Y.; Ma, Z.G.; Tian, G.L.; Wang, J.G.; Chen, X. Radical resection of hepatic polycystic echinococcosis complicated with hepatocellular carcinoma: A case report. World J. Clin. Cases 2021, 9, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Romic, B.; Romic, I.; Petrovic, I.; Romic, M.; Romic, R.; Romic, M.; Mance, M.; Pavlek, G.A. Synchronous Occurrence of Hepatocellular Carcinoma and Echinoccocal Liver Cyst—Can Parasite Promote Carcinogenesis? Literature Review and Classification Proposal. Chirurgia 2016, 111, 297–303. [Google Scholar]
- Bo, R.; Yasen, A.; Shao, Y.; Zhang, W.; Lin, R.; Jiang, T.; Wen, H.; Xiao, H.; Aji, T. Co-existence of hepatocellular carcinoma and cystic echinococcosis. Infect. Agent. Cancer 2020, 15, 5. [Google Scholar] [CrossRef]
- Guo, J.; Ma, C.; Song, X.; Tang, F.; Guo, L.; Mao, J.; Li, Y. Hepatocellular Carcinoma Complicated by Echinococcal Cyst: A Case Report. Front. Surg. 2022, 8, 816501. [Google Scholar] [CrossRef]
- Kostov, D.; Dragnev, N.; Patanov, R.; Kobakov, G. Hepatocellular carcinoma complicated with echinococcal cyst of the liver. Khirurgiia 2010, 4–5, 49–50. [Google Scholar]
- Kübeck, M.; Stöckl, V.; Stainer, W.; Schermaier, T.; Preisinger, J.; Schauer, W.; Hochleitner, U.; Höbling, W.; Barth, T.F.; Stadler, B.; et al. Cystic echinococcosis and hepatocellular carcinoma--a coincidence? A case report. Z. Gastroenterol. 2014, 52, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, T.; Shao, Y.; Wen, H. Cystic echinococcosis accompanied by hepatocellular carcinoma in a female herdsman. Int. J. Clin. Exp. Med. 2015, 8, 2985–2988. [Google Scholar] [PubMed]
- Oikonomopoulou, K.; Yu, H.; Wang, Z.; Vasiliou, S.K.; Brinc, D.; Christofi, G.; Theodorou, M.; Pavlou, P.; Hadjisavvas, A.; Demetriou, C.A.; et al. Association between Echinococcus granulosus infection and cancer risk—A pilot study in Cyprus. Clin. Chem. Lab. Med. 2016, 54, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- van Knapen, F. Echinococcus granulosus infection and malignancy. Br. Med. J. 1980, 281, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Brunetti, E.; Neumayr, A.; Maestri, M.; Goblirsch, S.; Tamarozzi, F. Cystic echinococcosis of the liver: A primer for hepatologists. World J. Hepatol. 2014, 6, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Battula, N.; Madanur, M.; Priest, O.; Srinivasan, P.; O’Grady, J.; Heneghan, M.A.; Bowles, M.; Muiesan, P.; Heaton, N.; Rela, M. Spontaneous rupture of hepatocellular carcinoma: A Western experience. Am. J. Surg. 2009, 197, 164–167. [Google Scholar] [CrossRef]
- Kim, H.C.; Yang, D.M.; Jin, W.; Park, S.J. The various manifestations of ruptured hepatocellular carcinoma: CT imaging findings. Abdom. Imaging 2008, 33, 633–642. [Google Scholar] [CrossRef]
- Pohnan, R.; Ryska, M.; Hytych, V.; Matej, R.; Hrabal, P.; Pudil, J. Echinococcosis mimicking liver malignancy: A case report. Int. J. Surg. Case Rep. 2017, 36, 55–58. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Ariizumi, S.; Shibuya, G.; Uemura, S.; Kato, T.; Yazawa, T.; Yamashita, S.; Omori, A.; Higuchi, R.; Takahashi, Y.; et al. Hepatocellular carcinoma with ring calcification mimicking hydatid disease: A case report. Surg. Case Rep. 2020, 6, 171. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Staging systems for hepatocellular carcinoma: Current status and future perspectives. World J. Hepatol. 2015, 7, 406–424. [Google Scholar] [CrossRef]
- WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003, 85, 253–261. [Google Scholar] [CrossRef]
- Yasen, A.; Wang, M.; Ran, B.; Lv, G.; Aji, T.; Xiao, H.; Shao, Y.; Wen, H. Echinococcus granulosus protoscoleces promotes proliferation and invasion of hepatocellular carcinoma cells. Cytotechnology 2021, 73, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, B.; Spiliotis, M.; Müller, J.; Scholl, S.; Müller, N.; Gottstein, B.; Hemphill, A. Echinococcus multilocularis phosphoglucose isomerase (EmPGI): A glycolytic enzyme involved in metacestode growth and parasite-host cell interactions. Int. J. Parasitol. 2010, 40, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Turhan, N.; Esendagli, G.; Ozkayar, O.; Tunali, G.; Sokmensuer, C.; Abbasoglu, O. Co-existence of Echinococcus granulosus infection and cancer metastasis in the liver correlates with reduced Th1 immune responses. Parasite Immunol. 2015, 37, 16–22. [Google Scholar] [CrossRef]
- Marchetti, A.; Ardizzoni, A.; Papotti, M.; Crinò, L.; Rossi, G.; Gridelli, C.; Barberis, M.; Maiorano, E.; Normanno, N.; Taddei, G.L.; et al. Recommendations for the analysis of ALK gene rearrangements in non-small-cell lung cancer: A consensus of the Italian Association of Medical Oncology and the Italian Society of Pathology and Cytopathology. J. Thorac. Oncol. 2013, 8, 352–358. [Google Scholar] [CrossRef]
- Bangaru, S.D.; Kozarsky, P.E.; Lee, D.J.; Sica, G.L.; Owonikoko, T.K. A Bystander Effect of Lung Cancer Chemotherapy on Chronic Echinococcal Disease. World J. Oncol. 2015, 6, 416–420. [Google Scholar] [CrossRef][Green Version]
- Yousofi, D.H.; Soozangar, N.; Khorami, S.; Taji, F.; Yousofi, M.; Shirzad, H. Hydatid Cyst Protoscolices Induce Cell Death in WEHI-164 Fibrosarcoma Cells and Inhibit the Proliferation of Baby Hamster Kidney Fibroblasts In Vitro. J. Parasitol. Res. 2012, 2012, 304183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovač, J.D.; Mitrović, M.; Janković, A.; Andrejević, M.; Bogdanović, A.; Zdujić, P.; Đinđić, U.; Dugalić, V. Hepatocellular Carcinoma Presenting Simultaneously with Echinococcal Cyst Mimicking a Single Liver Lesion in a Non-Cirrhotic Patient: A Case Report and Review of the Literature. Diagnostics 2022, 12, 1583. https://doi.org/10.3390/diagnostics12071583
Kovač JD, Mitrović M, Janković A, Andrejević M, Bogdanović A, Zdujić P, Đinđić U, Dugalić V. Hepatocellular Carcinoma Presenting Simultaneously with Echinococcal Cyst Mimicking a Single Liver Lesion in a Non-Cirrhotic Patient: A Case Report and Review of the Literature. Diagnostics. 2022; 12(7):1583. https://doi.org/10.3390/diagnostics12071583
Chicago/Turabian StyleKovač, Jelena Djokić, Milica Mitrović, Aleksandra Janković, Marko Andrejević, Aleksandar Bogdanović, Predrag Zdujić, Uroš Đinđić, and Vladimir Dugalić. 2022. "Hepatocellular Carcinoma Presenting Simultaneously with Echinococcal Cyst Mimicking a Single Liver Lesion in a Non-Cirrhotic Patient: A Case Report and Review of the Literature" Diagnostics 12, no. 7: 1583. https://doi.org/10.3390/diagnostics12071583
APA StyleKovač, J. D., Mitrović, M., Janković, A., Andrejević, M., Bogdanović, A., Zdujić, P., Đinđić, U., & Dugalić, V. (2022). Hepatocellular Carcinoma Presenting Simultaneously with Echinococcal Cyst Mimicking a Single Liver Lesion in a Non-Cirrhotic Patient: A Case Report and Review of the Literature. Diagnostics, 12(7), 1583. https://doi.org/10.3390/diagnostics12071583






