Influence of CT Image Matrix Size and Kernel Type on the Assessment of HRCT in Patients with SSC-ILD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Image Acquisition
2.3. Qualitative and Statistical Analysis
3. Results
3.1. Patient Characteristics
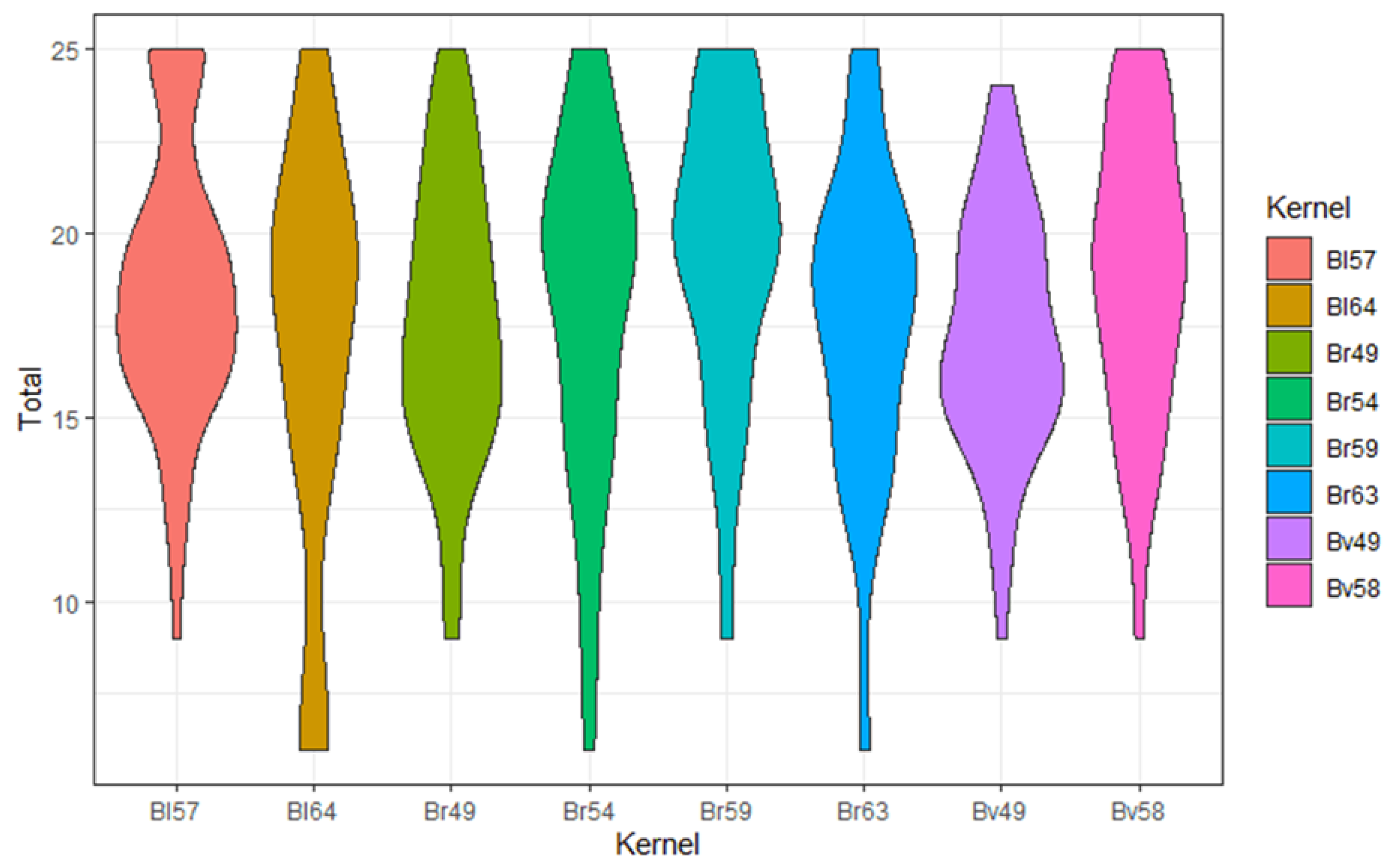
3.2. Comparison of Different Kernels
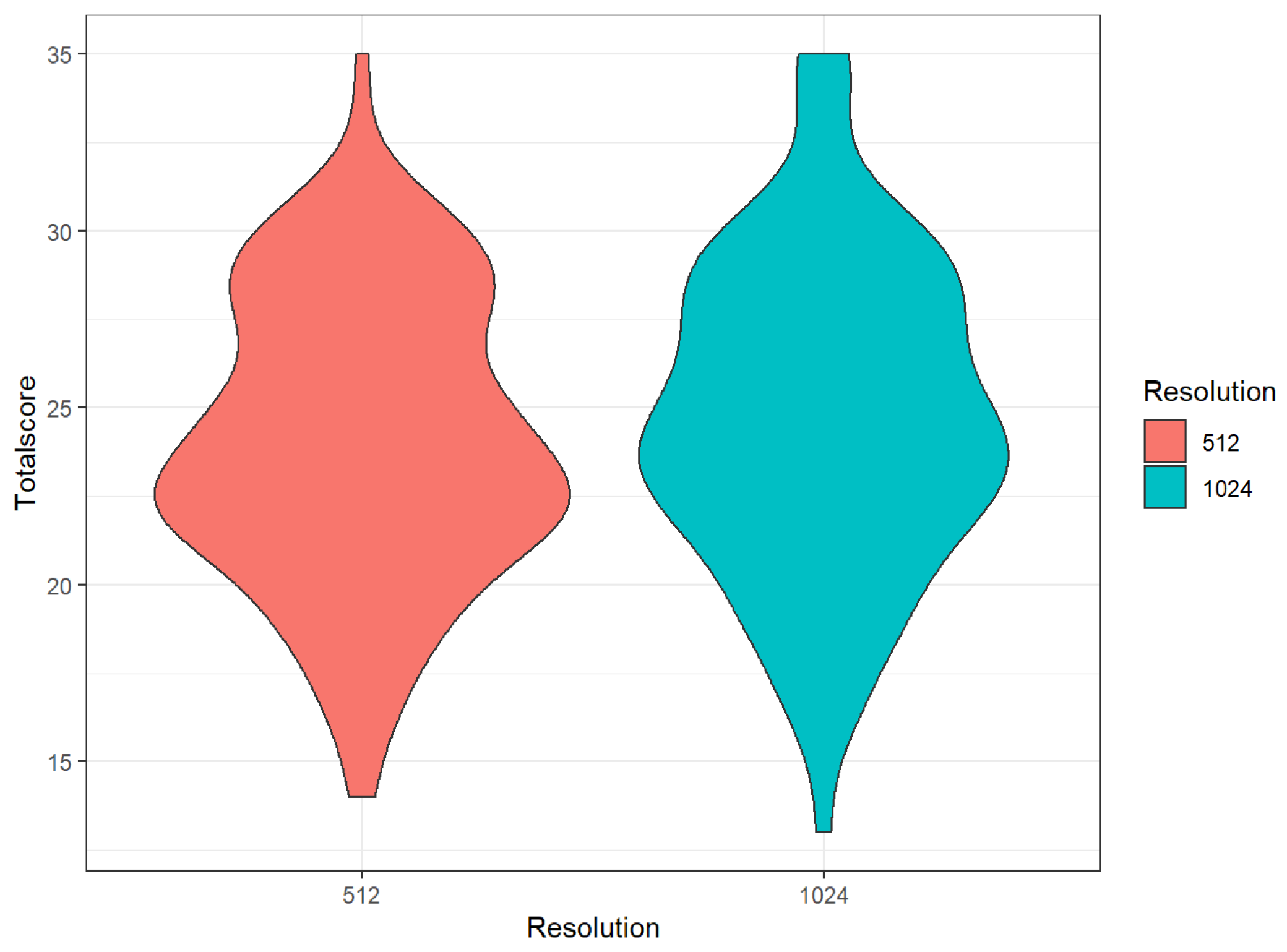
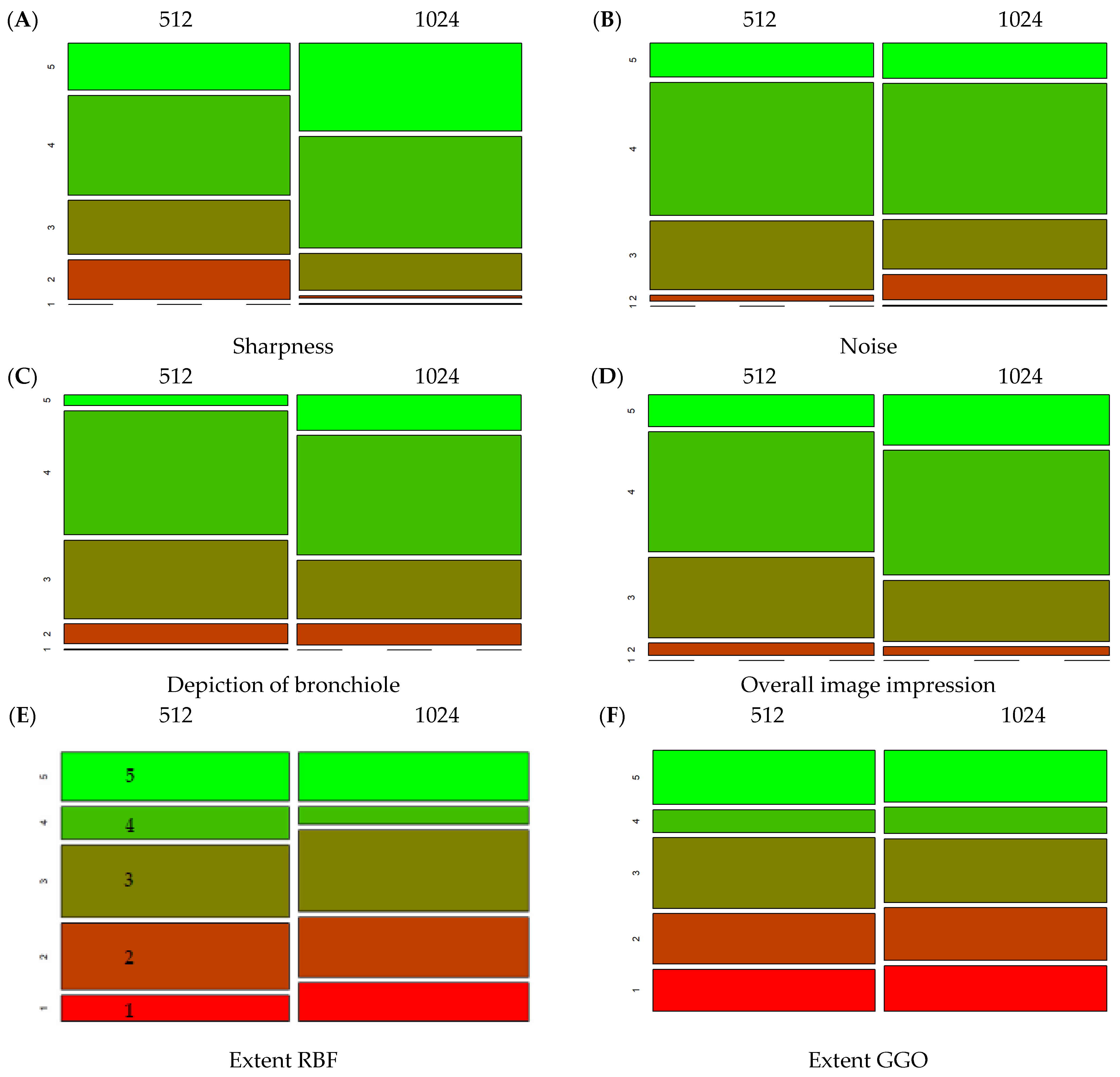
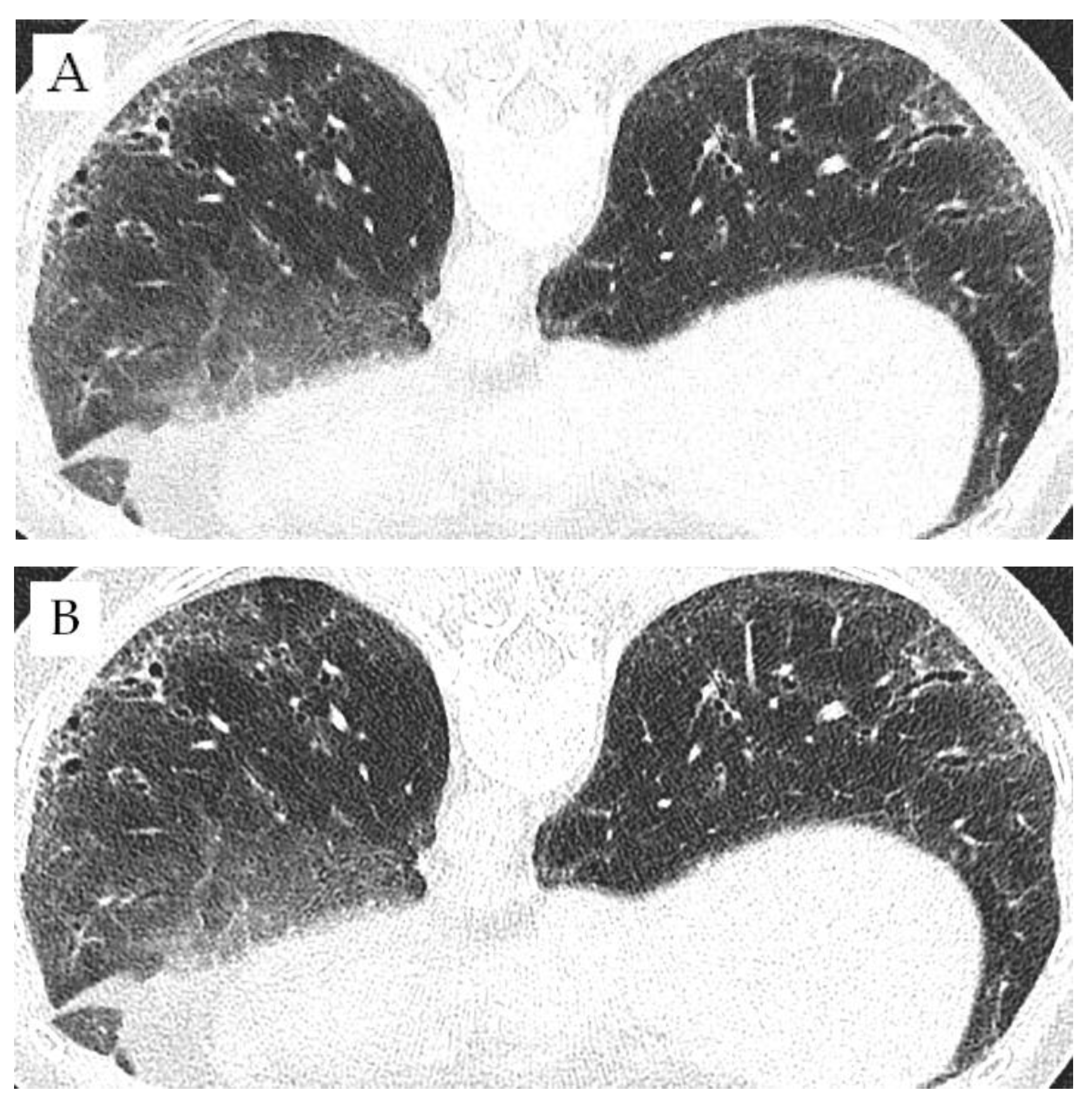
3.3. Comparison of Different Matrices
3.4. Intraclass Correlation and Field of View
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Le Pavec, J.; Launay, D.; Mathai, S.C.; Hassoun, P.M.; Humbert, M. Scleroderma lung disease. Clin. Rev. Allergy Immunol. 2011, 40, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, T.; Winklehner, A.; Nguyen, T.D.; Dobrota, R.; Baumueller, S.; Maurer, B.; Distler, O. Screening for interstitial lung disease in systemic sclerosis: Performance of high-resolution CT with limited number of slices: A prospective study. Ann. Rheum. Dis. 2014, 73, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Scirè, C.A.; Talarico, R.; Airo, P.; Alexander, T.; Allanore, Y.; Bruni, C.; Codullo, V.; Dalm, V.; De Vries-Bouwstra, J.; et al. Systemic sclerosis: State of the art on clinical practice guidelines. RMD Open 2019, 4, e000782. [Google Scholar] [CrossRef] [Green Version]
- Suliman, Y.A.; Dobrota, R.; Huscher, D.; Nguyen-Kim, T.D.L.; Maurer, B.; Jordan, S.; Speich, R.; Frauenfelder, T.; Distler, O. Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease. Arthritis Rheumatol. 2015, 67, 3256–3261. [Google Scholar] [CrossRef]
- Hata, A.; Yanagawa, M.; Honda, O.; Kikuchi, N.; Miyata, T.; Tsukagoshi, S.; Uranishi, A.; Tomiyama, N. Effect of Matrix Size on the Image Quality of Ultra-high-resolution CT of the Lung: Comparison of 512 × 512, 1024 × 1024, and 2048 × 2048. Acad. Radiol. 2018, 25, 869–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruaro, B.; Baratella, E.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Busca, A.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. High-Resolution Computed Tomography: Lights and Shadows in Improving Care for SSc-ILD Patients. Diagnostics 2021, 11, 1960. [Google Scholar] [CrossRef]
- Bussone, G.; Mouthon, L. Interstitial lung disease in systemic sclerosis. Autoimmun. Rev. 2011, 10, 248–255. [Google Scholar] [CrossRef]
- Goldin, J.G.; Lynch, D.A.; Strollo, D.C.; Suh, R.D.; Schraufnagel, D.E.; Clements, P.J.; Elashoff, R.M.; Furst, D.E.; Vasunilashorn, S.; McNitt-Gray, M.F.; et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008, 134, 358–367. [Google Scholar] [CrossRef]
- Wells, A.U.; Hansell, D.M.; Corrin, B.; Harrison, N.K.; Goldstraw, P.; Black, C.M.; Du Bois, R.M. High resolution computed tomography as a predictor of lung histology in systemic sclerosis. Thorax 1992, 47, 738–742. [Google Scholar] [CrossRef] [Green Version]
- Solomon, J.J.; Olson, A.L.; Fischer, A.; Bull, T.; Brown, K.K.; Raghu, G. Scleroderma lung disease. Eur. Respir. Rev. 2013, 22, 6–19. [Google Scholar] [CrossRef]
- Hartman, T.E.; Swensen, S.J.; Hansell, D.M.; Colby, T.V.; Myers, J.L.; Tazelaar, H.D.; Nicholson, A.G.; Wells, A.U.; Ryu, J.H.; Midthun, D.E.; et al. Nonspecific interstitial pneumonia: Variable appearance at high-resolution chest CT. Radiology 2000, 217, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Landini, N.; Sambataro, G.; Nardi, C.; Tofani, L.; Bruni, C.; Bellando-Randone, S.; Blagojevic, J.; Melchiorre, D.; Hughes, M.; et al. The role of chest CT in deciphering interstitial lung involvement: Systemic sclerosis versus COVID-19. Rheumatology 2022, 61, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Jimenez, S.A.; Wechsler, R. Significance of Ground-glass Opacity on HRCT in Long-term Follow-up of Patients With Systemic Sclerosis. J. Thorac. Imaging 2007, 22, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.J.; Weigt, S.S.; Belperio, J.A.; Brown, M.S.; Shi, Y.; Lai, J.H.; Goldin, J.G. Prediction of idiopathic pulmonary fibrosis progression using early quantitative changes on CT imaging for a short term of clinical 18-24-month follow-ups. Eur. Radiol. 2020, 30, 726–734. [Google Scholar] [CrossRef]
- Kawashima, H.; Ichikawa, K.; Takata, T.; Nagata, H.; Hoshika, M.; Akagi, N. Technical Note: Performance comparison of ultra-high-resolution scan modes of two clinical computed tomography systems. Med. Phys. 2020, 47, 488–497. [Google Scholar] [CrossRef]
- Wells, A.U. High-resolution computed tomography and scleroderma lung disease. Rheumatology 2008, 47, v59–v61. [Google Scholar] [CrossRef] [Green Version]
- Balestro, E.; Cocconcelli, E.; Giraudo, C.; Polverosi, R.; Biondini, D.; Lacedonia, D.; Bazzan, E.; Mazzai, L.; Rizzon, G.; Lococo, S.; et al. High-Resolution CT Change over Time in Patients with Idiopathic Pulmonary Fibrosis on Antifibrotic Treatment. J. Clin. Med. 2019, 8, 1469. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Sakamoto, S.; Kurosaki, A.; Kurihara, Y.; Satoh, K.; Usui, Y.; Nanki, T.; Arimura, Y.; Makino, H.; Okada, Y.; et al. Chest High-Resolution CT Findings of Microscopic Polyangiitis: A Japanese First Nationwide Prospective Cohort Study. Am. J. Roentgenol. 2019, 213, 104–114. [Google Scholar] [CrossRef]
- Tanaka, R.; Yoshioka, K.; Takagi, H.; Schuijf, J.D.; Arakita, K. Novel developments in non-invasive imaging of peripheral arterial disease with CT: Experience with state-of-the-art, ultra-high-resolution CT and subtraction imaging. Clin. Radiol. 2019, 74, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Kakinuma, R.; Moriyama, N.; Muramatsu, Y.; Gomi, S.; Suzuki, M.; Nagasawa, H.; Kusumoto, M.; Aso, T.; Muramatsu, Y.; Tsuchida, T.; et al. Ultra-High-Resolution Computed Tomography of the Lung: Image Quality of a Prototype Scanner. PLoS ONE 2015, 10, e0137165. [Google Scholar] [CrossRef]
- Kwan, A.C.; Pourmorteza, A.; Stutman, D.; Bluemke, D.A.; Lima, J.A.C. Next-Generation Hardware Advances in CT: Cardiac Applications. Radiology 2021, 298, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Euler, A.; Martini, K.; Baessler, B.; Eberhard, M.; Schoeck, F.; Alkadhi, H.; Frauenfelder, T. 1024-pixel image matrix for chest CT—Impact on image quality of bronchial structures in phantoms and patients. PLoS ONE 2020, 15, e0234644. [Google Scholar] [CrossRef] [PubMed]
- Minier, T.; Guiducci, S.; Bellando-Randone, S.; Bruni, C.; Lepri, G.; Czirják, L.; Distler, O.; Walker, U.A.; Fransen, J.; Allanore, Y.; et al. Preliminary analysis of the very early diagnosis of systemic sclerosis (VEDOSS) EUSTAR multicentre study: Evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 2087–2093. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- R Foundation for Statistical Computing. RStudio: Integrated Development for R; Version 1.4.1103; R Foundation for Statistical Computing: Boston, MA, USA, 2016. [Google Scholar]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files R Package Version 1.3.1. 2019. Available online: https://cran.r-project.org/package=readxl (accessed on 6 July 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Verlag, S., Ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; Borchers, H.W. DescTools: Tools for Descriptive Statistics; R Package Version 0.99.39; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Torsten Hothorn, F.B.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Gamer, M.; Lemon, J.; Singh, I. irr: Various Coefficients of Interrater Reliability and Agreement. R package version 0.84.1. 2019. Available online: https://cran.r-project.org/package=irr (accessed on 6 July 2022).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, D.J.; Koo, C.W.; Bartholmai, B.J.; Rajendran, K.; Weaver, J.M.; Halaweish, A.F.; Leng, S.; McCollough, C.H.; Fletcher, J.G. High-Resolution Chest Computed Tomography Imaging of the Lungs: Impact of 1024 Matrix Reconstruction and Photon-Counting Detector Computed Tomography. Investig. Radiol. 2019, 54, 129–137. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Chung, M.J.; Chong, S.; Sung, Y.M.; Lee, K.S. 1024 Matrix Image Reconstruction: Usefulness in High Resolution chest CT. J. Korean Radiol. Soc. 2006, 55, 565. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushong, S.C. Radiologic Science for Technologists: Physics, Biology, and Protection; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
| Comparison of Kernels | Classification |
|---|---|
| Sharpness | 1 = blurred, 5 = sharp |
| Noise | 1 = noisy, 5 = clear |
| Detection of pathologies | 1 = very bad, 5 = very good |
| Depiction of bronchiole | 1 = very bad, 5 = very good |
| Overall image impression | 1 = very bad, 5 = very good |
| Comparison of matrices | Classification |
| Sharpness | 1 = blurred, 5 = sharp |
| Noise | 1 = noisy, 5 = clear |
| Extent of reticulations/bronchiectasis/fibrosis | 1 = no such pathologies, 5 = moderate, up to much of such pathologies |
| Extent of ground-glass opacities (GGO) | 1 = no GGO, 5 = moderate up to much GGO |
| Depiction of bronchiole | 1 = very bad, 5 =very good |
| Overall image impression | 1 = very bad, 5 = very good |
| Demography | |
|---|---|
| Number of patients | 50; female: 43 (86%) |
| Age (in years; mean ± SD, range) | 54 ± 14, 23–85 |
| MRSS (mean ± SD, range) | 2.88 ± 4.72, 0–23 |
| Lung function | (mean ± SD) |
| TLC | 94.87 ± 21.89 |
| DLCO | 73.15 ± 19.55 |
| FEV1/FVC | 101.93 ± 9.22 |
| Pulmonary diagnoses | n (%) |
| St. a. HPT | 1 (2) |
| Latent TB infection | 2 (4) |
| Chronic bronchitis | 1 (2) |
| COPD II | 1 (2) |
| Smoking status | n (%) |
| Non-Smoker | 39 (78) |
| Ex-Smoker | 4 (8) |
| Smoker | 7 (14) |
| Bl57 | Bl64 | Br49 | Br54 | Br59 | Br63 | Bv49 | Bv58 | ||
|---|---|---|---|---|---|---|---|---|---|
| Sharpness | mean | 4.08 | 3.67 | 3.52 | 3.63 | 4.00 | 3.62 | 3.25 | 3.60 |
| sd | 0.87 | 1.40 | 1.03 | 1.17 | 0.96 | 1.09 | 0.86 | 1.05 | |
| n | 60 | 24 | 60 | 56 | 60 | 60 | 60 | 60 | |
| Noise | mean | 3.18 | 2.67 | 3.52 | 3.43 | 3.43 | 3.02 | 3.58 | 3.55 |
| sd | 1 | 1.13 | 1 | 1.09 | 0.95 | 1.03 | 0.98 | 0.96 | |
| n | 60 | 24 | 60 | 56 | 60 | 60 | 60 | 60 | |
| DoP | mean | 3.83 | 3.58 | 3.50 | 3.63 | 4.03 | 3.70 | 3.47 | 3.90 |
| sd | 0.83 | 1.06 | 0.89 | 0.89 | 0.96 | 0.91 | 0.70 | 0.88 | |
| n | 60 | 24 | 60 | 56 | 60 | 60 | 60 | 60 | |
| DoB | mean | 3.83 | 3.50 | 3.65 | 3.75 | 4.12 | 3.75 | 3.58 | 4.07 |
| sd | 0.98 | 1.10 | 0.71 | 1.07 | 0.96 | 1.00 | 0.81 | 0.86 | |
| n | 60 | 24 | 60 | 56 | 60 | 60 | 60 | 60 | |
| OII | mean | 3.67 | 3.42 | 3.53 | 3.55 | 3.90 | 3.50 | 3.38 | 3.82 |
| sd | 0.82 | 1.18 | 0.83 | 1.03 | 0.84 | 0.89 | 0.74 | 0.81 | |
| n | 60 | 24 | 60 | 56 | 60 | 60 | 60 | 60 | |
| Total | mean | 18.60 | 16.83 | 17.72 | 17.98 | 19.48 | 17.58 | 17.27 | 18.93 |
| sd | 3.77 | 5.53 | 3.77 | 4.64 | 3.99 | 4.20 | 3.23 | 3.91 | |
| n | 60 | 24 | 60 | 56 | 60 | 60 | 60 | 60 |
| 512 | 1024 | p (Wilcox.) | |||
|---|---|---|---|---|---|
| Mean | Sd | Mean | Sd | ||
| Sharpness | 3.64 | 0.98 | 4.18 | 0.76 | <0.01 |
| Noise | 3.81 | 0.70 | 3.72 | 0.86 | 0.09 |
| Depiction of bronchiole | 3.53 | 0.74 | 3.72 | 0.83 | <0.01 |
| Overall image impression | 3.70 | 0.76 | 3.89 | 0.77 | <0.01 |
| Extent RBF | 2.99 | 1.38 | 2.94 | 1.40 | 0.49 |
| Extent GGO | 3.05 | 1.27 | 2.91 | 1.31 | 0.04 |
| Total | 24.08 | 4.09 | 24.71 | 4.46 | <0.01 |
| Kernel | Matrix | ||
|---|---|---|---|
| Criterium | ICC | Criterium | ICC |
| Sharpness | 0.05 | Sharpness | 0.36 |
| Noise | 0.04 | Noise | 0.32 |
| DoB | 0.08 | DoB | 0.23 |
| DoP | 0.05 | R/B/F | 0.89 |
| GGO | 0.84 | ||
| OII | 0.02 | OII | 0.29 |
| Total | 0.05 | Total | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmer, B.D.; Blüthgen, C.; Bässler, B.; Martini, K.; Huber, F.A.; Ruby, L.; Schönenberger, A.; Frauenfelder, T. Influence of CT Image Matrix Size and Kernel Type on the Assessment of HRCT in Patients with SSC-ILD. Diagnostics 2022, 12, 1662. https://doi.org/10.3390/diagnostics12071662
Balmer BD, Blüthgen C, Bässler B, Martini K, Huber FA, Ruby L, Schönenberger A, Frauenfelder T. Influence of CT Image Matrix Size and Kernel Type on the Assessment of HRCT in Patients with SSC-ILD. Diagnostics. 2022; 12(7):1662. https://doi.org/10.3390/diagnostics12071662
Chicago/Turabian StyleBalmer, Bettina D., Christian Blüthgen, Bettina Bässler, Katharina Martini, Florian A. Huber, Lisa Ruby, Amadéa Schönenberger, and Thomas Frauenfelder. 2022. "Influence of CT Image Matrix Size and Kernel Type on the Assessment of HRCT in Patients with SSC-ILD" Diagnostics 12, no. 7: 1662. https://doi.org/10.3390/diagnostics12071662
APA StyleBalmer, B. D., Blüthgen, C., Bässler, B., Martini, K., Huber, F. A., Ruby, L., Schönenberger, A., & Frauenfelder, T. (2022). Influence of CT Image Matrix Size and Kernel Type on the Assessment of HRCT in Patients with SSC-ILD. Diagnostics, 12(7), 1662. https://doi.org/10.3390/diagnostics12071662






