A Narrative Review on LI-RADS Algorithm in Liver Tumors: Prospects and Pitfalls
Abstract
:1. Introduction
2. LI-RADS
2.1. US-LI-RADS
2.1.1. Technique
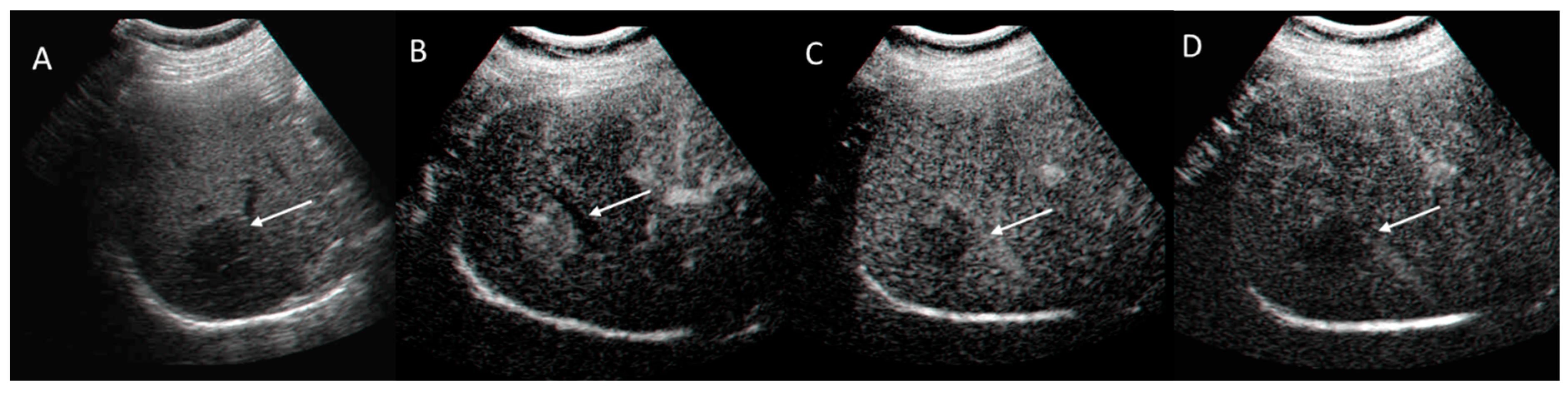
2.1.2. Ultrasound Category and Visualization Score
2.2. CT/MRI LI-RADS
2.2.1. CT/MRI Technique
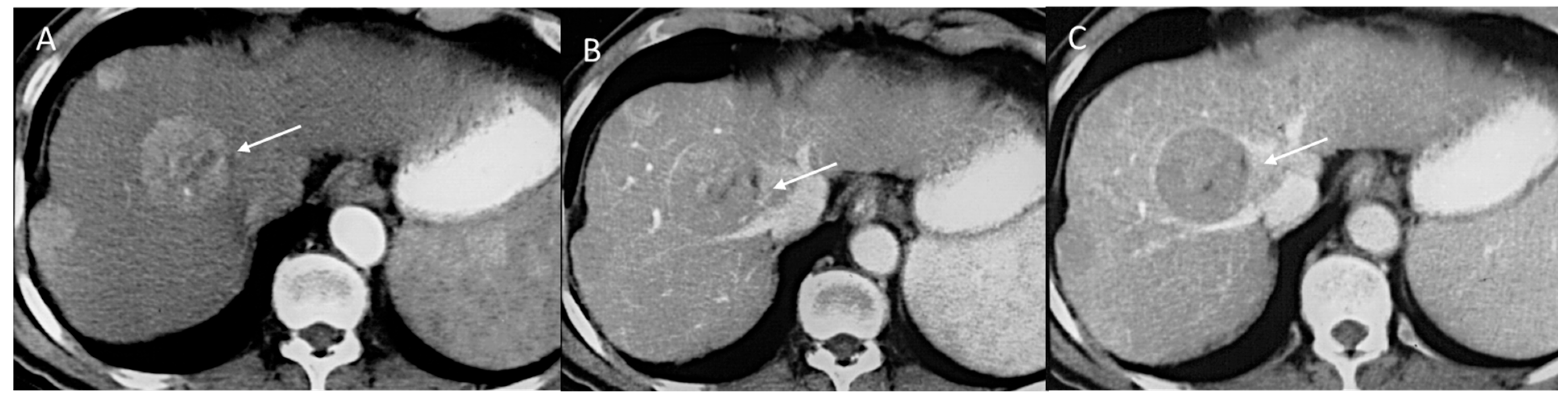
2.2.2. CT/MRI Categories
2.2.3. HCC Diagnosis
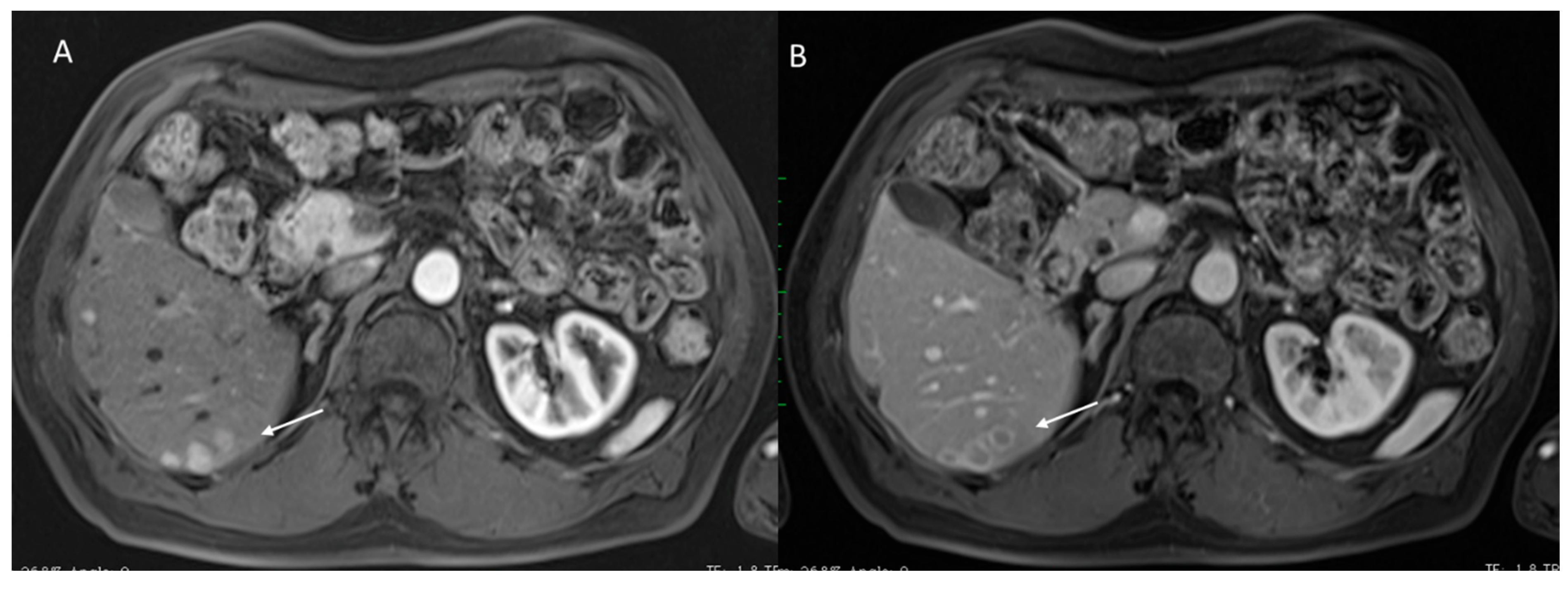
2.2.4. Ancillary Features
2.2.5. LR-M Category
2.3. CEUS-LI-RADS
2.3.1. Technique
2.3.2. CEUS-LI-RADS Categories
2.3.3. CEUS-LI-RADS vs. CT/MRI LI-RADS
2.4. LI-RADS Treatment Response Algorithm
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Cutolo, C.; Pradella, S.; Grazzini, G.; La Porta, M.; Brunese, M.C.; De Muzio, F.; et al. Diagnostic evaluation and ablation treatments assessment in hepatocellular carcinoma. Infect. Agent Cancer 2021, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Choi, J.A.; Choi, J.M.; Cho, E.S.; Kim, J.H.; Chung, J.J.; Yu, J.S. Sclerotic changes of cavernous hemangioma in the cirrhotic liver: Long-term follow-up using dynamic contrast-enhanced computed tomography. Radiol. Med. 2020, 125, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Bottari, A.; Silipigni, S.; Carerj, M.L.; Cattaf, A.; Maimone, S.; Marino, M.A.; Mazziotti, S.; Pitrone, A.; Squadrito, G.; Ascenti, G. Dual-source dual energy CT in the evaluation of hepatic fractional extracellular space in cirrhosis. Radiol. Med. 2020, 125, 7–14. [Google Scholar] [CrossRef]
- Bracci, S.; Dolciami, M.; Trobiani, C.; Izzo, A.; Pernazza, A.; D–Amati, G.; Manganaro, L.; Ricci, P. Quantitative CT texture analysis in predicting PD-L1 expression in locally advanced or metastatic NSCLC patients. Radiol. Med. 2021, 126, 1425–1433. [Google Scholar]
- Granata, V.; Petrillo, M.; Fusco, R.; Setola, S.V.; de Lutio di Castelguidone, E.; Catalano, O.; Piccirillo, M.; Albino, V.; Izzo, F.; Petrillo, A. Surveillance of HCC Patients after Liver RFA: Role of MRI with Hepatospecific Contrast versus Three-Phase CT Scan-Experience of High Volume Oncologic Institute. Gastroenterol. Res. Pract. 2013, 2013, 469097. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El-Serag, H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359. [Google Scholar] [CrossRef] [Green Version]
- Mokrane, F.Z.; Lu, L.; Vavasseur, A.; Otal, P.; Peron, J.M.; Luk, L.; Yang, H.; Ammari, S.; Saenger, Y.; Rousseau, H.; et al. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur. Radiol. 2020, 30, 558–570. [Google Scholar] [CrossRef]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol. Med. 2021. [Google Scholar]
- Granata, V.; Fusco, R.; Venanzio Setola, S.; Sandomenico, F.; Luisa Barretta, M.; Belli, A.; Palaia, R.; Tatangelo, F.; Grassi, R.; Izzo, F.; et al. Major and ancillary features according to LI-RADS in the assessment of combined hepatocellular-cholangiocarcinoma. Radiol. Oncol. 2020, 54, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Picone, C.; Vallone, P.; Belli, A.; Incollingo, P.; Albino, V.; Tatangelo, F.; Izzo, F.; et al. Microvascular invasion and grading in hepatocellular carcinoma: Correlation with major and ancillary features according to LIRADS. Abdom. Radiol. 2019, 44, 2788–2800. [Google Scholar] [CrossRef]
- Ganne-Carrié, N.; Chaffaut, C.; Bourcier, V.; Archambeaud, I.; Perarnau, J.M.; Oberti, F.; Roulot, D.; Moreno, C.; Louvet, A.; Dao, T.; et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J. Hepatol. 2018, 69, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Sempoux, C.; Balabaud, C.; Bioulac-Sage, P. Malignant transformation of hepatocellular adenoma. Hepatic Oncol. 2014, 1, 421–431. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Venanzio Setola, S.; Barretta, M.L.; Iasevoli, D.M.A.; Palaia, R.; Belli, A.; Patrone, R.; Tatangelo, F.; Grazzini, G.; et al. Diagnostic performance of LI-RADS in adult patients with rare hepatic tumors. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 399–414. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.M.; Catalano, O.; Filice, S.; Avallone, A.; Piccirillo, M.; Leongito, M.; Palaia, R.; Grassi, R.; Izzo, F.; et al. Uncommon neoplasms of the biliary tract: Radiological findings. Br. J. Radiol. 2017, 90, 20160561. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; de Lutio di Castelguidone, E.; Avallone, A.; Palaia, R.; Delrio, P.; Tatangelo, F.; Botti, G.; Grassi, R.; Izzo, F.; et al. Diagnostic performance of gadoxetic acid-enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol. 2019, 19, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petralia, G.; Summers, P.E.; Agostini, A.; Ambrosini, R.; Cianci, R.; Cristel, G.; Calistri, L.; Colagrande, S. Dynamic contrast-enhanced MRI in oncology: How we do it. Radiol. Med. 2020, 125, 1288–1300. [Google Scholar] [CrossRef]
- Ria, F.; Samei, E. Is regulatory compliance enough to ensure excellence in medicine? Radiol. Med. 2020, 125, 904–905. [Google Scholar] [CrossRef]
- Zhang, A.; Song, J.; Ma, Z.; Chen, T. Combined dynamic contrast-enhanced magnetic resonance imaging and difusion-weighted imaging to predict neoadjuvant chemotherapy efect in FIGO stage IB2-IIA2 cervical cancers. Radiol. Med. 2020, 125, 1233–1242. [Google Scholar] [CrossRef]
- Crimi, F.; Capelli, G.; Spolverato, G.; Bao, Q.R.; Florio, A.; Milite Rossi, S.; Cecchin, D.; Albertoni, L.; Campi, C.; Pucciarelli, S.; et al. MRI T2-weighted sequences based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). Radiol. Med. 2020, 125, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirienko, M.; Ninatti, G.; Cozzi, L.; Voulaz, E.; Gennaro, N.; Barajon, I.; Ricci, F.; Carlo-Stella, C.; Zucali, P.; Sollini, M.; et al. Computed tomography (CT)-derived radiomic features diferentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol. Med. 2020, 125, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, L.; Li, G.; Zhang, X.; Ren, J.; Shi, Z.; Li, J.; Yu, S. Computed tomography-based radiomics model for discriminating the risk stratifcation of gastrointestinal stromal tumors. Radiol. Med. 2020, 125, 465–473. [Google Scholar] [CrossRef]
- Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect. Agent Cancer 2017, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- De Muzio, F.; Cutolo, C.; Dell’Aversana, F.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Simonetti, I.; Dell’Aversana, F.; Grassi, F.; Bruno, F.; Belli, A.; et al. Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect. J. Clin. Med. 2022, 11, 2766. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Barabino, M.; Gurgitano, M.; Fochesato, C.; Angileri, S.A.; Franceschelli, G.; Santambrogio, R.; Mariani, N.M.; Opocher, E.; Carrafiello, G. LI-RADS to categorize liver nodules in patients at risk of HCC: Tool or a gadget in daily practice? Radiol. Med. 2021, 126, 5–13. [Google Scholar] [CrossRef]
- Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part I: Classification, diagnosis and staging. Dig. Liver Dis. 2020, 52, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part II: Treatment. Dig. Liver Dis. 2020, 52, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, M.; Di Nasso, M.; Morganti, R.; Faggioni, L.; Masi, G.; Falcone, A.; Neri, E. Application of the ESR iGuide clinical decision support system to the imaging pathway of patients with hepatocellular carcinoma and cholangiocarcinoma: Preliminary findings. Radiol. Med. 2020, 125, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Bicchierai, G.; Fusco, R.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6499–6528. [Google Scholar] [PubMed]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women With Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef]
- Gatti, M.; Calandri, M.; Bergamasco, L.; Darvizeh, F.; Grazioli, L.; Inchingolo, R.; Ippolito, D.; Rousset, S.; Veltri, A.; Fonio, P.; et al. Characterization of the arterial enhancement pattern of focal liver lesions by multiple arterial phase magnetic resonance imaging: Comparison between hepatocellular carcinoma and focal nodular hyperplasia. Radiol. Med. 2020, 125, 348–355. [Google Scholar] [CrossRef]
- Orlacchio, A.; Chegai, F.; Roma, S.; Merolla, S.; Bosa, A.; Francioso, S. Degradable starch microspheres transarterial chemoembolization (DSMs-TACE) in patients with unresectable hepatocellular carcinoma (HCC): Long-term results from a single-center 137-patient cohort prospective study. Radiol. Med. 2020, 125, 98–106. [Google Scholar] [CrossRef]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics (Basel) 2022, 12, 890. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gregucci, F.; Bonaparte, I.; Vitulano, N.; Surgo, A.; Mazzola, R.; Di Monaco, A.; Carbonara, R.; Alongi, F.; Langialonga, T.; et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2021, 126, 155–162, Epub 13 May 2020. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Belli, A.; Borzillo, V.; Palumbo, P.; Bruno, F.; Grassi, R.; Ottaiano, A.; Nasti, G.; Pilone, V.; et al. Conventional, functional and radiomics assessment for intrahepatic cholangiocarcinoma. Infect. Agent Cancer 2022, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Simonetti, I.; Cozzi, D.; Grazzini, G.; Grassi, F.; Belli, A.; Miele, V.; Izzo, F.; et al. An update on radiomics techniques in primary liver cancers. Infect. Agent Cancer 2022, 17, 6. [Google Scholar] [CrossRef]
- Barile, A.; Conti, L.; Lanni, G.; Calvisi, V.; Masciocchi, C. Evaluation of medial meniscus tears and meniscal stability: Weight-bearing MRI vs arthroscopy. Eur. J. Radiol. 2013, 82, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, G.M.; Ravanelli, M.; Roca, E.; Medicina, D.; Balzarini, P.; Pessina, C.; Vermi, W.; Berruti, A.; Maroldi, R.; Farina, D. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. Radiol. Med. 2021, 126, 786–794. [Google Scholar] [CrossRef]
- Iacobellis, F.; Di Serafino, M.; Brillantino, A.; Mottola, A.; Del Giudice, S.; Stavolo, C.; Festa, P.; Patlas, M.N.; Scaglione, M.; Romano, L. Role of MRI in early follow-up of patients with solid organ injuries: How and why we do it? Radiol. Med. 2021, 126, 1328–1334. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Guo, W.; Zeng, P.; Liu, Y.; Lang, N.; Yuan, H. A preliminary study using spinal MRI-based radiomics to predict high-risk cytogenetic abnormalities in multiple myeloma. Radio. Med. 2021, 126, 1226–1235. [Google Scholar]
- Qin, H.; Que, Q.; Lin, P.; Li, X.; Wang, X.R.; He, Y.; Chen, J.Q.; Yang, H. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): A comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol. Med. 2021, 126, 1312–1327. [Google Scholar]
- Gannon, C.J.; Izzo, F.; Aloia, T.A.; Pignata, S.; Nasti, G.; Vallone, P.; Orlando, R.; Scordino, F.; Curley, S.A. Can hepatocellular cancer screening increase the proportion of long-term survivors? Hepatogastroenterology 2009, 56, 1152–1156. [Google Scholar] [PubMed]
- Danti, G.; Berti, V.; Abenavoli, E.; Briganti, V.; Linguanti, F.; Mungai, F.; Pradella, S.; Miele, V. Diagnostic imaging of typical lung carcinoids: Relationship between MDCT, 111In-Octreoscan and 18F-FDG-PET imaging features with Ki-67 index. Radiol. Med. 2020, 125, 715–729. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Salvati, F.; Rossi, F.; Limbucci, N.; Pistoia, M.L.; Barile, A.; Masciocchi, C. Mucoid metaplastic-degeneration of anterior cruciate ligament. J. Sports Med. Phys. Fit. 2008, 48, 483–487. [Google Scholar]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Mufatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Barile, A.; Bruno, F.; Arrigoni, F.; Splendiani, A.; Di Cesare, E.; Zappia, M.; Guglielmi, G.; Masciocchi, C. Emergency and Trauma of the Ankle. In Seminars in Musculoskeletal Radiology; Thieme Medical Publishers: New York, NY, USA, 2017; Volume 21, pp. 282–289. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Brunese, L.; Brunese, M.C.; Carbone, M.; Ciccone, V.; Mercaldo, F.; Santone, A. Automatic PI-RADS assignment by means of formal methods. Radiol. Med. 2021, 127, 83–89. [Google Scholar] [CrossRef]
- van der Lubbe, M.F.; Vaidyanathan, A.; de Wit, M.; van den Burg, E.L.; Postma, A.A.; Bruintjes, T.D.; Bilderbeek-Beckers, M.A.L.; Dammeijer, P.F.M.; Bossche, S.V.; Van Rompaey, V.; et al. A non-invasive, automated diagnosis of Menière’s disease using radiomics and machine learning on conventional magnetic resonance imaging: A multicentric, case-controlled feasibility study. Radiol. Med. 2021, 127, 72–82. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Cassata, A.; Palaia, R.; Delrio, P.; Grassi, R.; Tatangelo, F.; Grazzini, G.; Izzo, F.; et al. Abbreviated MRI protocol for colorectal liver metastases: How the radiologist could work in pre surgical setting. PLoS ONE 2020, 15, e0241431. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Venanzio Setola, S.; Mattace Raso, M.; Avallone, A.; De Stefano, A.; Nasti, G.; Palaia, R.; Delrio, P.; Petrillo, A.; et al. Liver radiologic fndings of chemotherapy-induced toxicity in liver colorectal metastases patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9697–9706. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Maio, F.; Avallone, A.; Nasti, G.; Palaia, R.; Albino, V.; Grassi, R.; Izzo, F.; Petrillo, A. Qualitative assessment of EOB-GD-DTPA and Gd-BT-DO3A MR contrast studies in HCC patients and colorectal liver metastases. Infect. Agent Cancer 2019, 14, 40. [Google Scholar] [CrossRef]
- Chernyak, V.; Tang, A.; Do, R.K.G.; Kamaya, A.; Kono, Y.; Santillan, C.S.; Fowler, K.J.; Bashir, M.R.; Cunha, G.M.; Fetzer, D.T.; et al. Liver imaging: It is time to adopt standardized terminology. Eur. Radiol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Terzi, E.; Giamperoli, A.; Iavarone, M.; Leoni, S.; De Bonis, L.; Granito, A.; Forgione, A.; Tovoli, F.; Piscaglia, F. Prognosis of Single Early-Stage Hepatocellular Carcinoma (HCC) with CEUS Inconclusive Imaging (LI-RADS LR-3 and LR-4) Is No Better than Typical HCC (LR-5). Cancers 2022, 14, 336. [Google Scholar] [CrossRef]
- Masciocchi, C.; Arrigoni, F.; La Marra, A.; Mariani, S.; Zugaro, L.; Barile, A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br. J. Radiol. 2016, 89, 20150356. [Google Scholar] [CrossRef] [Green Version]
- Moura Cunha, G.; Chernyak, V.; Fowler, K.J.; Sirlin, C.B. Up-to-Date Role of CT/MRI LI-RADS in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Radiology. CT/MRI Liver Imaging Reporting and Data System v2018 Core. Available online: https://www.acr.org/ClinicalResources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LIRADS-v (accessed on 15 April 2020).
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Filice, F.; Leongito, M.; Palaia, R.; Izzo, F.; Petrillo, A. Major and ancillary magnetic resonance features of LI-RADS to assess HCC: An overview and update. Infect. Agent Cancer 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Belli, A.; Danti, G.; Bicci, E.; Cutolo, C.; Petrillo, A.; Izzo, F. Diffusion weighted imaging and diffusion kurtosis imaging in abdominal oncological setting: Why and when. Infect. Agent. Cancer 2022, 17, 25. [Google Scholar]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency ablation and microwave ablation in liver tumors: An update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [Green Version]
- Trombadori, C.M.L.; D’Angelo, A.; Ferrara, F.; Santoro, A.; Belli, P.; Manfredi, R. Radial Scar: A management dilemma. Radiol. Med. 2021, 126, 774–785. [Google Scholar] [CrossRef]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.M.; Giovagnoni, A. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Gaibazzi, N.; Tuttolomondo, D.; Fusco, S.; La Mura, V.; Peyvandi, F.; Aliberti, S.; Blasi, F.; Cozzi, D.; Carrafello, G.; et al. Deep vein thrombosis in COVID-19 patients in general wards: Prevalence and association with clinical and laboratory variables. Radiol. Med. 2021, 126, 722–728. [Google Scholar] [CrossRef]
- Zener, R.; Oreopoulos, G.; Beecroft, R.; Rajan, D.K.; Jaskolka, J.; Tan, K.T. Transabdominal Direct Sac Puncture Embolization of Type II Endoleaks after Endovascular Abdominal Aortic Aneurysm Repair. J. Vasc. Interv. Radiol. 2018, 29, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Castellana, M.; Virili, C.; Havre, R.F.; Bini, F.; Marinozzi, F.; D’Ambrosio, F.; Giorgino, F.; Giovanella, L.; Prosch, H.; et al. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: A systematic review and meta-analysis using histological standard of reference. Radiol. Med. 2020, 125, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Leongito, M.; Izzo, F.; Petrillo, A. Peribiliary liver metastases MR findings. Med. Oncol. 2017, 34, 124. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.T.; Wang, W.; Chen, L.D.; Ruan, S.M.; Chen, S.L.; Li, X.; Lu, M.D.; Xie, X.Y.; Kuang, M. Artifcial intelligence assists identifying malignant versus benign liver lesions using contrast-enhanced ultrasound. J. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Chammas, M.C.; Bordini, A.L. Contrast-enhanced ultrasonography for the evaluation of malignant focal liver lesions. Ultrasonography 2022, 41, 4–24. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Chen, K.; Wang, H.; Zhang, W.; Bao, J.; Wang, W. Diferentiation between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced ultrasound: A systematic review and meta-analysis. Clin. Hemorheol. Microcirc. 2021, 79, 293–309. [Google Scholar] [CrossRef]
- Xian, M.F.; Huang, Y.; Xie, W.X.; Pan, K.M.; Zeng, D.; Huang, H.; Li, M.D.; Xie, X.Y.; Kuang, M.; Lu, M.D.; et al. LR-M observations on contrastenhanced ultrasound: Detection of hepatocellular carcinoma using additional features in comparison with current LI-RADS criteria. AJR Am. J. Roentgenol. 2021, 219, 76–85. [Google Scholar] [CrossRef]
- Guo, H.L.; Zheng, X.; Cheng, M.Q.; Zeng, D.; Huang, H.; Xie, X.Y.; Lu, M.D.; Kuang, M.; Wang, W.; Xian, M.F.; et al. Contrast-Enhanced Ultrasound for Differentiation Between Poorly Diferentiated Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Ultrasound Med. 2021, 41, 1213–1225. [Google Scholar] [CrossRef]
- Barile, A.; Sabatini, M.; Iannessi, F.; Di Cesare, E.; Splendiani, A.; Calvisi, V.; Masciocchi, C. Pigmented villonodular synovitis (PVNS) of the knee joint: Magnetic resonance imaging (MRI) using standard and dynamic paramagnetic contrast media. Report of 52 cases surgically and histologically controlled. Radiol. Med. 2004, 107, 356–366. [Google Scholar]
- Kim, Y.Y.; Yeom, S.K.; Shin, H.; Choi, S.H.; Rhee, H.; Park, J.H.; Cho, E.S.; Park, S.; Lee, S.S.; Park, M.S. Clinical staging of mass-forming intrahepatic cholangiocarcinoma: Computed tomography versus magnetic resonance imaging. Hepatol. Commun. 2021, 5, 2009–2018. [Google Scholar] [CrossRef]
- Ichikawa, S.; Yamamoto, H.; Morita, T. Comparison of a Bayesian estimation algorithm and singular value decomposition algorithms for 80-detector row CT perfusion in patients with acute ischemic stroke. Radiol. Med. 2021, 126, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Rampado, O.; Depaoli, A.; Marchisio, F.; Gatti, M.; Racine, D.; Ruggeri, V.; Ruggirello, I.; Darvizeh, F.; Fonio, P.; Ropolo, R. Efects of diferent levels of CT iterative reconstruction on low-contrast detectability and radiation dose in patients of diferent sizes: An anthropomorphic phantom study. Radiol. Med. 2021, 126, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantifcation of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Ponnoprat, D.; Inkeaw, P.; Chaijaruwanich, J.; Traisathit, P.; Sripan, P.; Inmutto, N.; Na Chiangmai, W.; Pongnikorn, D.; Chitapanarux, I. Classifcation of hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on multi-phase CT scans. Med. Biol. Eng. Comput. 2020, 58, 2497–2515. [Google Scholar] [CrossRef]
- Tsunematsu, S.; Chuma, M.; Kamiyama, T.; Miyamoto, N.; Yabusaki, S.; Hatanaka, K.; Mitsuhashi, T.; Kamachi, H.; Yokoo, H.; Kakisaka, T.; et al. Intratumoral artery on contrast-enhanced computed tomography imaging: Diferentiating intrahepatic cholangiocarcinoma from poorly diferentiated hepatocellular carcinoma. Abdom. Imaging 2015, 40, 1492–1499. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Chen, W.X.; Wu, D.S.; Zhang, W.Y.; Zheng, L.R. Diferentiation of mass-forming intrahepatic cholangiocarcinoma from poorly diferentiated hepatocellular carcinoma: Based on the multivariate analysis of contrast-enhanced computed tomography fndings. Abdom. Radiol. 2016, 41, 978–989. [Google Scholar] [CrossRef]
- Ruys, A.T.; van Beem, B.E.; Engelbrecht, M.R.; Bipat, S.; Stoker, J.; Van Gulik, T.M. Radiological staging in patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2012, 85, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, S.; Isoda, H.; Shimizu, T.; Tamada, D.; Taura, K.; Togashi, K.; Onishi, H.; Motosugi, U. Distinguishing intrahepatic mass-forming biliary carcinomas from hepatocellular carcinoma by computed tomography and magnetic resonance imaging using the Bayesian method: A bi-center study. Eur. Radiol. 2020, 30, 5992–6002. [Google Scholar] [CrossRef]
- Chu, H.; Liu, Z.; Liang, W.; Zhou, Q.; Zhang, Y.; Lei, K.; Tang, M.; Cao, Y.; Chen, S.; Peng, S.; et al. Radiomics using CT images for preoperative prediction of futile resection in intrahepatic cholangiocarcinoma. Eur. Radiol. 2021, 31, 2368–2376. [Google Scholar] [CrossRef]
- Megibow, A.J. Clinical abdominal dual-energy CT: 15 years later. Abdom. Radiol. 2020, 45, 1198–1201. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Esposto Pirani, P.; Di Cesare, E.; Giovagnoni, A. The sub-millisievert era in CTCA: The technical basis of the new radiation dose approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef]
- Cunha, G.M.; Sirlin, C.B.; Fowler, K.J. Imaging diagnosis of hepatocellular carcinoma: LI-RADS. Chin. Clin. Oncol. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Brizi, M.G.; Perillo, F.; Cannone, F.; Tuzza, L.; Manfredi, R. The role of imaging in acute pancreatitis. Radiol. Med. 2021, 126, 1017–1029. [Google Scholar] [CrossRef]
- Assadsangabi, R.; Babaei, R.; Songco, C.; Ivanovic, V.; Bobinski, M.; Chen, Y.J.; Nabavizadeh, S.A. Multimodality oncologic evaluation of superfcial neck and facial lymph nodes. Radiol. Med. 2021, 126, 1074–1084. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Galdiero, R.; Setola, S.V.; Palaia, R.; Belli, A.; Silvestro, L.; Cozzi, D.; Brunese, L.; et al. Pancreatic cancer detection and characterization: State of the art and radiomics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3684–3699. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Setola, S.V.; de Lutio di Castelguidone, E.; Piccirillo, M.; Palaia, R.; Grassi, R.; Granata, F.; Izzo, F.; et al. Multidetector computer tomography in the pancreatic adenocarcinoma assessment: An update. Infect. Agent Cancer 2016, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.A.; Marrero, J.A. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Cuevas, C.; Fu, R.; Devine, B.; Wasson, N.; Ginsburg, A.; Zakher, B.; Pappas, M.; Graham, E.; Sullivan, S.D. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 162, 697–711. [Google Scholar] [CrossRef] [Green Version]
- da Silva, P.H.; Gomes, M.M.; de Matos, C.A.L.; de Souza, E.; Silva, I.S.; Gonzalez, A.M.; Torres, U.S.; Salazar, G.M.M.; D’Ippolito, G. HCC Detection on Surveillance US: Comparing Focused Liver Protocol Using US LI-RADS Technical Guidelines to a General Complete Abdominal US Protocol. J. Ultrasound Med. 2021, 40, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.K.; Fetzer, D.T.; Gabriel, H.; Seow, J.H.; Choi, H.H.; Maturen, K.E.; Wasnik, A.P.; Morgan, T.A.; Dahiya, N.; O’Boyle, M.K.; et al. Role of US LI-RADS in the LI-RADS Algorithm. Radiographics 2019, 39, 690–708. [Google Scholar] [CrossRef] [PubMed]
- Sevco, T.J.; Masch, W.R.; Maturen, K.E.; Mendiratta-Lala, M.; Wasnik, A.P.; Millet, J.D. Ultrasound (US) LI-RADS: Outcomes of Category US-3 Observations. AJR Am. J. Roentgenol. 2021, 217, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.A.; Maturen, K.E.; Dahiya, N.; Sun, M.R.M.; Kamaya, A. American College of Radiology Ultrasound Liver Imaging and Reporting Data System (US LI-RADS) Working Group. US LI-RADS: Ultrasound liver imaging reporting and data system for screening and surveillance of hepatocellular carcinoma. Abdom. Radiol. 2018, 43, 41–55. [Google Scholar] [CrossRef]
- Choi, H.H.; Rodgers, S.K.; Fetzer, D.T.; Wasnik, A.P.; Millet, J.D.; Morgan, T.A.; Dawkins, A.; Gabriel, H.; Kamaya, A. Ultrasound Liver Imaging Reporting and Data System (US LI-RADS): An Overview with Technical and Practical Applications. Acad. Radiol. 2021, 28, 1464–1476. [Google Scholar] [CrossRef]
- Kiri, L.; Abdolell, M.; Costa, A.F.; Keough, V.; Rowe, J.; Butt, R.; Clarke, S.E. US LI-RADS Visualization Score: Interobserver Variability and Association With Cause of Liver Disease, Sex, and Body Mass Index. Can. Assoc. Radiol. J. 2022, 73, 68–74. [Google Scholar] [CrossRef]
- MCunha, G.; Fowler, K.J.; Roudenko, A.; Taouli, B.; Fung, A.W.; Elsayes, K.M.; Marks, R.M.; Cruite, I.; Horvat, N.; Chernyak, V.; et al. How to Use LI-RADS to Report Liver CT and MRI Observations. Radiographics 2021, 41, 1352–1367. [Google Scholar] [CrossRef]
- Bertocchi, E.; Barugola, G.; Nicosia, L.; Mazzola, R.; Ricchetti, F.; Dell’Abate, P.; Alongi, F.; Rufo, G. A comparative analysis between radiation dose intensifcation and conventional fractionation in neoadjuvant locally advanced rectal cancer: A monocentric prospective observational study. Radiol. Med. 2020, 125, 990–998. [Google Scholar] [CrossRef]
- Agostini, A.; Floridi, C.; Borgheresi, A.; Badaloni, M.; Esposto Pirani, P.; Terilli, F.; Ottaviani, L.; Giovagnoni, A. Proposal of a low-dose, long-pitch, dualsource chest CT protocol on third-generation dual-source CT using a tin flter for spectral shaping at 100 kVp for CoronaVirus Disease 2019 (COVID-19) patients: A feasibility study. Radiol. Med. 2020, 125, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. Radiol. Med. 2020, 125, 384–397. [Google Scholar] [CrossRef]
- Yoon, J.H.; Chang, W.; Lee, E.S.; Lee, S.M.; Lee, J.M. Double low-dose dualenergy liver CT in patients at high-risk of HCC: A prospective, randomized. Investig. Radiol. 2020, 55, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Joob, B.; Wiwanitkit, V. Cholangiocarcinoma versus small liver abscess in dual source dual-energy CT quantitative parameters. Eur. J. Radiol. 2018, 99, 130. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.O.; Bae, K.; Cho, J.M.; Choi, H.C.; Choi, D.S. Diferentiation of small intrahepatic mass-forming cholangiocarcinoma from small liver abscess by dual source dual-energy CT quantitative parameters. Eur. J. Radiol. 2017, 92, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Shao, C.; Lv, Y.; Zhao, F. Tumor attenuation and quantitative analysis of perfusion parameters derived from triphasic CT scans in hepatocellular carcinoma: Relationship with histological grade. Medicine 2021, 100, e25627. [Google Scholar] [CrossRef]
- Perl, R.M.; Portugall, J.; Hinterleitner, C.; Hinterleitner, M.; Kloth, C.; Walter, S.S.; Bitzer, M.; Horger, M.S. Diferences between CT-perfusion and biphasic contrast-enhanced CT for detection and characterization of hepatocellular carcinoma: Potential explanations for discrepant cases. Anticancer Res. 2021, 41, 1451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Pang, G.; Li, X.; Yang, S.; Zhong, H. Value of perfusion parameters histogram analysis of triphasic CT in diferentiating intrahepatic mass forming cholangiocarcinoma from hepatocellular carcinoma. Sci. Rep. 2021, 11, 23163. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Lay Ergün, E.; Volkan-Salanci, B. Factors afecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, J.S.; Kim, K.H.; Moon, H.J.; Kim, S. Clinical signifcance of radiation dose-volume parameters and functional status on the patient reported quality of life changes after thoracic radiotherapy for lung cancer: A prospective study. Radiol. Med. 2021, 126, 466–473. [Google Scholar] [CrossRef]
- Mathew, R.P.; Sam, M.; Raubenheimer, M.; Patel, V.; Low, G. Hepatic hemangiomas: The various imaging avatars and its mimickers. Radiol. Med. 2020, 125, 801–815. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-derived data by contrast enhanced magnetic resonance in RAS mutations detection in colorectal liver metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef]
- Esposito, A.; Buscarino, V.; Raciti, D.; Casiraghi, E.; Manini, M.; Biondetti, P.; Forzenigo, L. Characterization of liver nodules in patients with chronic liver disease by MRI: Performance of the Liver Imaging Reporting and Data System (LI-RADS vol 2018) scale and its comparison with the Likert scale. Radiol. Med. 2020, 125, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, G.; Zucchetta, P.; Varotto, A.; Crimì, F.; Weber, M.; Cecchin, D.; Bisogno, G.; Spimpolo, A.; Giraudo, C.; Stramare, R. Volumetric histograms-based analysis of apparent difusion coefcients and standard uptake values for the assessment of pediatric sarcoma at staging: Preliminary results of a PET/MRI study. Radiol. Med. 2021, 126, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Petrillo, A. Introduction to special issue of radiology and imaging of cancer. Cancers 2020, 12, 2665. [Google Scholar] [CrossRef] [PubMed]
- Mirabile, A.; Lucarelli, N.M.; Sollazzo, E.P.; Stabile Ianora, A.A.; Sardaro, A.; Mirabile, G.; Lorusso, F.; Racanelli, V.; Maggialetti, N.; Scardapane, A. CT pulmonary angiography appropriateness in a single emergency department: Does the use of revised Geneva score matter? Radiol. Med. 2021, 126, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Bilreiro, C.; Soler, J.C.; Ayuso, J.R.; Caseiro-Alves, F.; Ayuso, C. Diagnostic value of morphological enhancement patterns in the hepatobiliary phase of gadoxetic acid-enhanced MRI to distinguish focal nodular hyperplasia from hepatocellular adenoma. Radiol. Med. 2021, 126, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Amato, D.M.; Albino, V.; Patrone, R.; Izzo, F.; Petrillo, A. Beyond the Vascular Profile: Conventional DWI, IVIM and Kurtosis in the Assessment of Hepatocellular Carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7284–7293. [Google Scholar]
- Granata, V.; Fusco, R.; Filice, S.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Petrillo, A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect. Agents Cancer 2018, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Konstantinidis, I.T.; Do, R.K.; Gultekin, D.H.; Gönen, M.; Schwartz, L.H.; Fong, Y.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Klimstra, D.S.; et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: A potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann. Surg. Oncol. 2014, 21, 2675–2683. [Google Scholar] [CrossRef] [Green Version]
- Albano, D.; Stecco, A.; Micci, G.; Sconfenza, L.M.; Colagrande, S.; Reginelli, A.; Grassi, R.; Carriero, A.; Midiri, M.; Lagalla, R.; et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: An Italian survey. Radiol. Med. 2021, 126, 299–305. [Google Scholar] [CrossRef]
- Taverna, C.; Novelli, L.; De Renzis, A.G.D.; Calistri, L.; Tomei, M.; Occhipinti, M.; Colagrande, S. The role of difusion-weighted and dynamic contrast enhancement perfusion-weighted imaging in the evaluation of salivary glands neoplasms. Radiol. Med. 2020, 125, 851–863. [Google Scholar] [CrossRef]
- Lian, S.; Zhang, C.; Chi, J.; Huang, Y.; Shi, F.; Xie, C. Diferentiation between nasopharyngeal carcinoma and lymphoma at the primary site using whole-tumor histogram analysis of apparent difusion coefcient maps. Radiol. Med. 2020, 125, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Zhang, K.; Liu, Y.; Cui, J.; Tao, J.; Wang, Y.; Wang, S. Invasive ductal breast cancer: Preoperative predict Ki-67 index based on radiomics of ADC maps. Radiol. Med. 2020, 125, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Fornell-Perez, R.; Vivas-Escalona, V.; Aranda-Sanchez, J.; GonzalezDominguez, M.C.; Rubio-Garcia, J.; Aleman-Flores, P.; Lozano-Rodriguez, A.; Porcel-de-Peralta, G.; Loro-Ferrer, J.F. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: The role of difusion-weighted imaging. Radiol. Med. 2020, 125, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Collins, D.J. Difusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [Green Version]
- Barnes, A.; Alonzi, R.; Blackledge, M.; Charles-Edwards, G.; Collins, D.J.; Cook, G.; Coutts, G.; Goh, V.; Graves, M.; Kelly, C.; et al. UK quantitative WB-DWI technical workgroup: Consensus meeting recommendations on optimisation, quality control, processing and analysis of quantitative whole-body difusion-weighted imaging for cancer. Br. J. Radiol. 2018, 91, 20170577. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef]
- Narquin, S.; Ingrand, P.; Azais, I.; Delwail, V.; Vialle, R.; Boucebci, S.; Tasu, J.P. Comparison of whole-body diffusion MRI and conventional radiological assessment in the staging of myeloma. Diagn. Interv. Imaging. 2013, 94, 629–636, Epub 15 May 2013. Erratum in: Diagn. Interv. Imaging. 2020, 101, 331. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Mattace Raso, M.; Gabelloni, M.; Avallone, A.; Ottaiano, A.; Tatangelo, F.; Brunese, M.C.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics 2022, 12, 1115. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G.; et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. J. Clin. Med. 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Dell’ Aversana, F.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB-MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’ Aversana, F.; Ottaiano, A.; Avallone, A.; Nasti, G.; Grassi, F.; et al. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers 2022, 14, 1110. [Google Scholar] [CrossRef] [PubMed]
- Boatright, C.; Peterson, J.; Williams, V.L.; Best, S.; Ash, R. LI-RADS v2018: Utilizing ancillary features on gadoxetate-enhanced MRI to modify final LI-RADS category. Abdom. Radiol. 2020, 45, 3136–3143. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Masedu, F.; De Cataldo, C.; Cannizzaro, E.; Bruno, F.; Pradella, S.; Arrigoni, F.; Valenti, M.; Splendiani, A.; Barile, A.; et al. Real-world clinical validity of cardiac magnetic resonance tissue tracking in primitive hypertrophic cardiomyopathy. Radiol. Med. 2021, 126, 1532–1543. [Google Scholar] [CrossRef]
- Barile, A. Correction to: Some thoughts and greetings from the new Editor-in-Chief. Radiol. Med. 2021, 126, 1377. [Google Scholar] [CrossRef]
- Cutolo, C.; De Muzio, F.; Fusco, R.; Simonetti, I.; Belli, A.; Patrone, R.; Grassi, F.; Dell’Aversana, F.; Pilone, V.; Petrillo, A.; et al. Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging. Diagnostics 2022, 12, 1323. [Google Scholar] [CrossRef]
- Liu, Y.I.; Shin, L.K.; Jeffrey, R.B.; Kamaya, A. Quantitatively defining washout in hepatocellular carcinoma. AJR Am. J. Roentgenol. 2013, 200, 84–89. [Google Scholar] [CrossRef]
- Mähringer-Kunz, A.; Steinle, V.; Düber, C.; Weinmann, A.; Koch, S.; Schmidtmann, I.; Schotten, S.; Hinrichs, J.B.; Graafen, D.; dos Santos, D.P.; et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int. 2019, 39, 324–331. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, C.B.; McInnes, M.D.F.; Salameh, J.P.; Levis, B.; Chernyak, V.; Sirlin, C.B.; Bashir, M.R.; Allen, B.C.; Burke, L.M.B.; Choi, J.Y.; et al. CT/MRI and CEUS LI-RADS Major Features Association with Hepatocellular Carcinoma: Individual Patient Data Meta-Analysis. Radiology 2022, 302, 326–335. [Google Scholar] [CrossRef]
- Motosugi, U.; Ichikawa, T.; Sou, H.; Sano, K.; Tominaga, L.; Muhi, A.; Araki, T. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology 2010, 256, 151–158. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Yoon, J.K.; Chung, Y.E.; Choi, J.Y.; Park, M.S. LI-RADS Major Features on MRI for Diagnosing Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J. Magn. Reason. Imaging 2021, 54, 518–525. [Google Scholar] [CrossRef]
- Tang, A.; Singal, A.G.; Mitchell, D.G.; Hecht, E.M.; Fowler, K.J.; Kulik, L.; Parikh, N.D.; Kono, Y.; Sirlin, C.B. Introduction to the Liver Imaging Reporting and Data System for Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, A.E.; Hecht, E.M.; Hahn, W.Y.; Kim, D.C.; Babb, J.S.; Lee, V.S.; West, A.B.; Krinsky, G.A. Importance of small (<or = 20-mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: Evaluation and comparison with whole explanted liver. Radiology 2005, 237, 938–944. [Google Scholar]
- Jang, H.J.; Kim, T.K.; Khalili, K.; Yazdi, L.; Menezes, R.; Park, S.H.; Sherman, M. Characterization of 1-to 2-cm liver nodules detected on hcc surveillance ultrasound according to the criteria of the American Association for the Study of Liver Disease: Is quadriphasic CT necessary? AJR Am. J. Roentgenol. 2013, 201, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Caruso, S.; Milazzo, M.; Mamone, G.; Marrone, G.; Miraglia, R.; Maruzzelli, L.; Carollo, V.; Minervini, M.I.; Vizzini, G.; et al. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: Prevalence of radiological vascular patterns and histological correlation with liver explants. Eur. Radiol. 2010, 20, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging reporting and data system (LI-RADS) version 2018: Imaging of hepatocellular carcinoma in at-risk patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Kamath, A.; Roudenko, A.; Hecht, E.; Sirlin, C.; Chernyak, V.; Fowler, K.; Mitchell, D.G. CT/MR LI-RADS 2018: Clinical implications and management recommendations. Abdominal. Radiol. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Paoletti, M.; Muzic, S.I.; Marchetti, F.; Farina, L.M.; Bastianello, S.; Pichiecchio, A. Diferential imaging of atypical demyelinating lesions of the central nervous system. Radiol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Shiri, I.; Hajianfar, G.; Oveisi, N.; Abdollahi, H.; Deevband, M.R.; Oveisi, M.; Zaidi, H. Noninvasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. Radiol. Med. 2020, 125, 754–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Meglio, N.D.; Roscio, D.D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDS™) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Pirovano, M.; Ciocca, M.; Gibelli, D.; Floridi, C.; Oliva, G. Radiomic analysis of the optic nerve at the frst episode of acute optic neuritis: An indicator of optic nerve pathology and a predictor of visual recovery? Radiol. Med. 2021, 126, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsoe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-V.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Jo, P.C.; Jang, H.J.; Burns, P.N.; Burak, K.W.; Kim, T.K.; Wilson, S.R. Integration of contrast-enhanced US into a multimodality approach to imaging of nodules in a cirrhotic liver: How i do it. Radiology 2017, 282, 317–331. [Google Scholar] [CrossRef]
- Kielar, A.Z.; Chernyak, V.; Bashir, M.R.; Do, R.K.; Fowler, K.J.; Mitchell, D.G.; Cerny, M.; Elsayes, K.M.; Santillan, C.; Kamaya, A.; et al. LIRADS 2017: An update. J. Magn. Reson. Imaging 2018, 47, 1459–1474. [Google Scholar] [CrossRef]
- An, C.; Lee, C.H.; Byun, J.H.; Lee, M.H.; Jeong, W.K.; Choi, S.H.; Kim, D.Y.; Lim, Y.-S.; Kim, Y.S.; Kim, J.H.; et al. Intraindividual comparison between gadoxetate-enhanced magnetic resonance imaging and dynamic computed tomography for characterizing focal hepatic lesions: A multicentre, multireader study Korean. J. Radiol. 2019, 20, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.Y.; Kim, M.J.; Kim, E.H.; Roh, Y.H.; An, C. Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: Performance of LI-RADS version 2018. Radiology 2019, 291, 72–80. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.M.; Sirlin, C.B. CT and MR imaging diagnosis staging of hepatocellular carcinoma: Part II Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef] [Green Version]
- Schima, W.; Heiken, J. LI-RADS v2017 for liver nodules: How we read and report. Cancer Imaging 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishigami, K.; Yoshimitsu, K.; Nishihara, Y.; Irie, H.; Asayama, Y.; Tajima, T.; Nishie, A.; Hirakawa, M.; Ushijima, Y.; Okamoto, D.; et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: Correlation with histopathologic findings. Radiology 2009, 250, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gaetano, A.M.; Catalano, M.; Pompili, M.; Marini, M.G.; Rodríguez Carnero, P.; Gullì, C.; Infante, A.; Iezzi, R.; Ponziani, F.R.; Cerrito, L.; et al. Critical analysis of major and ancillary features of LI-RADS v2018 in the differentiation of small (≤2 cm) hepatocellular carcinoma from dysplastic nodules with gadobenate dimeglumine-enhanced magnetic resonance imaging. Eur. Rev. Med. Pharmacol. Sci. 2019, 18, 7786–7801. [Google Scholar] [CrossRef]
- Tang, A.; Cruite, I.; Mitchell, D.G.; Sirlin, C.B. Hepatocellular carcinoma imaging systems: Why they exist, how they have evolved, and how they differ. Abdom. Radiol. 2018, 43, 3–12. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Y.; Chen, J.; Jiang, T.; Liu, W.; Rong, D.; Sun, L.; Zhang, L.; He, B.; Wang, J. Can modified LI-RADS increase the sensitivity of LI-RADS v2018 for the diagnosis of 10-19 mm hepatocellular carcinoma on gadoxetic acid-enhanced MRI? Abdom. Radiol. 2022, 47, 596–607. [Google Scholar] [CrossRef]
- Vernuccio, F.; Porrello, G.; Cannella, R.; Vernuccio, L.; Midiri, M.; Giannitrapani, L.; Soresi, M.; Brancatelli, G. Benign and malignant mimickers of infltrative hepatocellular carcinoma: Tips and tricks for diferential diagnosis on CT and MRI. Clin. Imaging 2021, 70, 33–45. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Meyer, M.; Choudhoury, K.R.; Gonzáles, F.; Schwartz, F.R.; Gupta, R.T.; Bashir, M.R.; Furlan, A.; Marin, D. LI-RADS: Diagnostic performance of hepatobiliary phase hypointensity and major imaging features of LR-3 and LR-4 lesions measuring 10–19 mm with arterial phase hyperenhancement. AJR Am. J. Roentgenol. 2019, 213, W57–W65. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Guarino, B.; Granata, F.; Tatangelo, F.; Avallone, A.; Piccirillo, M.; Palaia, R.; Izzo, F.; et al. Intravoxel incoherent motion (IVIM) in difusion-weighted imaging (DWI) for Hepatocellular carcinoma: Correlation with histologic grade. Oncotarget 2016, 7, 79357–79364. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Fusco, R.; Catalano, O.; Filice, S.; Amato, D.M.; Nasti, G.; Avallone, A.; Izzo, F.; Petrillo, A. Early assessment of colorectal cancer patients with liver metastases treated with antiangiogenic drugs: The role of intravoxel incoherent motion in difusion-weighted imaging. PLoS ONE 2015, 10, e0142876. [Google Scholar] [CrossRef]
- Shannon, B.A.; Ahlawat, S.; Morris, C.D.; Levin, A.S.; Fayad, L.M. Do contrastenhanced and advanced MRI sequences improve diagnostic accuracy for indeterminate lipomatous tumors? Radiol. Med. 2021, 127, 90–99. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kato, H.; Nagasawa, T.; Kaneko, Y.; Taguchi, K.; Ikeda, T.; Morita, H.; Miyazaki, T.; Matsuo, M. MR imaging fndings of musculoskeletal involvement in microscopic polyangiitis: A comparison with infammatory myopathy. Radiol. Med. 2021, 126, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Gibelli, D.; Martinenghi, C.; Giardini, D.; Soresina, M.; Menozzi, A.; Oliva, G.; Carrafello, G. Non-contrast magnetic resonance lymphography (NCMRL) in cancer-related secondary lymphedema: Acquisition technique and imaging fndings. Radiol. Med. 2021, 126, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Tang, Y.F.; Ruan, X.Z.; Huang, Q.L.; Sun, L.; Li, J. The value of Gd-BOPTAenhanced MRIs and DWI in the diagnosis of intrahepatic mass-forming cholangiocarcinoma. Neoplasma 2017, 64, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Kovač, J.D.; Galun, D.; Đurić-Stefanović, A.; Lilić, G.; Vasin, D.; Lazić, L.; Mašulović, D.; Šaranović, Đ. Intrahepatic mass-forming cholangiocarcinoma and solitary hypovascular liver metastases: Is the diferential diagnosis using difusion-weighted MRI possible? Acta Radiol. 2017, 58, 1417–1426. [Google Scholar] [CrossRef]
- Minutoli, F.; Pergolizzi, S.; Blandino, A.; Mormina, E.; Amato, E.; Gaeta, M. Efect of granulocyte colony-stimulating factor on bone marrow: Evaluation by intravoxel incoherent motion and dynamic contrast-enhanced magnetic resonance imaging. Radiol. Med. 2020, 125, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Cannella, R.; Vernuccio, F.; Sagreiya, H.; Choudhury, K.R.; Iranpour, N.; Marin, D.; Furlan, A. Liver Imaging Reporting and Data System (LI-RADS) v2018: Diagnostic value of ancillary features favoring malignancy in hypervascular observations ≥ 10 mm at intermediate (LR-3) and high probability (LR-4) for hepatocellular carcinoma. Eur. Radiol. 2020, 30, 3770–3781. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.S.; Bae, H.; Shin, J.; Yoon, J.K.; Kim, M.J. Application of Liver Imaging Reporting and Data System version 2018 ancillary features to upgrade from LR-4 to LR-5 on gadoxetic acid-enhanced MRI. Eur. Radiol. 2021, 31, 855–863. [Google Scholar] [CrossRef]
- Sano, K.; Ichikawa, T.; Motosugi, U.; Sou, H.; Muhi, A.M.; Matsuda, M.; Nakano, M.; Sakamoto, M.; Nakazawa, T.; Asakawa, M.; et al. Imaging study of early hepatocellular carcinoma: Usefulness of gadoxetic acid-enhanced MR imaging. Radiology 2011, 261, 834–844. [Google Scholar] [CrossRef]
- Song, J.S.; Choi, E.J.; Hwang, S.B.; Hwang, H.P. Choi HLI-RADS v2014 categorization of hepatocellular carcinoma: Intraindividual comparison between gadopentetate dimeglumine-enhanced MRI gadoxetic acid-enhanced MRI. Eur. Radiol. 2019, 29, 401–410. [Google Scholar] [CrossRef]
- Cerny, M.; Bergeron, C.; Billiard, J.S.; Murphy-Lavallee, J.; Olivie, D.; Berube, J.; Fan, B.; Castel, H.; Turcotte, S.; Perreault, P.; et al. LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features. Radiology 2018, 288, 118–128. [Google Scholar]
- Cortis, K.; Liotta, R.; Miraglia, R.; Caruso, S.; Tuzzolino, F.; Luca, A. Incorporating the hepatobiliary phase of gadobenate dimeglumine-enhanced MRI in the diagnosis of hepatocellular carcinoma: Increasing the sensitivity without compromising specificity. Acta Radiol. 2016, 57, 923–931. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Xie, S.; Chen, J.; Zhang, L.; Rong, D.; Kuang, S.; He, B.; Wang, J. The role of lesion hypointensity on gadobenate dimeglumine-enhanced hepatobiliary phase MRI as an additional major imaging feature for HCC classification using LI-RADS v2018 criteria. Eur. Radiol. 2021, 31, 7715–7724. [Google Scholar] [CrossRef]
- Iavarone, M.; Piscaglia, F.; Vavassori, S.; Galassi, M.; Sangiovanni, A.; Venerandi, L.; Forzenigo, L.V.; Golfieri, R.; Bolondi, L.; Colombo, M. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J. Hepatol. 2013, 58, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Lee, J.M.; Kim, S.H.; Han, J.K.; Choi, B.I. Intrahepatic mass-forming cholangiocarcinoma: Enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012, 264, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Péporté, A.R.; Sommer, W.H.; Nikolaou, K.; Reiser, M.F.; Zech, C.J. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. Eur. J. Radiol. 2013, 82, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Forner, A.; Reig, M.; Vilana, R.; de Lope, C.R.; Ayuso, C.; Bruix, J. Cholangiocarcinoma in cirrhosis: Absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology 2009, 50, 791–798. [Google Scholar] [CrossRef]
- Cannella, R.; Fraum, T.J.; Ludwig, D.R.; Borhani, A.A.; Tsung, A.; Furlan, A.; Fowler, K.J. Targetoid appearance on T2-weighted imaging and signs of tumor vascular involvement: Diagnostic value for differentiating HCC from other primary liver carcinomas. Eur. Radiol. 2021, 31, 6868–6878. [Google Scholar] [CrossRef]
- Sheng, R.F.; Zeng, M.S.; Rao, S.X.; Ji, Y.; Chen, L.L. MRI of small intrahepatic mass-forming cholangiocarcinoma and atypical small hepatocellular carcinoma (≤3 cm) with cirrhosis and chronic viral hepatitis: A comparative study. Clin. Imaging 2014, 38, 265–272. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.M.; Yoon, J.H. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology 2018, 288, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.M.; Han, J.K.; Kim, K.H.; Lee, J.Y.; Choi, B.I. Peripheral mass-forming cholangiocarcinoma in cirrhotic liver. AJR Am. J. Roentgenol. 2007, 189, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, M.J.; An, C. How to utilize LR-M features of the LI-RADS to improve the diagnosis of combined hepatocellular-cholangiocarcinoma on gadoxetate-enhanced MRI? Eur. Radiol. 2019, 29, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, S.S.; Park, S.H.; Kim, K.M.; Yu, E.; Park, Y.; Shin, Y.M.; Lee, M.G. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology 2019, 290, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Bashir, M.R.; Corwin, M.T.; Cruite, I.; Dietrich, C.F.; Do, R.; Ehman, E.C.; Fowler, K.J.; Hussain, H.K.; Jha, R.C.; et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018, 286, 29–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, K.J.; Potretzke, T.A.; Hope, T.A.; Costa, E.A.; Wilson, S.R. LI-RADS M (LR-M): Definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom. Radiol. 2018, 43, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Luo, Y.; Morelli, J.N.; Hu, X.; Shen, Y.; Hu, D. Differentiation of hepatocellular carcinoma from intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in high-risk patients matched to MR field strength: Diagnostic performance of LI-RADS version 2018. Abdom. Radiol. 2021, 46, 3168–3178. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, S.S.; Yu, E.; Kang, H.J.; Park, Y.; Kim, S.Y.; Lee, S.J.; Shin, Y.M.; Lee, M.G. Combined hepatocellular-cholangiocarcinoma: Gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J. Magn. Reson. Imaging 2017, 46, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lee, S.; Choi, J.Y.; Lim, J.S.; Park, M.S.; Kim, M.J. Diagnostic performance of the LR-M criteria and spectrum of LI-RADS imaging features among primary hepatic carcinomas. Abdom. Radiol. 2020, 45, 3743–3754. [Google Scholar] [CrossRef]
- Fanelli, F.; Cannavale, A.; Chisci, E.; Citone, M.; Falcone, G.M.; Michelagnoli, S.; Miele, V. Direct percutaneous embolization of aneurysm sac: A safe and effective procedure to treat post-EVAR type II endoleaks. Radiol. Med. 2021, 126, 258–263. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Randazzo, A.; Bruno, E.; Taibbi, A. Focal liver lesions in cirrhosis: Role of contrast-enhanced ultrasonography. World J. Radiol. 2022, 14, 70–81. [Google Scholar] [CrossRef]
- Sugimoto, K.; Saito, K.; Shirota, N.; Kamiyama, N.; Sakamaki, K.; Takahashi, H.; Wada, T.; Kakegawa, T.; Tomita, Y.; Abe, M.; et al. Comparison of modified CEUS LI-RADS with sonazoid and CT/MRI LI-RADS for diagnosis of hepatocellular carcinoma. Hepatol. Res. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Catalano, O.; Sandomenico, F.; Vallone, P.; Setola, S.V.; Granata, V.; Fusco, R.; Lastoria, S.; Mansi, L.; Petrillo, A. Contrast-Enhanced Ultrasound in the Assessment of Patients with Indeterminate Abdominal Findings at Positron Emission Tomography Imaging. Ultrasound Med. Biol. 2016, 42, 2717–2723. [Google Scholar] [CrossRef]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.; et al. Critical role of bevacizumab scheduling in combination with pre-surgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the BRANCH trial. Oncotarget 2015, 6, 30394–30407, PMCID:PMC4745808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Palaia, R.; Botti, G.; Tatangelo, F.; Granata, F.; Cascella, M.; Izzo, F.; et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE 2017, 12, e0179951. [Google Scholar] [CrossRef] [Green Version]
- Schellhaas, B.; Hammon, M.; Strobel, D.; Pfeifer, L.; Kielisch, C.; Goertz, R.S.; Cavallaro, A.; Janka, R.; Neurath, M.F.; Uder, M.; et al. Interobserver and intermodality agreement of standardized algorithms for non-invasive diagnosis of hepatocellular carcinoma in high-risk patients: CEUS-LI-RADS versus MRI-LI-RADS. Eur. Radiol. 2018, 28, 4254–4264, Epub 19 April 2018. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A pictorial review. Insights Imaging 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Lyshchik, A.; Piscaglia, F.; Cosgrove, D.; Jang, H.J.; Sirlin, C.; Dietrich, C.F.; Kim, T.K.; Willmann, J.K.; Kono, Y. CEUS LI-RADS: Algorithm, implementation, and key differences from CT/MRI. Abdom. Radiol. 2018, 43, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Jang, H.J.; Wilson, S.R. Benign liver masses: Imaging with microbubble contrast agents. Ultrasound Q. 2006, 22, 31–39. [Google Scholar] [PubMed]
- Vilgrain, V.; Boulos, L.; Vullierme, M.P.; Denys, A.; Terris, B.; Menu, Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics 2000, 20, 379–397. [Google Scholar] [CrossRef]
- Maruyama, H.; Takahashi, M.; Ishibashi, H.; Yoshikawa, M.; Yokosuka, O. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br. J. Radiol. 2012, 85, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Noh, S.Y.; Wilson, S.R.; Kono, Y.; Piscaglia, F.; Jang, H.J.; Lyshchik, A.; Dietrich, C.F.; Willmann, J.K.; Vezeridis, A.; et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017—a review of important differences compared to the CT/MRI system. Clin. Mol. Hepatol. 2017, 23, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.Y.; Li, J.W.; Ling, W.W.; Li, T.; Luo, Y.; Liu, J.B.; Lu, Q. Can contrast enhanced ultrasound differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma? World J. Gastroenterol. 2020, 26, 3938–3951. [Google Scholar] [CrossRef] [PubMed]
- Kielar, A.; Fowler, K.J.; Lewis, S.; Yaghmai, V.; Miller, F.H.; Yarmohammadi, H.; Kim, C.; Chernyak, V.; Yokoo, T.; Meyer, J.; et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom. Radiol. 2018, 43, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Piccirillo, M.; Pradella, S.; Giordano, M.; Cappabianca, S.; Brunese, L.; et al. Abbreviated MRI Protocol for the Assessment of Ablated Area in HCC Patients. Int. J. Environ. Res. Public Health 2021, 18, 3598. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Piccirillo, M.; Albino, V.; Palaia, R.; Belli, A.; Granata, V.; Setola, S.; Fusco, R.; Petrillo, A.; Orlando, R.; et al. Prospective screening increases the detection of potentially curable hepatocellular carcinoma: Results in 8900 high-risk patients. HPB 2013, 15, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Curley, S.A.; Izzo, F.; Abdalla, E.; Vauthey, J.N. Surgical treatment of colorectal cancer metastasis. Cancer Metastasis Rev 2004, 23, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, M.L.; Buonaguro, L.; Izzo, F.; Buonaguro, F.M. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget 2016, 7, 25087–25102. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Zignego, A.L.; Izzo, F.; Tornesello, M.L.; Buonaguro, F.M.; Mangia, A.; Sansonno, D.; et al. Genetic diversity of the KIR/HLA system and susceptibility to hepatitis C virus-related diseases. PLoS ONE 2015, 10, e0117420. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Cascella, M.; Fusco, R.; dell’Aprovitola, N.; Catalano, O.; Filice, S.; Schiavone, V.; Izzo, F.; Cuomo, A.; Petrillo, A. Immediate Adverse Reactions to Gadolinium-Based MR Contrast Media: A Retrospective Analysis on 10,608 Examinations. Biomed. Res. Int. 2016, 2016, 3918292. [Google Scholar] [CrossRef]
- Capone, F.; Costantini, S.; Guerriero, E.; Calemma, R.; Napolitano, M.; Scala, S.; Izzo, F.; Castello, G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur. Cytokine Netw. 2010, 21, 99–104. [Google Scholar] [CrossRef]
- Perrone, F.; Gallo, C.; Daniele, B.; Gaeta, G.B.; Izzo, F.; Capuano, G.; Adinolfi, L.E.; Mazzanti, R.; Farinati, F.; Elba, S.; et al. Cancer of Liver Italian Program (CLIP) Investigators. Tamoxifen in the treatment of hepatocellular carcinoma: 5-year results of the CLIP-1 multicentre randomised controlled trial. Curr. Pharm. Des. 2002, 8, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Rea, D.; Palma, G.; Luciano, A.; Cuomo, A.; Arra, C.; Izzo, F. Morphine Promotes Tumor Angiogenesis and Increases Breast Cancer Progression. Biomed. Res. Int. 2015, 2015, 161508. [Google Scholar] [CrossRef] [PubMed]
- Polesel, J.; Talamini, R.; Montella, M.; Maso, L.D.; Crovatto, M.; Parpinel, M.; Izzo, F.; Tommasi, L.G.; Serraino, D.; La Vecchia, C.; et al. Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur. J. Cancer 2007, 43, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Gallo, C.; Daniele, B.; Elba, S.; Giorgio, A.; Capuano, G.; Adinolfi, L.E.; De Sio, I.; Izzo, F.; Farinati, F.; et al. CLIP Investigators. Characteristics at presentation and outcome of hepatocellular carcinoma (HCC) in the elderly. A study of the Cancer of the Liver Italian Program (CLIP). Crit. Rev. Oncol. Hematol. 2006, 59, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, M.; McGinty, K.A.; Mervak, B.; Lerebours, R.; Li, C.; Shropshire, E.; Ronald, J.; Commander, L.; Hertel, J.; Luo, S.; et al. The LI-RADS version 2018 MRI treatment response algorithm: Evaluation of ablated hepatocellular carcinoma. Radiology 2020, 294, 320–326. [Google Scholar] [CrossRef]
- Shropshire, E.L.; Chaudhry, M.; Miller, C.M.; Allen, B.C.; Bozdogan, E.; Cardona, D.M.; King, L.Y.; Janas, G.L.; Do, R.K.; Kim, C.Y.; et al. LI-RADS Treatment Response Algorithm: Performance and Diagnostic Accuracy. Radiology 2019, 292, 226–234. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: Theoretical principles and clinical applications. Radiol. Med. 2019, 124, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Masciocchi, C.; Conti, L.; D’Orazio, F.; Conchiglia, A.; Lanni, G.; Barile, A. Errors in Musculoskeletal MRI. In Errors in Radiology; Romano, L., Pinto, A., Eds.; Springer: Milano, Italy, 2012. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Muzio, F.; Grassi, F.; Dell’Aversana, F.; Fusco, R.; Danti, G.; Flammia, F.; Chiti, G.; Valeri, T.; Agostini, A.; Palumbo, P.; et al. A Narrative Review on LI-RADS Algorithm in Liver Tumors: Prospects and Pitfalls. Diagnostics 2022, 12, 1655. https://doi.org/10.3390/diagnostics12071655
De Muzio F, Grassi F, Dell’Aversana F, Fusco R, Danti G, Flammia F, Chiti G, Valeri T, Agostini A, Palumbo P, et al. A Narrative Review on LI-RADS Algorithm in Liver Tumors: Prospects and Pitfalls. Diagnostics. 2022; 12(7):1655. https://doi.org/10.3390/diagnostics12071655
Chicago/Turabian StyleDe Muzio, Federica, Francesca Grassi, Federica Dell’Aversana, Roberta Fusco, Ginevra Danti, Federica Flammia, Giuditta Chiti, Tommaso Valeri, Andrea Agostini, Pierpaolo Palumbo, and et al. 2022. "A Narrative Review on LI-RADS Algorithm in Liver Tumors: Prospects and Pitfalls" Diagnostics 12, no. 7: 1655. https://doi.org/10.3390/diagnostics12071655
APA StyleDe Muzio, F., Grassi, F., Dell’Aversana, F., Fusco, R., Danti, G., Flammia, F., Chiti, G., Valeri, T., Agostini, A., Palumbo, P., Bruno, F., Cutolo, C., Grassi, R., Simonetti, I., Giovagnoni, A., Miele, V., Barile, A., & Granata, V. (2022). A Narrative Review on LI-RADS Algorithm in Liver Tumors: Prospects and Pitfalls. Diagnostics, 12(7), 1655. https://doi.org/10.3390/diagnostics12071655