STANDARD M10 SARS-CoV-2 Assay for Rapid Detection of SARS-CoV-2: Comparison of Four Real-Time PCR Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. STANDARD M10 SARS-CoV-2 as a Rapid RT-qPCR Assay
2.3. Xpert Xpress SARS-CoV-2 as a Rapid RT-qPCR Assay
2.4. Nucleic Acid Extraction for Conventional RT-qPCR Assay
2.5. STANDARD M nCoV Real-time Detection Kit as a Conventional RT-qPCR Assay
2.6. Allplex SARS-CoV-2 Assay as a Conventional RT-qPCR Assay
2.7. Statistical Analysis
3. Results
3.1. Performance of STANDARD M10 and Xpert Xpress SARS-CoV-2 Assays
3.2. Discordant Results
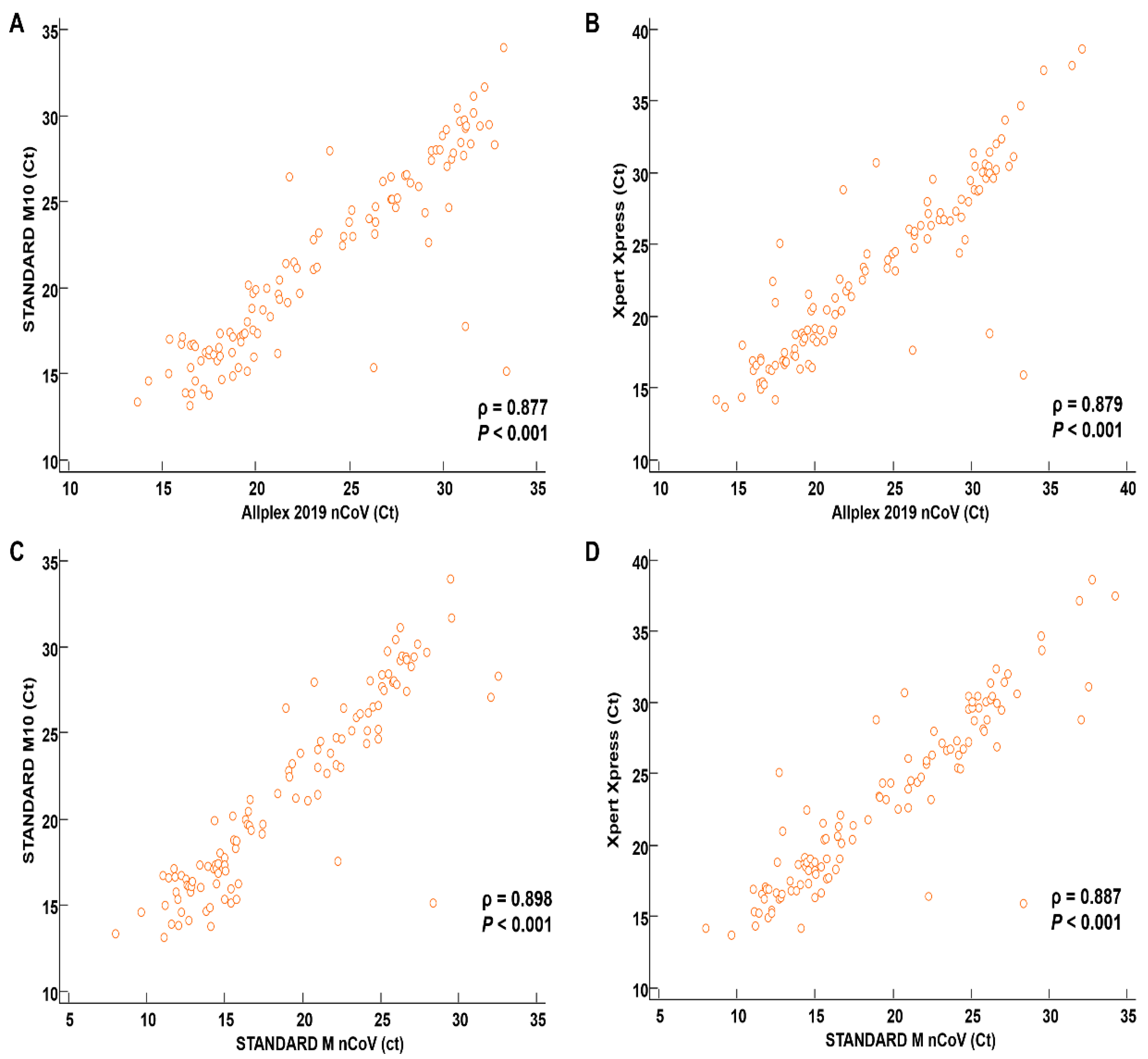
3.3. Correlation between SARS-CoV-2 Assays
3.4. Results for Variant Reference Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirotsu, Y.; Omata, M. SARS-CoV-2 B.1.1.7 lineage rapidly spreads and replaces R.1 lineage in Japan: Serial and stationary observation in a community. Infect. Genet. Evol. 2021, 95, 105088. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Omata, M. Discovery of a SARS-CoV-2 variant from the P.1 lineage harboring K417T/E484K/N501Y mutations in Kofu, Japan. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronnavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 April 2022).
- KCDC. KCDC Coronnavirus (COVID-19) Dashboard. Available online: http://ncov.mohw.go.kr/ (accessed on 1 April 2022).
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Catherine Muenker, M.; Moore, A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, A.; Perez, F.; Ngo Nsoga, M.T.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical evaluation of visby medical RT-PCR portable device for rapid detection of SARS-CoV-2. Diagnostics 2021, 11, 813. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.U. Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 4523–4531. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Alland, D.; Butler-Wu, S.M.; Pandey, U.; Perno, C.F.; Nava, A.; Carroll, K.C.; Mostafa, H.; Davies, E.; McEwan, A.; et al. Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test. J. Clin. Microbiol. 2020, 58, 709–720. [Google Scholar] [CrossRef]
- WHO. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 17 May 2022).
- European Centre for Disease Prevention and Control. Rapid Increase of a SARS-CoV-2 Variant with Multiple Spike Protein Mutations Observed in the United Kingdom. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf (accessed on 17 May 2022).
- New and Emerging Respiratory Virus Threats Advisory Group. NERVTAG Meeting on SARS-CoV-2 Variant under Investigation VUI-202012/01. Available online: https://app.box.com/s/3lkcbxepqixkg4mv640dpvvg978ixjtf/file/756963730457 (accessed on 17 May 2022).
- New and Emerging Respiratory Virus Threats Advisory Group. NERVTAG/SPI-M Extraordinary Meeting on SARS-CoV-2 Variant of Concern 202012/01 (Variant B.1.1.7). Available online: https://app.box.com/s/3lkcbxepqixkg4mv640dpvvg978ixjtf/file/756964987830 (accessed on 13 May 2022).
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021, 27, 620–621. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef]
- Islam, F.; Dhawan, M.; Nafady, M.H.; Emran, T.B.; Mitra, S.; Choudhary, O.P.; Akter, A. Understanding the omicron variant (B.1.1.529) of SARS-CoV-2: Mutational impacts, concerns, and the possible solutions. Ann. Med. Surg. 2022, 78, 103737. [Google Scholar] [CrossRef]
- CDC. COVID Data Tracker: Variant Proportions. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 17 May 2022).
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; El-Shall, N.A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ. Res. 2022, 209, 112816. [Google Scholar] [CrossRef]
- Wolters, F.; van de Bovenkamp, J.; van den Bosch, B.; van den Brink, S.; Broeders, M.; Chung, N.H.; Favie, B.; Goderski, G.; Kuijpers, J.; Overdevest, I.; et al. Multi-center evaluation of cepheid xpert(R) xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J. Clin. Virol. 2020, 128, 104426. [Google Scholar] [CrossRef]
- Farfour, E.; Lesprit, P.; Visseaux, B.; Pascreau, T.; Jolly, E.; Houhou, N.; Mazaux, L.; Asso-Bonnet, M.; Vasse, M.; on behalf of the SARS-CoV-2 Foch Hospital study group. The Allplex 2019-nCoV (Seegene) assay: Which performances are for SARS-CoV-2 infection diagnosis? Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1997–2000. [Google Scholar] [CrossRef]
- Freire-Paspuel, B.; Garcia-Bereguiain, M.A. Analytical and clinical evaluation of “AccuPower SARS-CoV-2 Multiplex RT-PCR kit (Bioneer, South Korea)” and “Allplex 2019-nCoV Assay (Seegene, South Korea)” for SARS-CoV-2 RT-PCR diagnosis: Korean CDC EUA as a quality control proxy for developing countries. Front. Cell. Infect. Microbiol. 2021, 11, 630552. [Google Scholar] [CrossRef]
- Burki, T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir. Med. 2022, 10, e17. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Natori, Y.; Nagakubo, Y.; Hosaka, K.; Sueki, H.; Amemiya, K.; Hayakawa, M.; Mochizuki, H.; et al. Direct comparison of Xpert Xpress, FilmArray Respiratory Panel, Lumipulse antigen test, and RT-qPCR in 165 nasopharyngeal swabs. BMC Infect. Dis. 2022, 22, 221. [Google Scholar] [CrossRef]
- Hong, K.H.; Lee, S.W.; Kim, T.S.; Huh, H.J.; Lee, J.; Kim, S.Y.; Park, J.S.; Kim, G.J.; Sung, H.; Roh, K.H.; et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020, 40, 351–360. [Google Scholar] [CrossRef]
- Zhen, W.; Smith, E.; Manji, R.; Schron, D.; Berry, G.J. Clinical evaluation of three sample-to-answer platforms for detection of SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00783-20. [Google Scholar] [CrossRef]
- Basu, A.; Zinger, T.; Inglima, K.; Woo, K.M.; Atie, O.; Yurasits, L.; See, B.; Aguero-Rosenfeld, M.E. Performance of Abbott ID Now COVID-19 Rapid Nucleic Acid Amplification Test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York City academic institution. J. Clin. Microbiol. 2020, 58, e01136-20. [Google Scholar] [CrossRef]
- Moran, A.; Beavis, K.G.; Matushek, S.M.; Ciaglia, C.; Francois, N.; Tesic, V.; Love, N. Detection of SARS-CoV-2 by use of the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. J. Clin. Microbiol. 2020, 58, e00772-20. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Scherberkova, I.; Whittier, S.; Green, D.A. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the rapid detection of SARS-CoV-2. J. Clin. Virol. 2020, 128, 104428. [Google Scholar] [CrossRef] [PubMed]
- Procop, G.W.; Brock, J.E.; Reineks, E.Z.; Shrestha, N.K.; Demkowicz, R.; Cook, E.; Ababneh, E.; Harrington, S.M. A comparison of five SARS-CoV-2 molecular assays with clinical correlations. Am. J. Clin. Pathol. 2021, 155, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; George, K.S. Evaluation of the COVID19 ID NOW EUA assay. J. Clin. Virol. 2020, 128, 104429. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Roh, K.H.; Hong, K.H.; Seong, M.W.; Ryoo, N.; Kim, H.S.; Lee, J.; Kim, S.Y.; Ryu, S.W.; Kim, M.N.; et al. COVID-19 molecular testing in Korea: Practical essentials and answers from experts based on experiences of Emergency Use Authorization assays. Ann. Lab. Med. 2020, 40, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lesbon, J.C.C.; Poleti, M.D.; de Mattos Oliveira, E.C.; Patane, J.S.L.; Clemente, L.G.; Viala, V.L.; Ribeiro, G.; Giovanetti, M.; de Alcantara, L.C.J.; de Lima, L.P.O.; et al. Nucleocapsid (N) gene mutations of SARS-CoV-2 can affect real-time RT-PCR diagnostic and impact false-negative results. Viruses 2021, 13, 2474. [Google Scholar] [CrossRef] [PubMed]
- Banada, P.; Green, R.; Banik, S.; Chopoorian, A.; Streck, D.; Jones, R.; Chakravorty, S.; Alland, D. A simple reverse transcriptase PCR melting-temperature assay to rapidly screen for widely circulating SARS-CoV-2 variants. J. Clin. Microbiol. 2021, 59, e0084521. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; In, J.W.; Lee, J.; Kim, S.Y.; Lee, K.A.; Kim, S.; An, Y.; Lee, D.; Sung, H.; Kim, J.S.; et al. Prevalence of a single-nucleotide variant of SARS-CoV-2 in Korea and its impact on the diagnostic sensitivity of the Xpert Xpress SARS-CoV-2 assay. Ann. Lab. Med. 2022, 42, 96–99. [Google Scholar] [CrossRef]
- Rong, K.; Cabrera, A.; Delport, J.; Schofield, S.; AlMutawa, F. Validation of the Cepheid Xpert® Xpress SARS-CoV-2 using upper and lower respiratory tract specimens. Eur. J. Microbiol. Immunol. 2022, 12, 18–21. [Google Scholar] [CrossRef]
| Performance 1 | Allplex 2019-nCoV as a Reference | STANDARD M nCoV as a Reference | ||
|---|---|---|---|---|
| STANDARD M10 SARS-CoV-2 | Xpert Xpress SARS-CoV-2 | STANDARD M10 SARS-CoV-2 | Xpert Xpress SARS-CoV-2 | |
| P/P (n) | 111 | 114 | 111 | 114 |
| P/N (n) | 3 | 0 | 3 | 0 |
| N/P (n) | 0 | 0 | 0 | 0 |
| N/N (n) | 101 | 101 | 101 | 101 |
| PPA (%) 1 | 97.4 (92.5–99.5) | 100.0 (96.8–100.0) | 97.4 (92.5–99.5) | 100.0 (96.8–100.0) |
| NPA (%) 1 | 100.0 (96.4–100.0) | 100.0 (96.4–100.0) | 100.0 (96.4–100.0) | 100.0 (96.4–100.0) |
| Total agreement (%)1 | 98.6 (96.0–99.7) | 100.0 (98.3–100.0) | 98.6 (96.0–99.7) | 100.0 (98.3–100.0) |
| Kappa value1 | 0.97 (0.94–1.00) | 1.00 (1.00–1.00) | 0.97 (0.94–1.00) | 1.00 (1.00–1.00) |
| Ct Value | Allplex 2019-nCoV as a Reference | STANDARD M nCOV RT-qPCR as a Reference | ||
|---|---|---|---|---|
| STANDARD M10 SARS-CoV-2 | Xpert Xpress SARS-CoV-2 | STANDARD M10 SARS-CoV-2 | Xpert Xpress SARS-CoV-2 | |
| <20 | 100.0% (n = 42) | 100.0% (n = 42) | 100.0% (n = 62) | 100.0% (n = 62) |
| 20–25 | 100.0% (n = 25) | 100.0% (n = 25) | 100.0% (n = 28) | 100.0% (n = 28) |
| 26–30 | 100.0% (n = 23) | 100.0% (n = 22) | 100.0% (n = 19) | 100.0% (n = 19) |
| >30 | 87.5% (n = 24) | 100.0% (n = 24) | 40.0% (n = 5) | 100.0% (n = 5) |
| Each Assay (Target Gene) | Ct Values |
|---|---|
| Allplex 2019-nCoV (E) | 36.5 (35.0–37.1) |
| Allplex 2019-nCoV (RdRP) | 37.0 (35.0–37.2) |
| Allplex 2019-nCoV (N) | 36.4 (34.7–36.8) |
| STANDARD M nCoV (E) | 32.8 (32.1–34.1) |
| STANDARD M nCoV (ORF1ab) | 32.2 (31.0–33.8) |
| Xpert Xpress (E) | 37.4 (37.2–38.4) |
| Xpert Xpress (N2) | 38.4 (36.8–39.0) |
| Target Gene in Each Assay | B.1.1.7 (Alpha) | B.1.351 (Beta) | P.1 (Gamma) | Wild-Type |
|---|---|---|---|---|
| Allplex 2019-nCoV (E) | 31.22 | 30.47 | 31.95 | 32.62 |
| Allplex 2019-nCoV (RdRP) | 32.79 | 30.39 | 31.77 | 31.69 |
| Allplex 2019-nCoV (N) | 32.13 | 31.83 | 33.11 | 33.6 |
| STANDARD M nCoV (E) | 28.19 | 28.19 | 29.48 | 29.26 |
| STANDARD M nCoV (ORF1ab) | 27.92 | 28.03 | 29.12 | 29.25 |
| STANDARD M10 (E) | 27.89 | 28.31 | 28.49 | 28.94 |
| STANDARD M10 (ORF1ab) | 27.6 | 27.99 | 28.21 | 28.08 |
| Xpert Xpress SARS-CoV-2 (E) | 30.1 | 30 | 30.7 | 31.1 |
| Xpert Xpress SARS-CoV-2 (N2) | 33.2 | 32.4 | 34.1 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Lee, N.; Lee, S.K.; Cho, E.-J.; Hyun, J.; Park, M.-J.; Song, W.; Kim, H.S. STANDARD M10 SARS-CoV-2 Assay for Rapid Detection of SARS-CoV-2: Comparison of Four Real-Time PCR Assays. Diagnostics 2022, 12, 1998. https://doi.org/10.3390/diagnostics12081998
Jeong S, Lee N, Lee SK, Cho E-J, Hyun J, Park M-J, Song W, Kim HS. STANDARD M10 SARS-CoV-2 Assay for Rapid Detection of SARS-CoV-2: Comparison of Four Real-Time PCR Assays. Diagnostics. 2022; 12(8):1998. https://doi.org/10.3390/diagnostics12081998
Chicago/Turabian StyleJeong, Seri, Nuri Lee, Su Kyung Lee, Eun-Jung Cho, Jungwon Hyun, Min-Jeong Park, Wonkeun Song, and Hyun Soo Kim. 2022. "STANDARD M10 SARS-CoV-2 Assay for Rapid Detection of SARS-CoV-2: Comparison of Four Real-Time PCR Assays" Diagnostics 12, no. 8: 1998. https://doi.org/10.3390/diagnostics12081998
APA StyleJeong, S., Lee, N., Lee, S. K., Cho, E.-J., Hyun, J., Park, M.-J., Song, W., & Kim, H. S. (2022). STANDARD M10 SARS-CoV-2 Assay for Rapid Detection of SARS-CoV-2: Comparison of Four Real-Time PCR Assays. Diagnostics, 12(8), 1998. https://doi.org/10.3390/diagnostics12081998






