Watch the Mime Carefully! A Refractory Interstitial Lung Disease
Abstract
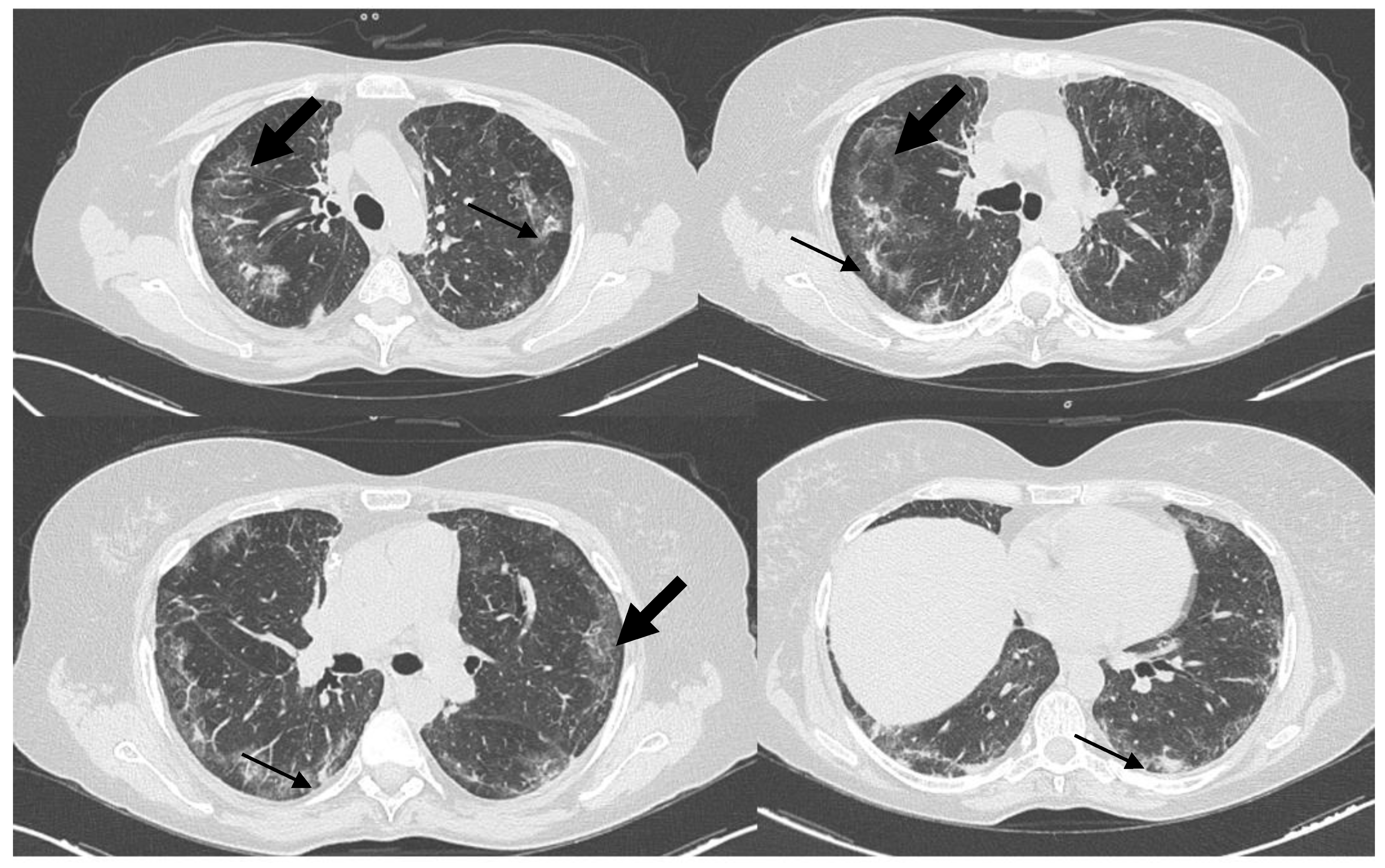
:
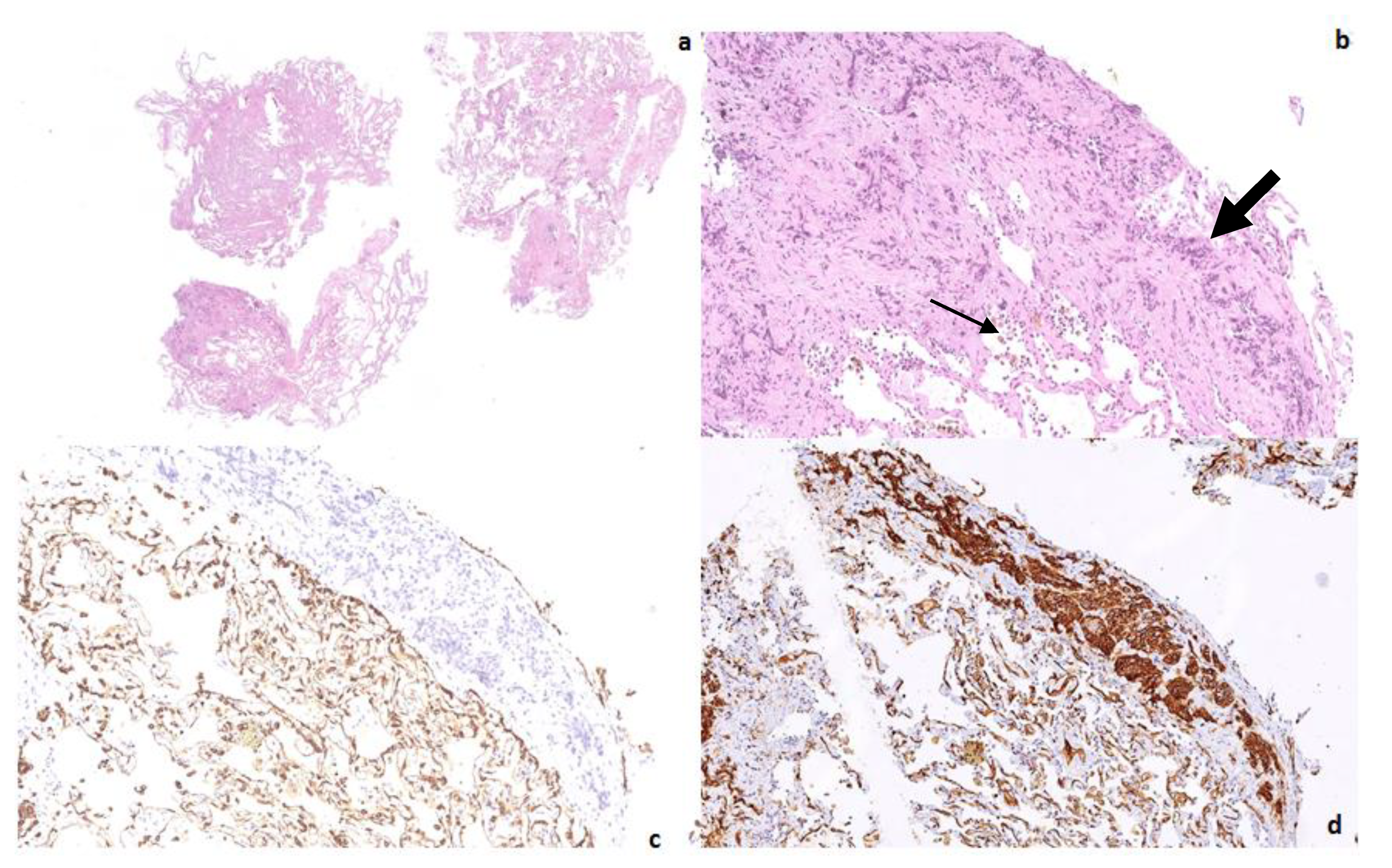
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, J.; Zhang, J. Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: A report of four cases and review of the literature. Oncol. Lett. 2014, 8, 2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, K.; Massad, M.; Pollak, C.; Rubin, C.; Yeh, J.; Wang, J.; Edelman, G.; Yeh, J.; Prasad, S.; Weinberg, G. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: Insights from an internet registry in the study of a rare cancer. Chest 2011, 140, 1312–1318. [Google Scholar] [CrossRef] [Green Version]
- Dail, D.H.; Liebow, A.A.; Gmelich, J.T.; Friedman, P.J.; Miyai, K.; Myer, W.; Patterson, S.D.; Hammar, S.P. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer 1983, 51, 452–464. [Google Scholar] [CrossRef]
- Mizuno, Y. Pulmonary epithelioid hemangioendothelioma. Gen. Thorac. Cardiovasc. Surg. 2011, 59, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Luburich, P.; Ayuso, M.C.; Picado, C.; Serra-Batllés, J.; Ramirez, J.F.; Solé, M. CT of pulmonary epithelioid hemangioendothelioma. J. Comp. Assist. Tomogr. 1994, 18, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.Y.; Jin, G.Y.; Lee, Y.C.; Lee, H.B.; Kang, M.J.; Choi, H.Y.; Chung, M.J. Pulmonary epithelioid hemangioendothelioma: A tumor presented as a single cavitary mass. J. Korean Med. Sci. 2003, 18, 599–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, R.D.; Sousa, M.; Trinidad, C.; Pinto, E.; Badiola, I.A. New Insights about Pulmonary Epithelioid Hemangioendothelioma: Review of the Literature and Two Case Reports. Case Rep. Radiol. 2017, 2017, 5972940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardaro, A.; Bardoscia, L.; Petruzzelli, M.F.; Portaluri, M. Epithelioid hemangioendothelioma: An overview and update on a rare vascular tumor. Oncol. Rev. 2014, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronin, P.; Arenberg, D. Pulmonary Epithelioid Hemangioendothelioma: An Unusual Case and a Review of the Literature. Chest 2004, 125, 789–793. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziano, P.; Fuso, P.; Carbonelli, C. Watch the Mime Carefully! A Refractory Interstitial Lung Disease. Diagnostics 2022, 12, 1743. https://doi.org/10.3390/diagnostics12071743
Graziano P, Fuso P, Carbonelli C. Watch the Mime Carefully! A Refractory Interstitial Lung Disease. Diagnostics. 2022; 12(7):1743. https://doi.org/10.3390/diagnostics12071743
Chicago/Turabian StyleGraziano, Paolo, Paolo Fuso, and Cristiano Carbonelli. 2022. "Watch the Mime Carefully! A Refractory Interstitial Lung Disease" Diagnostics 12, no. 7: 1743. https://doi.org/10.3390/diagnostics12071743
APA StyleGraziano, P., Fuso, P., & Carbonelli, C. (2022). Watch the Mime Carefully! A Refractory Interstitial Lung Disease. Diagnostics, 12(7), 1743. https://doi.org/10.3390/diagnostics12071743





