Chronic Fatigue Syndrome in Patients with Deteriorated Iron Metabolism
Abstract
:1. Introduction
2. Methods
3. Assessment of Fatigue
4. Clinical Presentation of Iron Disturbances
4.1. Iron Deficiency
4.1.1. The Pathophysiology of Fatigue in Iron Deficiency
4.1.2. Gender Differences in Iron Deficiency Anemia
4.1.3. Iron Deficiency and Fatigue in Women of Reproductive Age
4.1.4. The Importance of Fatigue among Patients with Heavy Menstrual Bleeding (HMB)
4.1.5. Fatigue in Anemia of Chronic Diseases
4.1.6. Fatigue in Aplastic Anemia, Nocturnal Paroxysmal Hemoglobinuria, and Myelodysplastic Syndrome
4.1.7. Fatigue in Elderly Population
| Name of Clinical State | Brief Description of Presented Clinical States |
|---|---|
| Iron deficiency and fatigue in women of reproductive age | Women of childbearing age are particularly vulnerable to iron deficiency due to increased requirements during pregnancy and the loss of this nutrient during menstruation or childbirth. Other causes of iron deficiency may be an inadequate diet low in iron and high in substances that inhibit iron absorption from the gastrointestinal tract. Iron deficiency among women of reproductive age is a common phenomenon observed worldwide. In the USA, Japan, and Europe, the prevalence is 10–20% [85,86]. |
| Fatigue among patients with heavy menstrual bleeding | According to the NICE (National Institute for Health and Care Excellence’s) definition, it is excessive blood loss at the time of expected menstruation that disrupts the physical, emotional, social, and material elements of a woman’s quality of life, which may occur alone or in combination with other symptoms [65]. The prevalence of this disorder is estimated to be between 27.2% and 54% amongst young women [87,88,89]. |
| Anemia of chronic diseases | Anemia can have various origins; however, it is mostly caused by iron deficiency or chronic disease. Iron deficiency anemia is characterized by decreased hemoglobin synthesis, leading to the development of microcytic and hypochromic erythrocytes [90]. In contrast, anemia of chronic diseases is characterized by the normal iron content in the body with inadequate iron distribution, leading to the development of normocytic and normochromic erythrocytes [91]. Moreover, in the anemia of chronic diseases, the current inflammation results in decreased production and release of EPO, which is responsible for enhancing erythrocyte formation in the bone marrow [92].
|
| Hematological disorders |
|
| Elderly | WHO (World Health Organization) defines multimorbidity as the presence of two or more chronic diseases. It is estimated that this condition may affect up to 95% of people aged ≥65 years [98]. The risk factors for multimorbidity are unknown, but it is speculated that aging may be one of the significant factors. Frailty syndrome is another significant risk factor. It is a syndrome occurring in chronically ill patients that includes unintentional weight loss (≥5 kg per year), fatigue, muscle weakness, slowed gait, and low physical activity. Frailty syndrome is associated with chronic inflammation of unknown cause leading to fatigue, decreased muscle mass, and decreased activity. |
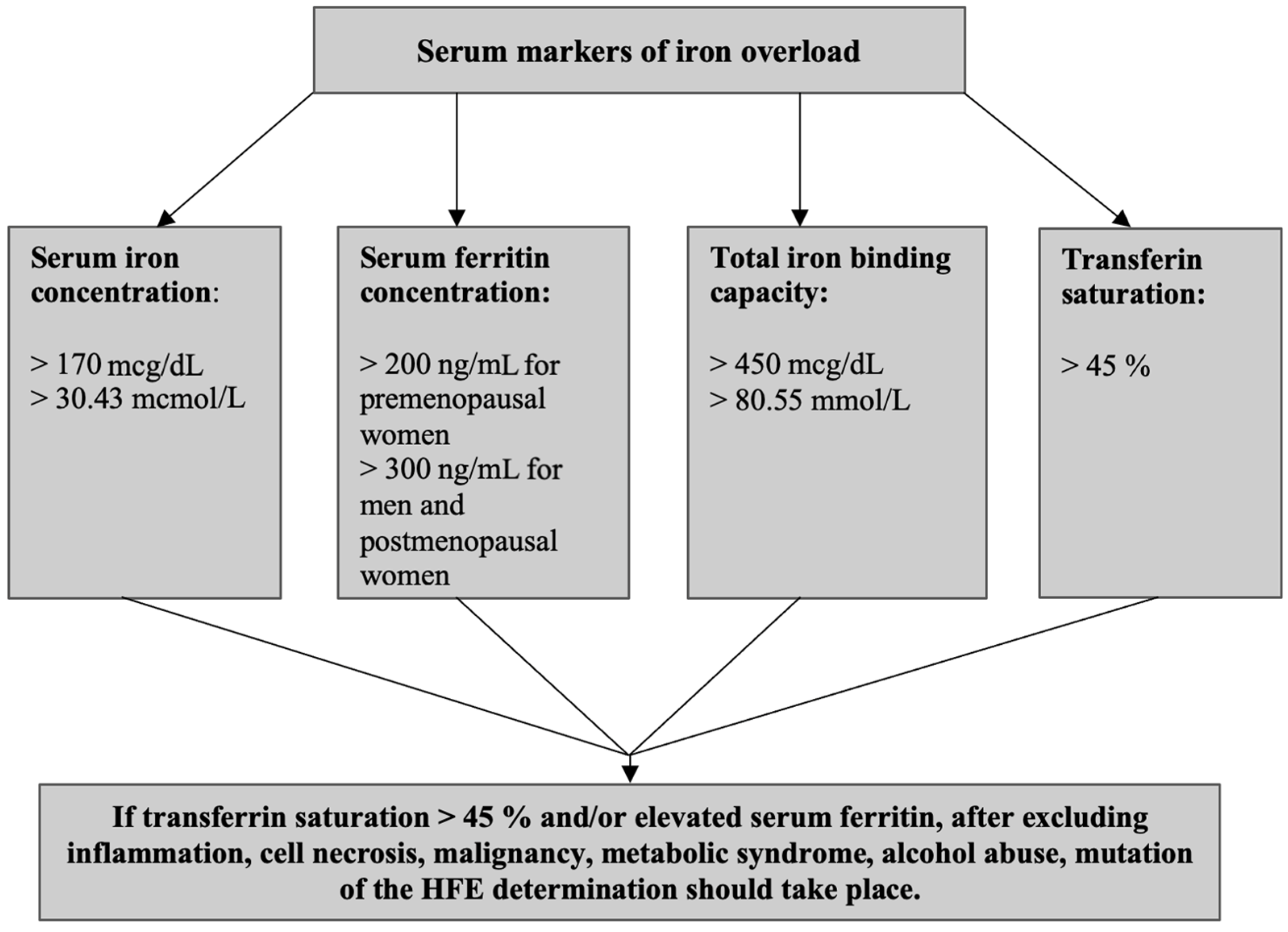
4.2. Iron Overload Disorders
4.2.1. The Pathophysiology of Fatigue in Iron Overload
4.2.2. The Clinical Implications of Iron Overload
4.2.3. Fatigue in Hereditary Hemochromatosis
4.2.4. Fatigue Related to Frequent Transfusions and Ineffective Erythropoiesis
| Name of Disease | Brief Description of the Disease |
|---|---|
| Hereditary hemochromatosis | It is a genetic disease in 80% based on HFE-gene mutation, leading to increased accumulation of iron in body tissues, resulting in the generation of oxidative stress and damage to many organs. Cirrhosis, diabetes, and dark skin color were the main symptoms of HH before the era of HFE gen revealing [100]. An introduction of genetic tests in patients with abnormal iron management parameters to routine clinical practice makes it possible to diagnose HH early before the patients demonstrate symptoms of advanced diseases. Instead of the above-mentioned classic triad, one of the early symptoms noticed by patients with HH is the feeling of severe, chronic fatigue, which very often significantly decreases the quality of life. The treatment of choice for hereditary hemochromatosis are venesections. Treatment performed at the appropriate frequency significantly reduces fatigue, ferritin, and iron [109]. Venesections are more effective in reducing iron levels than chelating drugs [100]. Additionally, the applied treatment significantly improves the function of the heart muscle [109,114]. |
| Beta-thalassemia | It is one of the genetically determined (mutation of genes located on chromosome 11) hemolytic anemia resulting from a disturbance in the synthesis of hemoglobin beta chains [115]. There are three groups of β-thalassemia: minor, intermedia, and major. Beta thalassemia major is the most severe form of beta-thalassemia. Severe symptoms of hemolytic anemia may appear already after six months of age and require treatment by regular red blood cell transfusions and sometimes chelation therapy [115]. Beta-thalassemia intermedia is a milder form of the disease. |
| Sideroblastic anemia | It is a group of congenital and acquired diseases characterized by the presence of peripheral microcytic anemia in the blood and an image of ring sideroblasts in the bone marrow (iron overloaded mitochondria surround erythroblast nuclei) [116]. |
| Congenital dyserythropoietic anemias (CDA) | It is a group of rarely diagnosed anemia of unknown etiology, characterized by increased ineffective erythropoiesis, multinuclear erythroblast nuclei in the marrow, and secondary iron accumulation in tissues. Abnormal erythroblasts are destroyed in the bone marrow, resulting in elevated serum bilirubin and LDH levels [117]. The classification considers three types of CDA. In types I and III, CDA macrocytes are present, while type II CDA is characterized by normocytosis. Anemia is usually mild to moderate and manifests itself at different times in life. |
5. Discussion
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naess, H.; Nyland, H.I.; Thomassen, L.; Aarseth, J.; Myhr, K.M. Fatigue at long-term follow-up in young adults with cerebral infarction. Cereb. Dis. 2005, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Tiesinga, L.J.; Dassen, T.W.; Halfens, R.J. Fatigue: A summary of the definitions, dimensions, and indicators. Nurs. Diagn. 1996, 7, 51–62. [Google Scholar] [CrossRef]
- Jonefjäll, B.; Simrén, M.; Lasson, A.; Öhman, L.; Strid, H. Psychological distress, iron deficiency, active disease and female gender are independent risk factors for fatigue in patients with ulcerative colitis. United Eur. Gastroenterol. J. 2018, 6, 148–158. [Google Scholar] [CrossRef]
- Hillson, R. Fatigue and Tiredness in Diabetes. Pract. Diabetes 2020, 37, 45–46. [Google Scholar] [CrossRef]
- Franssen, P.M.; Bültmann, U.; Kant, I.; van Amelsvoort, L.G. The association between chronic diseases and fatigue in the working population. J. Psychosom. Res. 2003, 54, 339–344. [Google Scholar] [CrossRef]
- Ream, E.; Richardson, A. Fatigue: A concept analysis. Int. J. Nurs. Stud. 1996, 33, 519–529. [Google Scholar] [CrossRef]
- Swain, M. Fatigue in chronic disease. Clin. Sci. 2000, 99, 1–8. [Google Scholar] [CrossRef]
- Efficace, F.; Gaidano, G.; Breccia, M.; Criscuolo, M.; Cottone, F.; Caocci, G.; Bowen, D.; Lübbert, M.; Angelucci, E.; Stauder, R.; et al. Prevalence, severity and correlates of fatigue in newly diagnosed patients with myelodysplastic syndromes. Br. J. Haematol. 2015, 168, 361–370. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Moser, D.K.; Westlake, C.; Pike, N.; Ter-Galstanyan, A.; Dracup, K. Correlates of fatigue in patients with heart failure. Prog. Cardiovasc. Nurs. 2008, 23, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Johnsen, S.P.; Watt, T.; Harder, I.; Kirkevold, M.; Andersen, G. Dimensions of post-stroke fatigue: A two-year follow-up study. Cereb. Dis. 2008, 26, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Lower, E.E.; De Vries, J. Sarcoidosis-associated fatigue. Eur. Respir. J. 2012, 40, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ferentinos, P.; Kontaxakis, V.; Havaki-Kontaxaki, B.; Dikeos, D.; Lykouras, L. Psychometric evaluation of the Fatigue Severity Scale in patients with major depression. Qual. Life Res. 2011, 20, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rosti-Otajärvi, E.; Hämäläinen, P.; Wiksten, A.; Hakkarainen, T.; Ruutiainen, J. Validity and reliability of the Fatigue Severity Scale in Finnish multiple sclerosis patients. Brain Behav. 2017, 7, e00743. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Van der Steeg, A.F.; Roukema, J.A. Psychometric properties of the Fatigue Assessment Scale (FAS) in women with breast problems. Int. J. Clin. Health Psychol. 2010, 10, 125–139. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef]
- Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: A systematic review. Arthritis Rheum. 2007, 57, 1348–1357. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Elbers, R.G.; van Wegen, E.E.; Verhoef, J.; Kwakkel, G. Reliability and structural validity of the Multidimensional Fatigue Inventory (MFI) in patients with idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 532–536. [Google Scholar] [CrossRef]
- Da Costa, D.; Dritsa, M.; Bernatsky, S.; Pineau, C.; Ménard, H.A.; Dasgupta, K.; Keschani, A.; Rippen, N.; Clarke, A.E. Dimensions of fatigue in systemic lupus erythematosus: Relationship to disease status and behavioral and psychosocial factors. J. Rheumatol. 2006, 33, 1282–1288. [Google Scholar]
- Michielsen, H.J.; De Vries, J.; Drent, M.; Peros-Golubicic, T. Psychometric qualities of the Fatigue Assessment Scale in Croatian sarcoidosis patients. Sarcoidosis Vasc. Diffuse Lung Dis. 2005, 22, 133–138. [Google Scholar]
- Smith, O.R.; van den Broek, K.C.; Renkens, M.; Denollet, J. Comparison of fatigue levels in patients with stroke and patients with end-stage heart failure: Application of the fatigue assessment scale. J. Am. Geriatr. Soc. 2008, 56, 1915–1919. [Google Scholar] [CrossRef]
- Tseng, B.Y.; Billinger, S.A.; Gajewski, B.J.; Kluding, P.M. Exertion fatigue and chronic fatigue are two distinct constructs in people post-stroke. Stroke 2010, 41, 2908–2912. [Google Scholar] [CrossRef] [Green Version]
- Smith, O.R.; Michielsen, H.J.; Pelle, A.J.; Schiffer, A.A.; Winter, J.B.; Denollet, J. Symptoms of fatigue in chronic heart failure patients: Clinical and psychological predictors. Eur. J. Heart Fail. 2007, 9, 922–927. [Google Scholar] [CrossRef]
- Wang, X.S.; Hao, X.S.; Wang, Y.; Guo, H.; Jiang, Y.-Q.; Mendoza, T.R.; Cleeland, C.S. Validation study of the Chinese version of the Brief Fatigue Inventory (BFI-C). J. Pain Symptom Manag. 2004, 27, 322–332. [Google Scholar] [CrossRef]
- Lin, C.C.; Chang, A.P.; Chen, M.L.; Cleeland, C.S.; Mendoza, T.R.; Wang, X.S. Validation of the Taiwanese version of the Brief Fatigue Inventory. J. Pain Symptom Manag. 2006, 32, 52–59. [Google Scholar] [CrossRef]
- Catania, G.; Bell, C.; Ottonelli, S.; Marchetti, M.; Bryce, J.; Grossi, A.; Costantini, M. Cancer-related fatigue in Italian cancer patients: Validation of the Italian version of the Brief Fatigue Inventory (BFI). Support Care Cancer 2013, 21, 413–419. [Google Scholar] [CrossRef]
- Chandran, V.; Bhella, S.; Schentag, C.; Gladman, D.D. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann. Rheum. Dis. 2007, 66, 936–939. [Google Scholar] [CrossRef]
- Butt, Z.; Lai, J.S.; Rao, D.; Heinemann, A.W.; Bill, A.; Cella, D. Measurement of fatigue in cancer, stroke, and HIV using the Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-F) scale. J. Psychosom. Res. 2013, 74, 64–68. [Google Scholar] [CrossRef]
- Lai, J.S.; Beaumont, J.L.; Ogale, S.; Brunetta, P.; Cella, D. Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J. Rheumatol. 2011, 38, 672–679. [Google Scholar] [CrossRef]
- Loge, J.H.; Ekeberg, O.; Kaasa, S. Fatigue in the general Norwegian population: Normative data and associations. J. Psychosom. Res. 1998, 45, 53–65. [Google Scholar] [CrossRef]
- Cella, M.; Chalder, T. Measuring fatigue in clinical and community settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tench, C.; Bentley, D.; Vleck, V.; McCurdie, I.; White, P.; D’Cruz, D. Aerobic fitness, fatigue, and physical disability in systemic lupus erythematosus. J. Rheumatol. 2002, 29, 474–481. [Google Scholar] [PubMed]
- Lwin, C.T.; Bishay, M.; Platts, R.G.; Booth, D.A.; Bowman, S.J. The assessment of fatigue in primary Sjögren’s syndrome. Scand. J. Rheumatol. 2003, 32, 33–37. [Google Scholar] [CrossRef]
- Jakobsson, S.; Taft, C.; Östlund, U.; Ahlberg, K. Performance of the Swedish version of the Revised Piper Fatigue Scale. Eur. J. Oncol Nurs. 2013, 17, 808–813. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Cuesta-Vargas, A.I.; Fernández-De-Las-Peñas, C.; Piper, B.F.; Arroyo-Morales, M. The Piper Fatigue Scale-Revised: Translation and psychometric evaluation in Spanish-speaking breast cancer survivors. Qual. Life Res. 2014, 23, 271–276. [Google Scholar] [CrossRef]
- Varbobitis, I.; Kokkotis, G.; Gizis, M.; Perlepe, N.; Laoudi, E.; Bletsa, M.; Bekiari, D.; Koutsounas, I.; Kounadis, G.; Xourafas, V.; et al. The IBD-F Patient Self-Assessment Scale Accurately Depicts the Level of Fatigue and Predicts a Negative Effect on the Quality of Life of Patients with IBD in Clinical Remission. Inflamm. Bowel. Dis. 2021, 27, 826–835. [Google Scholar] [CrossRef]
- Norton, C.; Czuber-Dochan, W.; Bassett, P.; Berliner, S.; Bredin, F.; Darvell, M.; Forbes, A.; Gay, M.; Ream, E.; Terry, H. Assessing fatigue in inflammatory bowel disease: Comparison of three fatigue scales. Aliment Pharm. Ther. 2015, 42, 203–211. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Coates, A.; Mountjoy, M.; Burr, J. Incidence of Iron Deficiency and Iron Deficient Anemia in Elite Runners and Triathletes. Clin. J. Sport Med. 2017, 27, 493–498. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Neidlein, S.; Wirth, R.; Pourhassan, M. Iron deficiency, fatigue and muscle strength and function in older hospitalized patients. Eur. J. Clin. Nutr. 2021, 75, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Konomi, A. Iron deficiency without anaemia is a potential cause of fatigue: Meta-analyses of randomised controlled trials and cross-sectional studies. Br. J. Nutr. 2017, 117, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Gugger, A.; de Godoi Rezende Costa Molino, C.; Wieczorek, M.; Chocano-Bedoya, P.O.; Abderhalden, L.A.; Schaer, D.J.; Spahn, D.R.; Orav, E.J.; Vellas, B.; da Silva, J.A.P.; et al. Prevalence and incidence of iron deficiency in European community-dwelling older adults: An observational analysis of the DO-HEALTH trial. Aging Clin. Exp. Res. 2022. [Google Scholar] [CrossRef]
- Röhrig, G.; Becker, I.; Schulz, R.J.; Lenzen-Großimlinghaus, R.; Willschrei, P.; Gebauer, S.; Modreker, M.; Jäger, M.; Wirth, R. Association between hematologic parameters and functional impairment among geriatric inpatients: Data of a prospective cross-sectional multicenter study (“GeriPrävalenz2013”). Maturitas 2016, 90, 37–41. [Google Scholar] [CrossRef]
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef]
- De Falco, L.; Sanchez, M.; Silvestri, L.; Kannengiesser, C.; Muckenthaler, M.; Iolascon, A.; Gouya, L.; Camaschella, C.; Beaumont, C. Iron refractory iron deficiency anemia. Haematologica 2013, 98, 845–853. [Google Scholar] [CrossRef]
- Bouri, S.; Martin, J. Investigation of iron deficiency anaemia. Clin. Med. 2018, 18, 242–244. [Google Scholar] [CrossRef]
- Elstrott, B.; Khan, L.; Olson, S.; Raghunathan, V.; DeLoughery, T.; Shatzel, J.J. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur. J. Haematol. 2020, 104, 153–161. [Google Scholar] [CrossRef]
- Asif, N.; Ijaz, A.; Rafi, T.; Haroon, Z.H.; Bashir, S.; Ayyub, M. Diagnostic Accuracy of Serum Iron and Total Iron Binding Capacity (TIBC) in Iron Deficiency State. J. Coll. Phys. Surg. Pak. 2016, 26, 958–961. [Google Scholar]
- Dignass, A.; Farrag, K.; Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018, 2018, 9394060. [Google Scholar] [CrossRef]
- Low, C.L.; Bailie, G.R.; Eisele, G. Sensitivity and specificity of transferrin saturation and serum ferritin as markers of iron status after intravenous iron dextran in hemodialysis patients. Ren. Fail. 1997, 19, 781–788. [Google Scholar] [CrossRef]
- Choi, J.W. Sensitivity, specificity, and predictive value of serum soluble transferrin receptor at different stages of iron deficiency. Ann. Clin. Lab. Sci. 2005, 35, 435–439. [Google Scholar]
- Davies, K.J.; Maguire, J.J.; Brooks, G.A.; Dallman, P.R.; Packer, L. Muscle Mitochondrial Bioenergetics, Oxygen Supply, and Work Capacity during Dietary Iron Deficiency and Repletion. Am. J. Physiol. 1982, 242, E418–E427. [Google Scholar] [CrossRef]
- Augusto, V.; Padovani, C.; Eduardo, G.; Campos, R. Skeletal Muscle Fiber Types in C57BL6J Mice. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Rineau, E.; Gueguen, N.; Procaccio, V.; Geneviève, F.; Reynier, P.; Henrion, D.; Lasocki, S. Iron Deficiency without Anemia Decreases Physical Endurance and Mitochondrial Complex I Activity of Oxidative Skeletal Muscle in the Mouse. Nutrients 2021, 13, 1056. [Google Scholar] [CrossRef]
- Brownlie, T.; Utermohlen, V.; Hinton, P.S.; Giordano, C.; Haas, J.D. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am. J. Clin. Nutr. 2002, 75, 734–742. [Google Scholar] [CrossRef]
- Brutsaert, T.D.; Hernandez-Cordero, S.; Rivera, J.; Viola, T.; Hughes, G.; Haas, J.D. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am. J. Clin. Nutr. 2003, 77, 441–448. [Google Scholar] [CrossRef]
- Levi, M.; Simonetti, M.; Marconi, E.; Brignoli, O.; Cancian, M.; Masotti, A.; Pegoraro, V.; Heiman, F.; Cricelli, C.; Lapi, F. Gender differences in determinants of iron-deficiency anemia: A population-based study conducted in four European countries. Ann. Hematol. 2019, 98, 1573–1582. [Google Scholar] [CrossRef]
- Greig, A.J.; Patterson, A.J.; Collins, C.E.; Chalmers, K.A. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: A systematic review. J. Nutr. Sci. 2013, 2, e14. [Google Scholar] [CrossRef]
- Patterson, A.J.; Brown, W.J.; Roberts, D.C. Dietary and supplement treatment of iron deficiency results in improvements in general health and fatigue in Australian women of childbearing age. J. Am. Coll. Nutr. 2001, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Thomsen, L.L.; Langhoff-Roos, J. Intravenous iron isomaltoside treatment of women suffering from severe fatigue after postpartum hemorrhage. J. Matern. Fetal Neonatal. Med. 2019, 32, 2797–2804. [Google Scholar] [CrossRef]
- Napolitano, M.; Dolce, A.; Celenza, G.; Grandone, E.; Perilli, M.G.; Siragusa, S.; Carta, G.; Orecchioni, A.; Mariani, G. Iron-dependent erythropoiesis in women with excessive menstrual blood losses and women with normal menses. Ann. Hematol. 2014, 93, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bourgeois, T.; Klima, J.; Berlan, E.D.; Fisher, A.N.; O’Brien, S.H. Iron deficiency and fatigue in adolescent females with heavy menstrual bleeding. Haemophilia 2013, 19, 225–230. [Google Scholar] [CrossRef]
- Kocaoz, S.; Cirpan, R.; Degirmencioglu, A.Z. The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pak. J. Med. Sci. 2019, 35, 365–370. [Google Scholar] [CrossRef]
- Gregg, L.P.; Bossola, M.; Ostrosky-Frid, M.; Hedayati, S.S. Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar] [CrossRef]
- Auerbach, M.; Henry, D.; Derman, R.J.; Achebe, M.M.; Thomsen, L.L.; Glaspy, J. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am. J. Hematol. 2019, 94, 1007–1014. [Google Scholar] [CrossRef]
- Bhandari, S.; Kalra, P.A.; Berkowitz, M.; Belo, D.; Thomsen, L.L.; Wolf, M. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: The FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol. Dial. Transplant. 2021, 36, 111–120. [Google Scholar] [CrossRef]
- Mercadal, L.; Metzger, M.; Casadevall, N.; Haymann, J.P.; Karras, A.; Boffa, J.-J.; Flamant, M.; Vrtovsnik, F.; Stengel, B.; Froissart, M.; et al. Timing and determinants of erythropoietin deficiency in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 35–42. [Google Scholar] [CrossRef]
- Goldenberg, B.A.; Graff, L.A.; Clara, I.; Zarychanski, R.; Walker, J.R.; Carr, R.; Rogala, L.; Miller, N.; Bernstein, C.N. Is iron deficiency in the absence of anemia associated with fatigue in inflammatory bowel disease? Am. J. Gastroenterol. 2013, 108, 1392–1397. [Google Scholar] [CrossRef]
- Graff, L.A.; Vincent, N.; Walker, J.R.; Clara, I.; Carr, R.; Ediger, J.; Miller, N.; Rogala, L.; Rawsthorne, P.; Lix, L.; et al. A population based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm. Bowel. Dis. 2011, 17, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Graff, L.A.; Clara, I.; Walker, J.R.; Lix, L.; Carr, R.; Miller, N.; Rogala, L.; Bernstein, C.N. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin. Gastroenterol. Hepatol. 2013, 11, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.R.; Kupper, N.; de Jonge, P.; Denollet, J. Distinct trajectories of fatigue in chronic heart failure and their association with prognosis. Eur. J. Heart Fail. 2010, 12, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.A.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Rationale and design of the CONFIRM-HF study: A double-blind, randomized, placebo-controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Heart Fail. 2014, 1, 52–58. [Google Scholar] [CrossRef]
- O’Higgins, C.M.; Brady, B.; O’Connor, B.; Walsh, D.; Reilly, R.B. The pathophysiology of cancer-related fatigue: Current controversies. Support Care Cancer 2018, 26, 3353–3364. [Google Scholar] [CrossRef]
- Oliva, E.N.; Finelli, C.; Santini, V.; Poloni, A.; Liso, V.; Cilloni, D.; Impera, S.; Terenzi, A.; Levis, A.; Cortelezzi, A.; et al. Quality of life and physicians’ perception in myelodysplastic syndromes. Am. J. Blood Res. 2012, 2, 136–147. [Google Scholar] [PubMed]
- Capuron, L.; Schroecksnadel, S.; Féart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Layé, S.; Fuchs, D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef]
- Poluri, A.; Mores, J.; Cook, D.B.; Findley, T.W.; Cristian, A. Fatigue in the elderly population. Phys. Med. Rehabil. Clin. N. Am. 2005, 16, 91–108. [Google Scholar] [CrossRef]
- Torossian, M.; Jacelon, C.S. Chronic Illness and Fatigue in Older Individuals: A Systematic Review. Rehabil. Nurs. 2021, 46, 125–136. [Google Scholar] [CrossRef]
- Krayenbuehl, P.A.; Battegay, E.; Breymann, C.; Furrer, J.; Schulthess, G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, 118, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, P.; Druais, P.L.; Waldvogel, S.; Favrat, B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: A randomized controlled trial. Can. Med. Assoc. J. 2012, 184, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, S.; Pedrazzini, B.; Vaucher, P.; Bize, R.; Cornuz, J.; Tissot, J.D.; Favrat, B. Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: A randomized controlled trial. BMC Med. 2012, 10, 8. [Google Scholar] [CrossRef]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: A systematic review of randomised controlled trials. BMJ Open. 2018, 8, e019240. [Google Scholar] [CrossRef]
- World Health Organization. The Prevalence of Anaemia in Women: A Tabulation of Available Information; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Scrimshaw, N.S. Functional consequences of iron-deficiency in human-populations. J. Nutr. Sci. Vitaminol. 1984, 30, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.S.; Marions, L.B.; Edlund, M.G. Heavy menstrual bleeding significantly affects quality of life. Acta Obs. Gynecol. Scand. 2014, 93, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bruinvels, G.; Burden, R.; Brown, N.; Richards, T.; Pedlar, C. The prevalence and impact of heavy menstrual bleeding (menorrhagia) in elite and non-elite athletes. PLoS ONE 2016, 11, e0149881. [Google Scholar] [CrossRef] [PubMed]
- Kazemijaliseh, H.; Ramezani Tehrani, F.; Behboudi-Gandevani, S.; Khalili, D.; Hosseinpoanah, F.; Azizi, F. A Population-based study of the prevalence of abnormal uterine bleeding and its related factors among Iranian reproductive-age women: An updated data. Arch. Iran Med. 2017, 20, 558–563. [Google Scholar]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Markopoulos, K.; Albertini, R.; di Sabatino, A.; Biagi, F.; Ciccocioppo, R.; Arbustini, E.; Corazza, G.R. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica 2008, 93, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Doehner, W.; Comin-Colet, J.; IRON CORE Group. Iron deficiency in chronic heart failure: Case-based practical guidance. ESC Heart Fail. 2018, 5, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Rollison, D.E.; Howlader, N.; Smith, M.T.; Strom, S.S.; Merritt, W.D.; Ries, L.A.; Edwards, B.K.; List, A.F. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008, 112, 45–52. [Google Scholar] [CrossRef]
- Escalante, C.P.; Chisolm, S.; Song, J.; Richardson, M.; Salkeld, E.; Aoki, E.; Garcia-Manero, G. Fatigue, symptom burden, and health-related quality of life in patients with myelodysplastic syndrome, aplastic anemia, and paroxysmal nocturnal hemoglobinuria. Cancer Med. 2019, 8, 543–553. [Google Scholar] [CrossRef]
- Gulbis, B.; Eleftheriou, A.; Angastiniotis, M.; Ball, S.; Surrallés, J.; Castella, M.; Heimpel, H.; Hill, A.; Corrons, J.-L.V. Epidemiology of rare anaemias in Europe. Adv. Exp. Med. Biol. 2010, 686, 375–396. [Google Scholar] [PubMed]
- Borowitz, M.J.; Craig, F.E.; Digiuseppe, J.A.; Illingworth, A.J.; Rosse, W.; Sutherland, D.R.; Wittwer, C.T.; Richards, S.J. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytom. B Clin. Cytom. 2010, 78, 211–230. [Google Scholar] [CrossRef]
- Navickas, R.; Petric, V.K.; Feigl, A.B.; Seychell, M. Multimorbidity: What do we know? What should we do? J. Comorb. 2016, 6, 4–11. [Google Scholar] [CrossRef]
- Knyszyńska, A.; Radecka, A.; Zabielska, P.; Łuczak, J.; Karakiewicz, B.; Lubkowska, A. The Role of Iron Metabolism in Fatigue, Depression, and Quality of Life in Multiple Sclerosis Patients. Int. J. Environ. Res. Public Health 2020, 17, 6818. [Google Scholar] [CrossRef]
- Daniłowicz-Szymanowicz, L.; Świątczak, M.; Sikorska, K.; Starzyński, R.R.; Raczak, A.; Lipiński, P. Pathogenesis, Diagnosis, and Clinical Implications of Hereditary Hemochromatosis-The Cardiological Point of View. Diagnostics 2021, 11, 1279. [Google Scholar] [CrossRef]
- Siddique, A.; Kowdley, K.V. Review article: The iron overload syndromes. Aliment Pharm. Ther. 2012, 35, 876–893. [Google Scholar] [CrossRef]
- Alessandrino, E.P.; Amadori, S.; Barosi, G.; Cazzola, M.; Grossi, A.; Liberato, L.N.; Locatelli, F.; Marchetti, M.; Morra, E.; Rebulla, P.; et al. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A Statement Ital. Soc. Hematol. Haematol. 2002, 87, 1286–1306. [Google Scholar]
- Schipper, H.M. Neurodegeneration with brain iron accumulation—Clinical syndromes and neuroimaging. Biochim. Biophys. Acta. 2012, 1822, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H.M.; Koller, C.; Taher, A. Red blood cell transfusions and iron overload in the treatment of patients with myelodysplastic syndromes. Cancer 2008, 112, 1089–1095. [Google Scholar] [CrossRef]
- Rozwadowska, K.; Daniłowicz-Szymanowicz, L.; Fijałkowski, M.; Sikorska, K.; Gałąska, R.; Kozłowski, D.; Gruchała, M.; Raczak, G. Can two-dimensional speckle tracking echocardiography be useful for left ventricular assessment in the early stages of hereditary haemochromatosis? Echocardiography 2018, 35, 1772–1781. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hereditary hemochromatosis—A new look at an old disease. N. Engl. J. Med. 2004, 350, 2383–2397. [Google Scholar] [CrossRef]
- Casanova-Esteban, P.; Guiral, N.; Andrés, E.; Gonzalvo, C.; Mateo-Gallego, R.; Giraldo, P.; Paramo, J.A.; Civeira, F. Effect of phlebotomy on lipid metabolism in subjects with hereditary hemochromatosis. Metabolism 2011, 60, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.C.; Hustead, T.R. Causes and evaluation of mildly elevated liver transaminase levels. Am. Fam. Phys. 2011, 84, 1003–1008. [Google Scholar]
- Świątczak, M.; Sikorska, K.; Raczak, G.; Daniłowicz-Szymanowicz, L. Nonroutine use of 2-dimensional speckle tracking echocardiography and fatigue assessment to monitor the effects of therapeutic venesections in a patient with newly diagnosed hereditary hemochromatosis. Kardiol. Pol. 2020, 78, 786–787. [Google Scholar] [CrossRef]
- Barton, J.C.; Acton, R.T. Diabetes in HFE Hemochromatosis. J. Diabetes Res. 2017, 2017, 9826930. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef]
- While, A.E.; Mullen, J. Living with sickle cell disease: The perspective of young people. Br. J. Nurs. 2004, 13, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tabei, S.M.; Mazloom, M.; Shahriari, M.; Zareifar, S.; Azimi, A.; Hadaegh, A.; Karimi, M. Determining and surveying the role of carnitine and folic acid to decrease fatigue in β-thalassemia minor subjects. Pediatr. Hematol. Oncol. 2013, 30, 742–747. [Google Scholar] [CrossRef]
- Byrne, D.; Walsh, J.P.; Daly, C.; McKiernan, S.; Norris, S.; Murphy, R.T.; King, G. Improvements in cardiac function detected using echocardiography in patients with hereditary haemochromatosis. Ir. J. Med. Sci. 2020, 189, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Paramore, C.; Levine, L.; Bagshaw, E.; Ouyang, C.; Kudlac, A.; Larkin, M. Patient- and Caregiver-Reported Burden of Transfusion-Dependent β-Thalassemia Measured Using a Digital Application. Patient 2021, 14, 197–208. [Google Scholar] [CrossRef]
- Camaschella, C. Hereditary sideroblastic anemias: Pathophysiology, diagnosis, and treatment. Semin. Hematol. 2009, 46, 371–377. [Google Scholar] [CrossRef]
- Iolascon, A.; Heimpel, H.; Wahlin, A.; Tamary, H. Congenital dyserythropoietic anemias: Molecular insights and diagnostic approach. Blood 2013, 122, 2162–2166. [Google Scholar] [CrossRef]
- Woodward, M.; Debold, E.P. Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity through Distinct Molecular Mechanisms. Front. Physiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.G. Fatigue in liver disease: Pathophysiology and clinical management. Can. J. Gastroenterol. 2006, 20, 181–188. [Google Scholar] [CrossRef]
- Swain, M.G.; Jones, D.E.J. Fatigue in chronic liver disease: New insights and therapeutic approaches. Liver Int. 2019, 39, 6–19. [Google Scholar] [CrossRef]
- Fritschi, C.; Quinn, L. Fatigue in patients with diabetes: A review. J. Psychosom. Res. 2010, 69, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name of the Scale | Examples of Use |
|---|---|
| Fatigue Severity Scale (FSS) |
|
| Multidimensional Fatigue Inventory (MFI) |
|
| Fatigue Assessment Scale (FAS) |
|
| Brief Fatigue Inventory (BFI) |
|
| Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-f) |
|
| Chalder Fatigue Scale (CFQ) |
|
| Revised Piper Fatigue Scale (PFS) |
|
| Inflammatory Bowel Disease Fatigue Scale (IBD-F) |
|
| Multidimensional Assessment of Fatigue (MAF) |
|
| Short-Form 36 Questionnaire (SF-36) |
|
| Serum Iron (umol/L) | Ferritin Concentration (ug/L) | Iron Transferrin Saturation (%) | Total Iron-Binding Capacity (umol/L) | Serum Soluble Transferrin Receptor Concentration (mg/L) | |
|---|---|---|---|---|---|
| Normal values | 10–30 | 10–200 in women; 15–400 in men | 17–44% | 40–80 in women; 45–75 in men | 1.9–4.4 in women; 2.2–5 in men |
| Iron deficiency anemia | ↓ | 17–44% | ↓ | ↑ | ↑ |
| Latent iron deficiency | Normal/↓ | 40–80 in women; 45–75 in men | Normal/↓ | ↑ | ↑ |
| Iron deficiency anemia refractory to iron therapy | ↓ | 1.9–4.4 in women; 2.2–5 in men | ↓ | ↑ | ↑ |
| Anemia of chronic diseases | ↓ | 10–30 | ↓ | ↓ | Normal |
| Iron Metabolism Parameter | Sensitivity and Specificity |
|---|---|
| Serum iron (SF) | In the diagnosis of IDA, it provides a specificity of 38.67% and a sensitivity of 63.5% [50]. |
| Ferritin concentration | Serum ferritin at a cut-off limit of 41 ng/mL has a sensitivity and specificity of 98% and 98%, respectively [51]. |
| Iron transferrin saturation | There is a lack of data in the literature regarding sensitivity and specificity in IDA. In a study by Low et al. conducted in 1997, it was shown that for transferrin saturation values <20%, the sensitivity was 74%, and the specificity was 36% [52]. |
| Total iron-binding capacity | Provides sensitivity of 64.5% with a specificity of 42.8% in the diagnosis of IDA [50]. |
| Serum soluble transferrin receptor concentration | A study of 72 patients with advanced IDA performed by Choi et al. revealed sensitivity and specificity of 70.8% and 90.6%, respectively [53]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątczak, M.; Młodziński, K.; Sikorska, K.; Raczak, A.; Lipiński, P.; Daniłowicz-Szymanowicz, L. Chronic Fatigue Syndrome in Patients with Deteriorated Iron Metabolism. Diagnostics 2022, 12, 2057. https://doi.org/10.3390/diagnostics12092057
Świątczak M, Młodziński K, Sikorska K, Raczak A, Lipiński P, Daniłowicz-Szymanowicz L. Chronic Fatigue Syndrome in Patients with Deteriorated Iron Metabolism. Diagnostics. 2022; 12(9):2057. https://doi.org/10.3390/diagnostics12092057
Chicago/Turabian StyleŚwiątczak, Michał, Krzysztof Młodziński, Katarzyna Sikorska, Alicja Raczak, Paweł Lipiński, and Ludmiła Daniłowicz-Szymanowicz. 2022. "Chronic Fatigue Syndrome in Patients with Deteriorated Iron Metabolism" Diagnostics 12, no. 9: 2057. https://doi.org/10.3390/diagnostics12092057
APA StyleŚwiątczak, M., Młodziński, K., Sikorska, K., Raczak, A., Lipiński, P., & Daniłowicz-Szymanowicz, L. (2022). Chronic Fatigue Syndrome in Patients with Deteriorated Iron Metabolism. Diagnostics, 12(9), 2057. https://doi.org/10.3390/diagnostics12092057







