Abstract
Fibrous dysplasia (FD) is a benign fibro-osseous lesion that frequently involves the craniofacial bones and femur. Malignant transformation of FD is a rare occurrence. We report a 38-year-old woman with osteosarcoma (OS) arising from FD of the femur. Magnetic resonance imaging revealed a well-defined lesion in the medulla of the femur, with cortical thinning and local bone destruction. Wide excision of the femur was performed. Grossly, the inner part of the mass was hard and tan-gray in color, and the outer part of the mass adjacent to the cortex showed myxoid discoloration with infiltrative borders. Microscopically, most of the tumor consisted of curvilinear woven bone and fibrous stroma with bland spindle cells, which transitioned to the outer portion of the tumor, showing cellular proliferation of pleomorphic cells with frequent mitotic activity. Next-generation sequencing revealed GNAS and TP53 mutations, and the diagnosis of OS arising from FD was strongly supported. This case highlights the characteristic images and molecular features of the malignant transformation of FD.
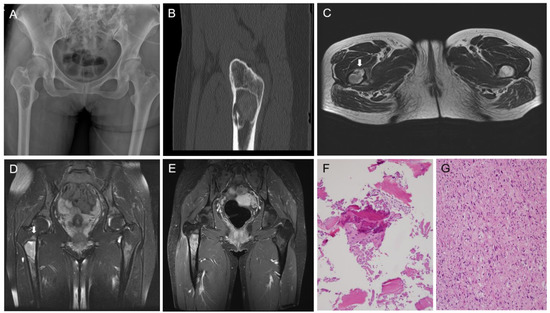
Figure 1.
Fibrous dysplasia (FD) is a benign fibro-osseous lesion with varying proportions of fibrous and osseous components. Depending on whether FD affects one or multiple bones, it is divided into monostotic and polyostotic types [1]. The craniofacial bones and femur are commonly involved in monostotic FD [2]. Malignant transformation of FD rarely occurs, and osteosarcoma (OS) is the most common histological subtype [3,4]. To date, there have been few reports demonstrating the molecular profiles of OS in FD [5]. Herein, we describe the clinicopathological, immunohistochemical, and molecular features of OS arising from FD of the proximal femur in a middle-aged woman. A detailed case presentation is presented in Figure 1 and Figure 2. As the degree of nuclear atypia and tumor cellularity in the outer portion suggested high-grade OS, we considered dedifferentiated low-grade central osteosarcoma (LGCOS) and OS arising in FD as the main differential diagnoses. LGCOS can be differentiated from FD by the presence of the following histologic features: infiltrative growth, cellularity, cytologic atypia, and mitotic activity [6,7]. Infiltrative growth, such as permeation or extension to the cortical bone and adjacent soft tissue, is an important gross feature of LGCOS. Microscopically, increased cellularity and intersecting fascicular patterns of spindle cells are usually detected in LGCOS but not in FD. LGCOS shows focal nuclear atypia, including hyperchromatic, enlarged nuclei, and irregular nuclear membranes. In addition to the morphological features, investigation of the underlying molecular profiles is helpful for differential diagnosis. While FD frequently harbors GNAS mutations [8,9], LGCOS frequently shows MDM2 and/or CDK4 amplification [10]. We conducted next-generation sequencing (NGS) analysis to determine whether the high-grade OS was dedifferentiation from LGCOS or malignant transformation from FD. DNA sequence-change analysis revealed GNAS (p.R201H, c.602G > A) and TP53 mutations (p.W53Cfs*70, c.159delG). Copy number variation analysis revealed a gain of PDGFRA, KIT, and CTNND2 and a loss of CDKN2A. TP53 mutations and CDKN2A deletions have been reported in primary OS or secondary OS associated with FD [5,11]. Our NGS results strongly supported the diagnosis of malignant transformation of FD. Following surgical resection and pathological diagnosis, the patient underwent Adriamycin-based chemotherapy and was alive without evidence of disease recurrence for 6 months. This case highlights the macroscopic, microscopic, and molecular characteristics of the malignant transformation of FD into OS. The diagnostic approaches, characteristic images, and interpretations of ancillary tests demonstrated in this case would help pathologists and clinicians make accurate diagnoses of malignant transformation of FD and their differential diagnosis from dedifferentiated LGCOS, which is essential for establishing an optimal treatment plan. A 38-year-old woman with no significant medical history was referred with stabbing pain in right hip joint. The pain occurred during load-bearing activities such as walking for 4 months, and became worse even after taking medicine. (A) X-ray shows a well-defined intramedullary lesion, 5.6 × 3.3 × 2.5 cm in size. (B) CT scan highlighted cortical destruction. (C) T2-weighted axial MR image reveals focal cortical destruction with thinning and endosteal erosion (arrow) in the anterior aspect of the femur. (D) Fat-saturated T2-weighted coronal MR image reveals a well-defined intramedullary lesion with peritumoral bone edema (arrow) in the meta-diaphysis of the femur. (E) Gadolinium-enhanced T1-weighted coronal MR image reveals heterogenous enhancement. (F) CT-guided bone biopsy was performed because pain and radiographic finding of cortical thinning were atypical features for fibrous dysplasia and suggested the possibility of malignancy. The first biopsy specimen includes mainly blood clots with some bland-looking spindle cells and woven bone. (G) The second biopsy reveals atypical spindle cells with moderate-to-high cellularity and frequent mitotic figures.
Figure 1.
Fibrous dysplasia (FD) is a benign fibro-osseous lesion with varying proportions of fibrous and osseous components. Depending on whether FD affects one or multiple bones, it is divided into monostotic and polyostotic types [1]. The craniofacial bones and femur are commonly involved in monostotic FD [2]. Malignant transformation of FD rarely occurs, and osteosarcoma (OS) is the most common histological subtype [3,4]. To date, there have been few reports demonstrating the molecular profiles of OS in FD [5]. Herein, we describe the clinicopathological, immunohistochemical, and molecular features of OS arising from FD of the proximal femur in a middle-aged woman. A detailed case presentation is presented in Figure 1 and Figure 2. As the degree of nuclear atypia and tumor cellularity in the outer portion suggested high-grade OS, we considered dedifferentiated low-grade central osteosarcoma (LGCOS) and OS arising in FD as the main differential diagnoses. LGCOS can be differentiated from FD by the presence of the following histologic features: infiltrative growth, cellularity, cytologic atypia, and mitotic activity [6,7]. Infiltrative growth, such as permeation or extension to the cortical bone and adjacent soft tissue, is an important gross feature of LGCOS. Microscopically, increased cellularity and intersecting fascicular patterns of spindle cells are usually detected in LGCOS but not in FD. LGCOS shows focal nuclear atypia, including hyperchromatic, enlarged nuclei, and irregular nuclear membranes. In addition to the morphological features, investigation of the underlying molecular profiles is helpful for differential diagnosis. While FD frequently harbors GNAS mutations [8,9], LGCOS frequently shows MDM2 and/or CDK4 amplification [10]. We conducted next-generation sequencing (NGS) analysis to determine whether the high-grade OS was dedifferentiation from LGCOS or malignant transformation from FD. DNA sequence-change analysis revealed GNAS (p.R201H, c.602G > A) and TP53 mutations (p.W53Cfs*70, c.159delG). Copy number variation analysis revealed a gain of PDGFRA, KIT, and CTNND2 and a loss of CDKN2A. TP53 mutations and CDKN2A deletions have been reported in primary OS or secondary OS associated with FD [5,11]. Our NGS results strongly supported the diagnosis of malignant transformation of FD. Following surgical resection and pathological diagnosis, the patient underwent Adriamycin-based chemotherapy and was alive without evidence of disease recurrence for 6 months. This case highlights the macroscopic, microscopic, and molecular characteristics of the malignant transformation of FD into OS. The diagnostic approaches, characteristic images, and interpretations of ancillary tests demonstrated in this case would help pathologists and clinicians make accurate diagnoses of malignant transformation of FD and their differential diagnosis from dedifferentiated LGCOS, which is essential for establishing an optimal treatment plan. A 38-year-old woman with no significant medical history was referred with stabbing pain in right hip joint. The pain occurred during load-bearing activities such as walking for 4 months, and became worse even after taking medicine. (A) X-ray shows a well-defined intramedullary lesion, 5.6 × 3.3 × 2.5 cm in size. (B) CT scan highlighted cortical destruction. (C) T2-weighted axial MR image reveals focal cortical destruction with thinning and endosteal erosion (arrow) in the anterior aspect of the femur. (D) Fat-saturated T2-weighted coronal MR image reveals a well-defined intramedullary lesion with peritumoral bone edema (arrow) in the meta-diaphysis of the femur. (E) Gadolinium-enhanced T1-weighted coronal MR image reveals heterogenous enhancement. (F) CT-guided bone biopsy was performed because pain and radiographic finding of cortical thinning were atypical features for fibrous dysplasia and suggested the possibility of malignancy. The first biopsy specimen includes mainly blood clots with some bland-looking spindle cells and woven bone. (G) The second biopsy reveals atypical spindle cells with moderate-to-high cellularity and frequent mitotic figures.
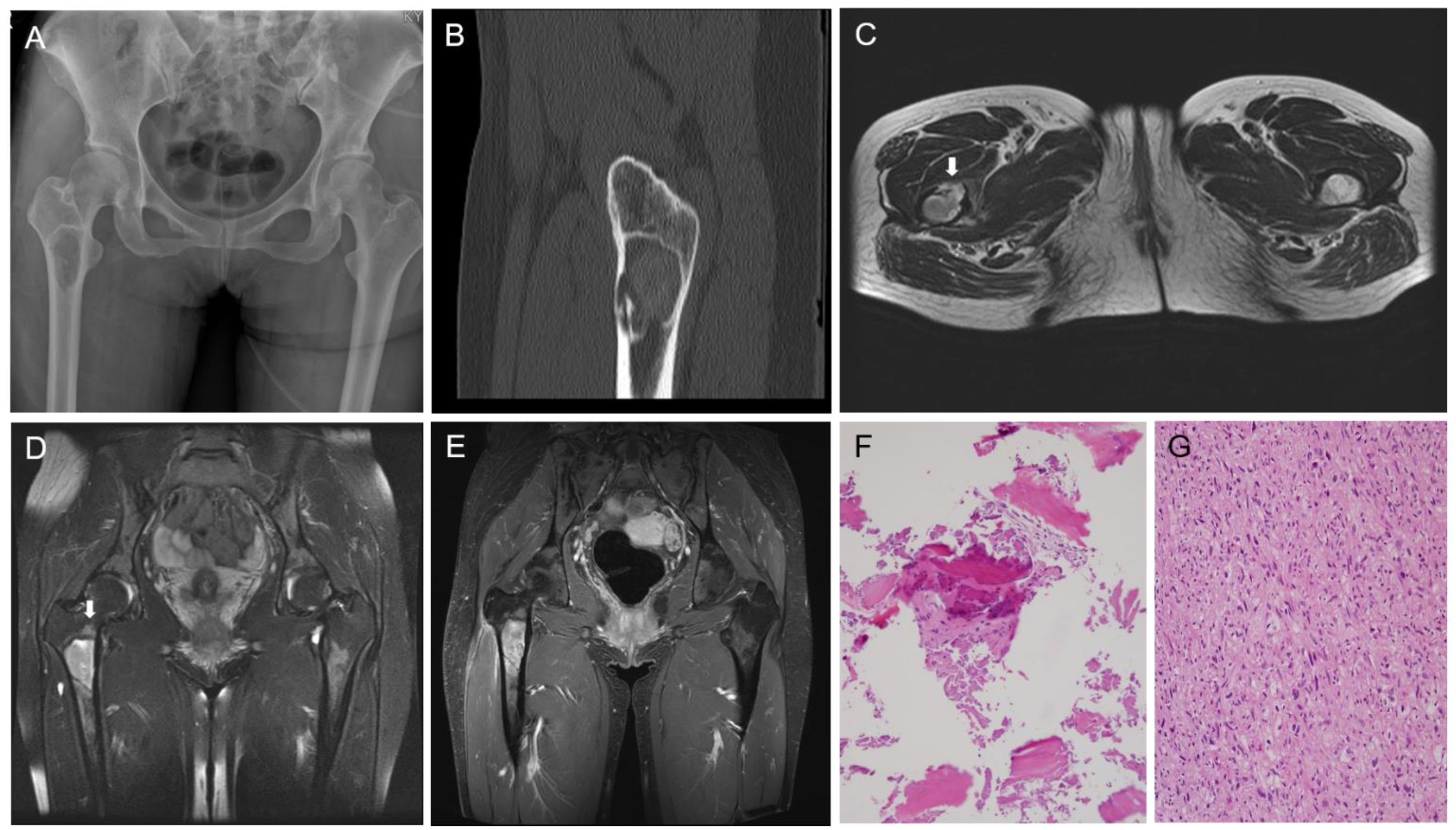
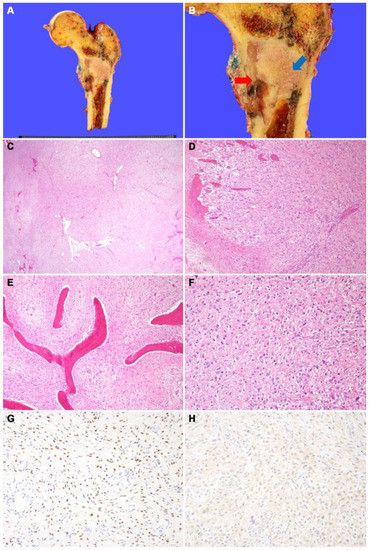
Figure 2.
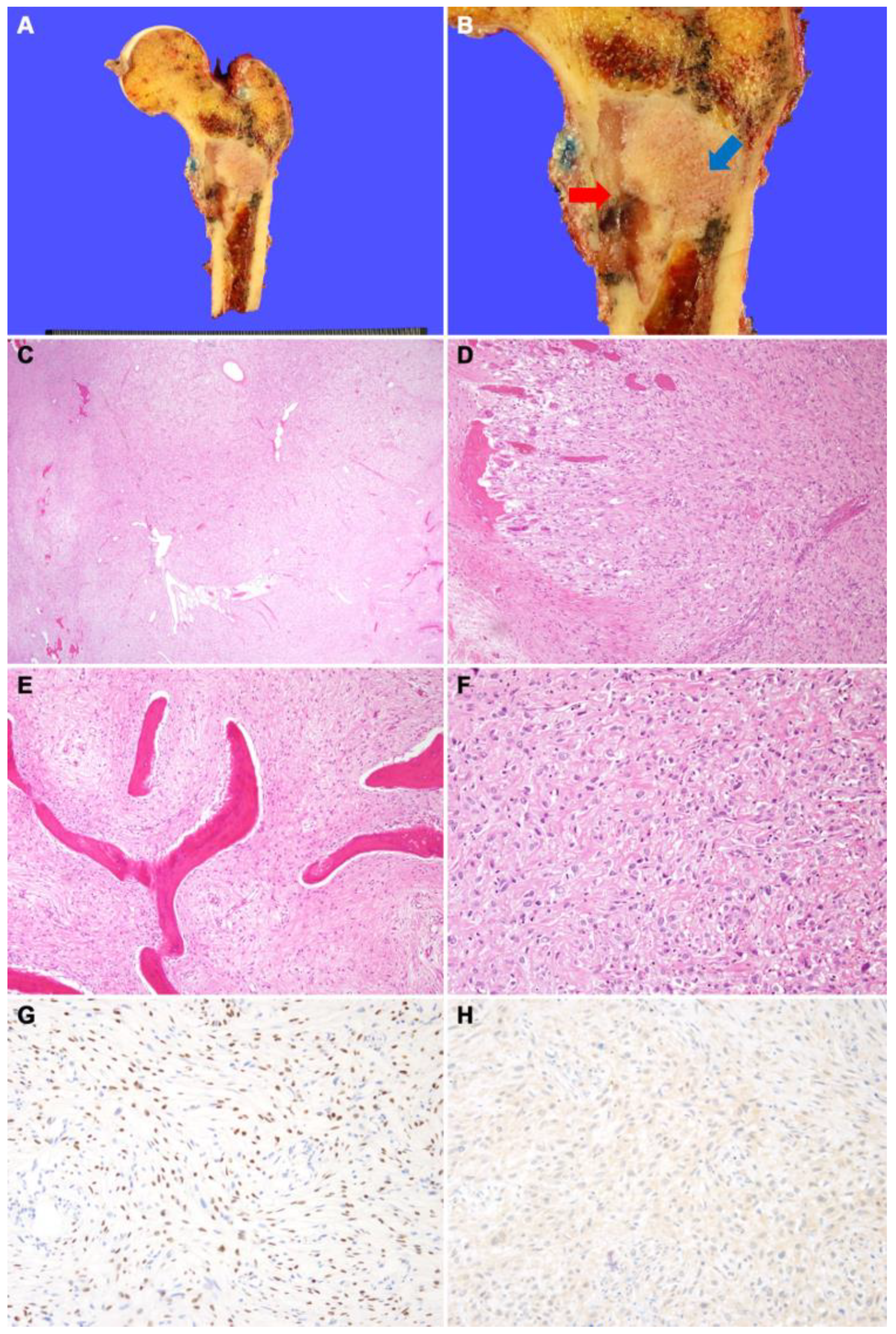
(A) Wide excision of proximal femur was performed. (B) Grossly, fibrous dysplasia portion is seen as a well-demarcated tan-gray mass with hard texture in the metaphysis of the femur (blue arrow). The osteosarcoma portion of the mass shows gray myxoid discoloration and fragile texture (red arrow). This portion was contiguous with cortical bone destruction and periosteal soft-tissue extension. (C) On low magnification, the tumors consist of an inner loosely cellular portion containing woven bone (right) and an outer highly cellular portion without woven bone (left). (D) The tumors destroyed the cortical bone and extended into the skeletal muscle. (E) On high magnification, the inner part of the tumor consists of curvilinear woven bone and fibrous stroma with bland spindle cells. (F) The outer part of the tumor shows highly cellular proliferation of pleomorphic cells with irregularly shaped hyperchromatic nuclei. Malignant osteoid interlaced the tumor cells. (G) SATB2 immunostaining demonstrates diffuse nuclear positivity. (H) CDK4 immunostaining shows negative result, suggesting a lack of MDM2 and/or CDK4 amplification.
Figure 2.
(A) Wide excision of proximal femur was performed. (B) Grossly, fibrous dysplasia portion is seen as a well-demarcated tan-gray mass with hard texture in the metaphysis of the femur (blue arrow). The osteosarcoma portion of the mass shows gray myxoid discoloration and fragile texture (red arrow). This portion was contiguous with cortical bone destruction and periosteal soft-tissue extension. (C) On low magnification, the tumors consist of an inner loosely cellular portion containing woven bone (right) and an outer highly cellular portion without woven bone (left). (D) The tumors destroyed the cortical bone and extended into the skeletal muscle. (E) On high magnification, the inner part of the tumor consists of curvilinear woven bone and fibrous stroma with bland spindle cells. (F) The outer part of the tumor shows highly cellular proliferation of pleomorphic cells with irregularly shaped hyperchromatic nuclei. Malignant osteoid interlaced the tumor cells. (G) SATB2 immunostaining demonstrates diffuse nuclear positivity. (H) CDK4 immunostaining shows negative result, suggesting a lack of MDM2 and/or CDK4 amplification.
Author Contributions
Conceptualization, H.G.K., J.H.B. and K.N.; formal analysis, H.G.K.; investigation, K.N.; writing—original draft preparation, H.G.K., J.H.B. and K.N.; writing—review and editing, H.G.K. and K.N.; visualization, H.G.K.; supervision, H.G.K. and K.N.; project administration, H.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (NRF-2020R1G1A1003692).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of Kyung Hee University Hospital (Seoul, Korea) (8 April 2022).
Informed Consent Statement
All subjects provided informed consent for inclusion before they participated in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available because of privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest regarding this study.
References
- Sugiura, Y.; Kanda, H.; Motoi, N.; Nomura, K.; Inamura, K.; Okada, E.; Matsumoto, H.; Shimoji, T.; Matsumoto, S.; Nakayama, J.; et al. Osteosarcoma arising in fibrous dysplasia, confirmed by mutational analysis of GNAS gene. Pathol. Res. Pract. 2018, 214, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Board, W. Soft Tissue and Bone Tumours; International Agency for Research on Cancer: Lyon, France, 2020; pp. 472–474. [Google Scholar]
- Li, Z.; Raynald; Wang, Z.; Qian, H. Malignant transformation of craniofacial fibrous dysplasia: A systematic review of overall survival. Neurosurg. Rev. 2020, 43, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, P.; Sim, F.H.; Bond, J.R.; Unni, K.K. Malignancies in fibrous dysplasia. Cancer 1994, 73, 1411–1424. [Google Scholar] [CrossRef]
- Shi, R.; Li, X.; Zhang, J.; Chen, F.; Ma, M.; Feng, Y.; Li, T. Clinicopathological and genetic study of a rare occurrence: Malignant transformation of fibrous dysplasia of the jaws. Mol. Genet. Genom. Med. 2022, 10, e1861. [Google Scholar] [CrossRef] [PubMed]
- Inwards, C.Y. Low-Grade Central Osteosarcoma versus Fibrous Dysplasia. Pathol. Case Rev. 2001, 6, 22–27. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Jahanshahi, G.; Dehghan Marvasti, F. Diagnostic challenges of low-grade central osteosarcoma of jaw: A literature review. J. Dent. 2015, 16, 62–67. [Google Scholar]
- Tabareau-Delalande, F.; Collin, C.; Gomez-Brouchet, A.; Decouvelaere, A.V.; Bouvier, C.; Larousserie, F.; Marie, B.; Delfour, C.; Aubert, S.; Rosset, P.; et al. Diagnostic value of investigating GNAS mutations in fibro-osseous lesions: A retrospective study of 91 cases of fibrous dysplasia and 40 other fibro-osseous lesions. Mod. Pathol. 2013, 26, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas-Souza, C.; De Andrea, C.; Bihl, M.; Kovac, M.; Pillay, N.; Forshew, T.; Gutteridge, A.; Ye, H.; Amary, M.F.; Tirabosco, R.; et al. GNAS mutations are not detected in parosteal and low-grade central osteosarcomas. Mod. Pathol. 2015, 28, 1336–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciot, R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
