Anti-Carbamylated Protein (Anti-CarP) Antibodies in Patients Evaluated for Suspected Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Immunoassays
2.3. Statistical Analysis
3. Results
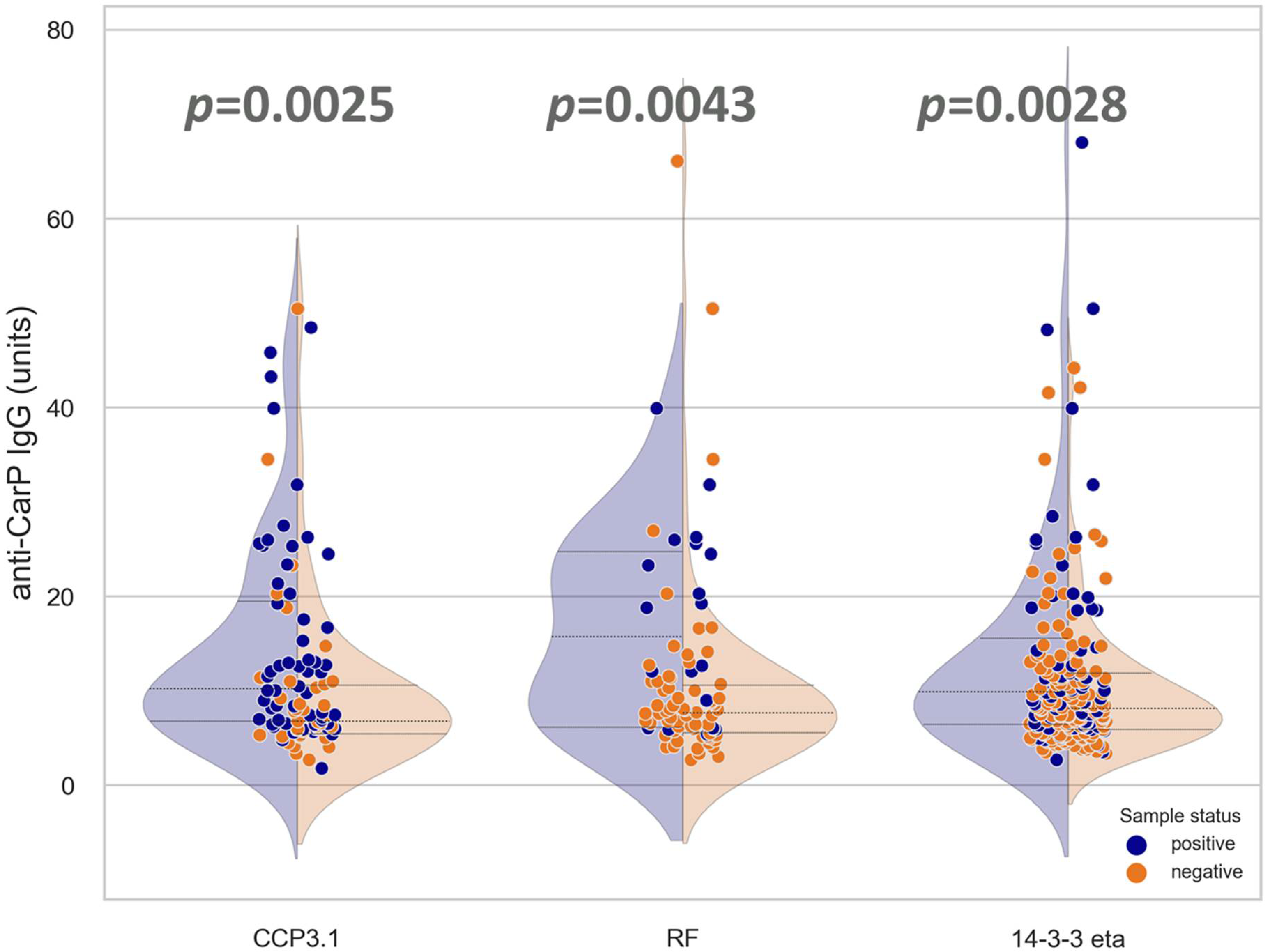
3.1. Anti-CarP Antibodies Are Found in Both ACPA-Positive and ACPA-Negative Patient Samples
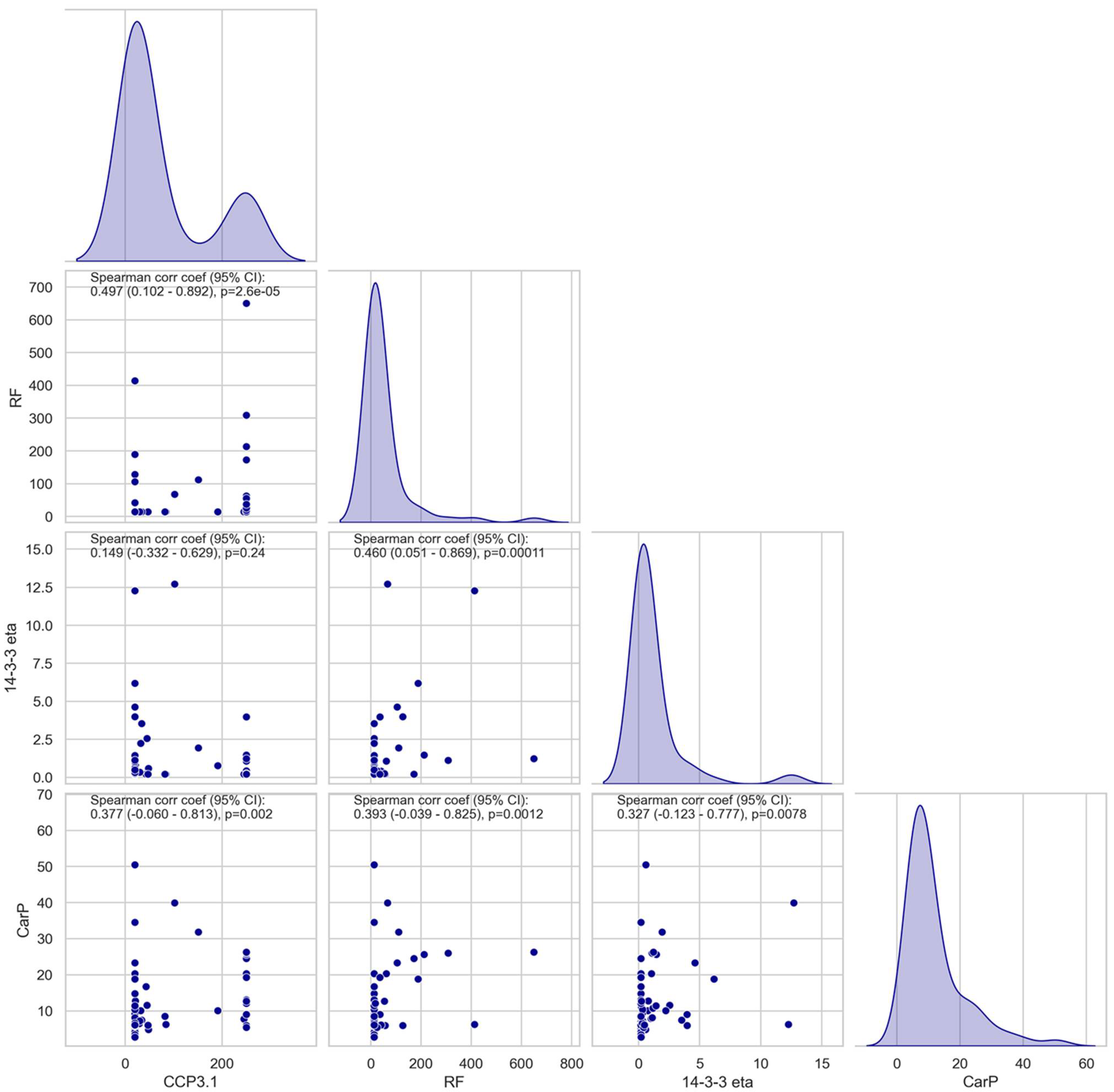
3.2. Association and Correlation of Anti-CarP Antibodies with Anti-CCP3.1, RF, or 14-3-3 Eta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.A.; Janssen, G.M.C.; van Veelen, P.; Levarht, N.E.W.; van der Helm-van Mil, A.H.; Cerami, A.; Huizinga, T.W.J.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 17372–17377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truchetet, M.E.; Dublanc, S.; Barnetche, T.; Vittecoq, O.; Mariette, X.; Richez, C.; Blanco, P.; Mahler, M.; Contin-Bordes, C.; Schaeverbeke, T.; et al. Association of the Presence of Anti-Carbamylated Protein Antibodies in Early Arthritis with a Poorer Clinical and Radiologic Outcome: Data from the French ESPOIR Cohort. Arthritis Rheumatol. 2017, 69, 2292–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, J.H.; Verheul, M.K.; Barton, A.; MacGregor, A.J.; Lunt, M.; Toes, R.; Symmons, D.; A Trouw, L.; Verstappen, S.M. Anticarbamylated protein antibodies are associated with long-term disability and increased disease activity in patients with early inflammatory arthritis: Results from the Norfolk Arthritis Register. Ann. Rheum. Dis. 2016, 75, 1139–1144. [Google Scholar] [CrossRef]
- Shi, J.; van Steenbergen, H.W.; van Nies, J.A.; Levarht, E.W.; Huizinga, T.W.; van der Helm-van Mil, A.H.; Toes, R.E.M.; Trouw, L.A. The specificity of anti-carbamylated protein antibodies for rheumatoid arthritis in a setting of early arthritis. Arthritis Res. Ther. 2015, 17, 339. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.W.; Trouw, L.A.; Shi, J.; Toes, R.E.; Huizinga, T.W.; Demoruelle, M.K.; Kolfenbach, J.R.; Zerbe, G.O.; Deane, K.D.; Edison, J.D.; et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J. Rheumatol. 2015, 42, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; van de Stadt, L.A.; Levarht, E.W.; Huizinga, T.W.; Hamann, D.; van Schaardenburg, D.; Toes, R.; Trouw, L. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Stoop, J.N.; Liu, B.S.; Shi, J.; Jansen, D.T.; Hegen, M.; Huizinga, T.W.J.; Trouw, L.A.; Toes, R.E.M. Antibodies specific for carbamylated proteins precede the onset of clinical symptoms in mice with collagen induced arthritis. PLoS ONE 2014, 9, e102163. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.C.; Anink, J.; Shi, J.; Levarht, E.W.; Reinards, T.H.; Otten, M.H.; van Tol, M.J.D.; der Zijde, C.M.J.-V.; Brinkman, D.M.C.; Allaart, C.F.; et al. Anticarbamylated protein (anti-CarP) antibodies are present in sera of juvenile idiopathic arthritis (JIA) patients. Ann. Rheum. Dis. 2013, 72, 2053–2055. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; van de Stadt, L.A.; Levarht, E.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A.; van Schaardenburg, D. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 911–915. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Sciandrone, M.; Perricone, C.; Galvan, G.; Cipriano, E.; Galligari, A.; Levato, T.; Colasanti, T.; Massaro, L.; Natalucci, F.; et al. Biomarkers of erosive arthritis in systemic lupus erythematosus: Application of machine learning models. PLoS ONE 2018, 13, e0207926. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.; Webb, T.; Seaman, A.; Infantino, M.; Meacci, F.; Manfredi, M.; Benucci, M.; Lakos, G.; Favalli, E.G.; Schioppo, T.; et al. Anti-CarP antibodies as promising marker to measure joint damage and disease activity in patients with rheumatoid arthritis. Immunol. Res. 2015, 61, 24–30. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; Twisk, J.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; De Gast, T.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A.C. Increased levels of C-reactive protein in serum from blood donors before the onset of rheumatoid arthritis. Arthritis Rheum. 2004, 50, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.M.T.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A.C. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Nell, V.P.; Machold, K.P.; Eberl, G.; Stamm, T.A.; Uffmann, M.; Smolen, J.S. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology 2004, 43, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finckh, A.; Liang, M.H.; van Herckenrode, C.M.; de Pablo, P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006, 55, 864–872. [Google Scholar] [CrossRef]
- Reed, E.; Hedström, A.K.; Hansson, M.; Mathsson-Alm, L.; Brynedal, B.; Saevarsdottir, S.; Cornillet, M.; Jakobsson, P.-J.; Holmdahl, R.; Skriner, K.; et al. Presence of autoantibodies in “seronegative” rheumatoid arthritis associates with classical risk factors and high disease activity. Arthritis Res. Ther. 2020, 22, 170. [Google Scholar] [CrossRef]
- Trouw, L.A.; Mahler, M. Closing the serological gap: Promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun. Rev. 2012, 12, 318–322. [Google Scholar] [CrossRef]
- Brink, M.; Hansson, M.; Mathsson-Alm, L.; Wijayatunga, P.; Verheul, M.K.; Trouw, L.A.; Holmdahl, R.; Rönnelid, J.; Klareskog, L.; Rantapää-Dahlqvist, S. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 43. [Google Scholar] [CrossRef] [Green Version]
- Ajeganova, S.; van Steenbergen, H.W.; Verheul, M.K.; Forslind, K.; Hafström, I.; Toes, R.E.M.; Huizinga, T.W.J.; Svensson, B.; A Trouw, L.; Mil, A.H.M. The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: A study exploring replication and the added value to ACPA and rheumatoid factor. Ann. Rheum. Dis. 2017, 76, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Bralo, L.; Perez-Pampin, E.; Regueiro, C.; Montes, A.; Varela, R.; Boveda, M.D.; Gomez-Reino, J.J.; González, A. Anti-carbamylated protein autoantibodies associated with mortality in Spanish rheumatoid arthritis patients. PLoS ONE 2017, 12, e0180144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabo, S.; Hashimoto, M.; Ito, S.; Furu, M.; Ito, H.; Fujii, T.; Yoshifuji, H.; Imura, Y.; Nakashima, R.; Murakami, K.; et al. Carbamylated albumin is one of the target antigens of anti-carbamylated protein antibodies. Rheumatology 2017, 56, 1217–1226. [Google Scholar] [CrossRef]
- Dekkers, J.S.; Verheul, M.K.; Stoop, J.N.; Liu, B.; Ioan-Facsinay, A.; van Veelen, P.; De Ru, A.H.; Janssen, G.M.C.; Hegen, M.; Rapecki, S.; et al. Breach of autoreactive B cell tolerance by post-translationally modified proteins. Ann. Rheum. Dis. 2017, 76, 1449–1457. [Google Scholar] [CrossRef]
- Trouw, L.A.; Rispens, T.; Toes, R.E.M. Beyond citrullination: Other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Verheul, M.K.; Yee, A.; Seaman, A.; Janssen, G.M.; van Veelen, P.A.; Drijfhout, J.W.; Toes, R.E.; Mahler, M.; Trouw, L.A. Identification of carbamylated alpha 1 anti-trypsin (A1AT) as an antigenic target of anti-CarP antibodies in patients with rheumatoid arthritis. J. Autoimmun. 2017, 80, 77–84. [Google Scholar] [CrossRef]
- Li, L.; Deng, C.; Chen, S.; Zhang, S.; Wu, Z.; Hu, C.; Zhang, F.; Li, Y. Meta-Analysis: Diagnostic Accuracy of Anti-Carbamylated Protein Antibody for Rheumatoid Arthritis. PLoS ONE 2016, 11, e0159000. [Google Scholar] [CrossRef] [Green Version]
- Kolarz, B.; Ciesla, M.; Rosenthal, A.K.; Dryglewska, M.; Majdan, M. The value of anti-CarP and anti-PAD4 as markers of rheumatoid arthritis in ACPA/RF negative rheumatoid arthritis patients. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720x21989868. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Naides, S.J.; Bykerk, V.; Siminovitch, K.A.; van Schaardenburg, D.; Boers, M.; Landewé, R.; van der Heijde, D.; Tak, P.-P.; Genovese, M.C.; et al. Serum 14-3-3eta is a novel marker that complements current serological measurements to enhance detection of patients with rheumatoid arthritis. J. Rheumatol. 2014, 41, 2104–2113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liang, Y.; Feng, L.; Cui, L. Diagnostic performance of 14-3-3η and anti-carbamylated protein antibodies in Rheumatoid Arthritis in Han population of Northern China. Clin. Chim. Acta 2020, 502, 102–110. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricchiuti, V.; Chun, K.Y.; Yang, J.M.; Aure, M.A.; Gomez, L.; Norman, G.L.; Mahler, M. Anti-Carbamylated Protein (Anti-CarP) Antibodies in Patients Evaluated for Suspected Rheumatoid Arthritis. Diagnostics 2022, 12, 1661. https://doi.org/10.3390/diagnostics12071661
Ricchiuti V, Chun KY, Yang JM, Aure MA, Gomez L, Norman GL, Mahler M. Anti-Carbamylated Protein (Anti-CarP) Antibodies in Patients Evaluated for Suspected Rheumatoid Arthritis. Diagnostics. 2022; 12(7):1661. https://doi.org/10.3390/diagnostics12071661
Chicago/Turabian StyleRicchiuti, Vincent, Kelly Y. Chun, Jane M. Yang, Mary Ann Aure, Luis Gomez, Gary L. Norman, and Michael Mahler. 2022. "Anti-Carbamylated Protein (Anti-CarP) Antibodies in Patients Evaluated for Suspected Rheumatoid Arthritis" Diagnostics 12, no. 7: 1661. https://doi.org/10.3390/diagnostics12071661
APA StyleRicchiuti, V., Chun, K. Y., Yang, J. M., Aure, M. A., Gomez, L., Norman, G. L., & Mahler, M. (2022). Anti-Carbamylated Protein (Anti-CarP) Antibodies in Patients Evaluated for Suspected Rheumatoid Arthritis. Diagnostics, 12(7), 1661. https://doi.org/10.3390/diagnostics12071661






