Echocardiographic Quantification of Superior Vena Cava (SVC) Flow in Neonates: Pilot Study of Modified Technique
Abstract
:1. Introduction
2. Methods
2.1. PCMRI Acquisition
2.2. Echocardiographic Measures
2.2.1. Method 1—Traditional Superior Vena Cava Flow Technique
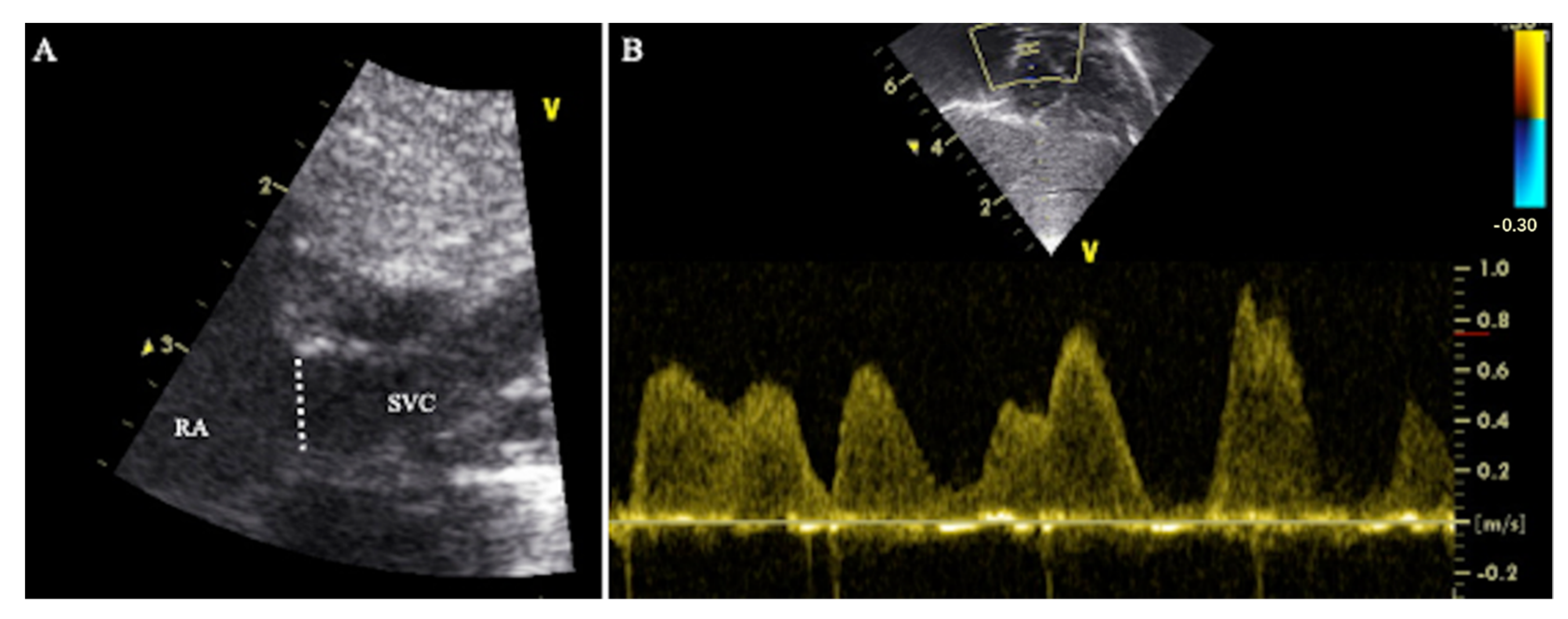
2.2.2. Method 2—Modified Superior Vena Cava Flow Technique
2.2.3. Method 3—Modified Superior Vena Cava Flow Technique
2.3. Statistical Analysis
3. Results
3.1. Method 1
3.2. Method 2
3.3. Method 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kluckow, M.; Evans, N. Superior vena cava flow in newborn infants: A novel marker of systemic blood flow. Arch. Dis. Child Fetal Neonatal Ed. 2000, 82, F182–F187. [Google Scholar] [CrossRef] [PubMed]
- De Waal, K.; Kluckow, M. Superior vena cava flow: Role, assessment and controversies in the management of perinatal perfusion. Semin Fetal Neonatal Med. 2020, 25, 101122. [Google Scholar] [CrossRef] [PubMed]
- Kluckow, M. The Pathophysiology of Low Systemic Blood Flow in the Preterm Infant. Front. Pediatr. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Kluckow, M.; Evans, N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2000, 82, F188–F194. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Evans, N.; Kluckow, M.; Bowen, J.R.; Rieger, I. Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics 2007, 120, 372–380. [Google Scholar] [CrossRef]
- De Waal, K.A. The methodology of Doppler-derived central blood flow measurements in newborn infants. Int. J. Pediatr. 2012, 2012, 680162. [Google Scholar] [CrossRef]
- Lee, A.; Liestol, K.; Nestaas, E.; Brunvand, L.; Lindemann, R.; Fugelseth, D. Superior vena cava flow: Feasibility and reliability of the off-line analyses. Arch. Dis. Child Fetal Neonatal Ed. 2010, 95, F121–F125. [Google Scholar] [CrossRef]
- Groves, A.M.; Kuschel, C.; Knight, D.B.; Skinner, J.R. Echocardiographic assessment of blood flow volume in the superior vena cava and descending aorta in the newborn infant. Arch. Dis. Child Fetal Neonatal Ed. 2008, 93, F24–F28. [Google Scholar] [CrossRef]
- Mertens, L.; Seri, I.; Marek, J.; Arlettaz, R.; Barker, P.; McNamara, P.; Moon-Grady, A.J.; Coon, P.D.; Noori, S.; Simpson, J.; et al. Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: Practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J. Am. Soc. Echocardiogr. 2011, 24, 1057–1078. [Google Scholar]
- Ficial, B.; Finnemore, A.E.; Cox, D.J.; Broadhouse, K.; Price, A.; Durighel, G.; Ekitzidou, G.; Hajnal, J.; Edwards, A.D.; Groves, A.M. Validation study of the accuracy of echocardiographic measurements of systemic blood flow volume in newborn infants. J. Am. Soc. Echocardiogr. 2013, 26, 1365–1371. [Google Scholar] [CrossRef]
- Harabor, A.; Fruitman, D. Comparison between a suprasternal or high parasternal approach and an abdominal approach for measuring superior vena cava Doppler velocity in neonates. J. Ultrasound Med. 2012, 31, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Ficial, B.; Bonafiglia, E.; Padovani, E.M.; Prioli, M.; Finnemore, A.; Cox, D.J.; Broadhouse, K.M.; Price, A.; Durighel, G.; Groves, A.M. A modified echocardiographic approach improves reliability of superior vena caval flow quantification. Arch. Dis. Child Fetal Neonatal Ed. 2017, 102, F7–F11. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Chiesa, G.; Durighel, G.; Goldring, S.T.; Fitzpatrick, J.; Uribe, S.; Razavi, R.; Hajnal, J.; Edwards, A.D. Functional cardiac MRI in preterm and term newborns. Arch. Dis. Cild Fetal Neonatal Ed. 2011, 96, F86–F91. [Google Scholar] [CrossRef] [PubMed]
- Broadhouse, K.M.; Price, A.N.; Durighel, G.; Cox, D.J.; Finnemore, A.E.; Edwards, A.D.; Hajnal, J.V.; Groves, A.M. Assessment of PDA shunt and systemic blood flow in newborns using cardiac MRI. NMR Biomed. 2013, 26, 1135–1141. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Osborn, D.A.; Evans, N.; Kluckow, M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch. Dis. Child Fetal Neonatal Ed. 2004, 89, F168–F173. [Google Scholar] [CrossRef]
- Groves, A.M.; Kuschel, C.; Knight, D.B.; Skinner, J.R. Relationship between blood pressure and blood flow in newborn preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2008, 93, F29–F32. [Google Scholar] [CrossRef]
- Tyszczuk, L.; Meek, J.; Elwell, C.; Wyatt, J.S. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics 1998, 102 Pt 1, 337–341. [Google Scholar] [CrossRef]
- Batton, B.; Li, L.; Newman, N.S.; Das, A.; Watterberg, K.L.; Yoder, B.A.; Faix, R.G.; Laughon, M.M.; Stoll, B.J.; Higgins, R.D.; et al. Early blood pressure, antihypotensive therapy and outcomes at 18–22 months’ corrected age in extremely preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2016, 101, F201–F206. [Google Scholar] [CrossRef]
- De Boode, W.P.; van der Lee, R.; Horsberg Eriksen, B.; Nestaas, E.; Dempsey, E.; Singh, Y.; Austin, T.; El-Khuffash, A. The role of Neonatologist Performed Echocardiography in the assessment and management of neonatal shock. Pediatr. Res. 2018, 84, 57–67. [Google Scholar] [CrossRef]
- Mertens, L.; Seri, I.; Marek, J.; Arlettaz, R.; Barker, P.; McNamara, P.; Moon-Grady, A.J.; Coon, P.D.; Noori, S.; Simpson, J.; et al. Targeted neonatal echocardiography in the neonatal intensive care unit: Practice guidelines and recommendations for training. Eur. J. Echocardiogr. 2011, 12, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Stranak, Z.; On behalf of the HIP consortium; Semberova, J.; Barrington, K.; O’Donnell, C.; Marlow, N.; Naulaers, G.; Dempsey, E. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur. J. Pediatr. 2014, 173, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Corsini, I.; On Behalf of Study Group of Neonatal Cardiology of the Italian Society of Neonatology; Ficial, B.; Fiocchi, S.; Schena, F.; Capolupo, I.; Cerbo, R.M.; Condò, M.; Doni, D.; La Placa, S.; et al. Neonatologist performed echocardiography (NPE) in Italian neonatal intensive care units: A national survey. Ital. J. Pediatr. 2019, 45, 131. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.R.; Giesinger, R.E.; Stanford, A.H.; Ashwath, R.; McNamara, P.J. Assessment of superior vena cava flow and cardiac output in different patterns of patent ductus arteriosus shunt. Echocardiography 2021, 38, 1524–1533. [Google Scholar] [CrossRef]
- Barone, G.; Pittiruti, M.; Biasucci, D.G.; Elisei, D.; Iacobone, E.; La Greca, A.; Marinosci, G.Z.; D’Andrea, V. Neo-ECHOTIP: A structured protocol for ultrasound-based tip navigation and tip location during placement of central venous access devices in neonates. J. Vasc. Access 2021, 11297298211007703. [Google Scholar] [CrossRef]
- Siekmann, M.; Lothes, T.; König, R.; Wirtz, C.R.; Coburger, J. Experimental study of sector and linear array ultrasound accuracy and the influence of navigated 3D-reconstruction as compared to MRI in a brain tumor model. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 471–478. [Google Scholar] [CrossRef]
- Jiang, J.; Strother, C.; Johnson, K.; Baker, S.; Consigny, D.; Wieben, O.; Zagzebski, J. Comparison of blood velocity measurements between ultrasound Doppler and accelerated phase-contrast MR angiography in small arteries with disturbed flow. Phys. Med. Biol. 2011, 56, 1755–1773. [Google Scholar] [CrossRef]
- Hoskins, P.R. Accuracy of maximum velocity estimates made using Doppler ultrasound systems. Br. J. Radiol. 1996, 69, 172–177. [Google Scholar] [CrossRef]
- Guidi, G.; Licciardello, C.; Falteri, S. Intrinsic spectral broadening (ISB) in ultrasound Doppler as a combination of transit time and local geometrical broadening. Ultrasound Med. Biol. 2000, 26, 853–862. [Google Scholar] [CrossRef]
- Seitz, J.; Strotzer, M.; Wild, T.; Nitz, W.R.; Voelk, M.; Lenhart, M.; Feuerbach, S. Quantification of blood flow in the carotid arteries: Comparison of Doppler ultrasound and three different phase-contrast magnetic resonance imaging sequences. Investig. Radiol. 2001, 36, 642–647. [Google Scholar] [CrossRef]
- Kluckow, M.R.; Evans, N.J. Superior vena cava flow is a clinically valid measurement in the preterm newborn. J. Am. Soc. Echocardiogr. 2014, 27, 794. [Google Scholar] [CrossRef] [PubMed]
- Azarine, A.; Garçon, P.; Stansal, A.; Canepa, N.; Angelopoulos, G.; Silvera, S.; Sidi, D.; Marteau, V.; Zins, M. Four-dimensional Flow MRI: Principles and Cardiovascular Applications. Radiographics 2019, 39, 632–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Method 1 | Method 2 | Method 3 | |
|---|---|---|---|
| Equipment: | |||
| - VTI | Sector probe | Sector probe | Sector probe |
| - SVC CSA | Sector probe | Sector probe | High frequency linear probe |
| Echo views: | |||
| - VTI | Subcostal | Suprasternal | Suprasternal |
| - SVC CSA | Long axis parasternal | Short axis parasternal | Short axis parasternal |
| Off-line analysis: | |||
| - VTI | From peak velocity | From peak velocity | From mean velocity |
| - SVC CSA | Calculated from diameter | Directly traced | Directly traced |
| Cohort (N = 7) | |
|---|---|
| Gestation (wk) | 32.5 [24.7–37] |
| Birth weight (g) | 1625 [640–2688] |
| Female | 2 (28.5%) |
| Cesarean delivery | 4 (57.1%) |
| 5-Min Apgar score | 9 [7–10] |
| Postnatal age at scan (days) | 7 [2–74] |
| Corrected gestation (wk) at scan | 34.8 [31.7–37.2] |
| Weight (g) at scan | 1870 [970–2660] |
| Non-invasive respiratory support | 3 (42.8%) |
| No respiratory support | 4 (57.1%) |
| SVC Flow Assessment by Echocardiography | SVC Flow Assessment by PCMRI | |||
|---|---|---|---|---|
| Method 1 | Method 2 | Method 3 | ||
| SVC diameter (mm) | 4.1 (0.7) | NA | NA | NA |
| SVC cross sectional area (mm2) | 11 (2) | 15 (4) | 20 (4) | 23 (6) |
| SVC VTI or stroke distance (cm) | 17.7 (3.2) | 12.6 (2.9) | 7.1 (1.5) | 6.6 (1.4) |
| HR (bpm) | 143 (10) | 139 (9) | 139 (9) | 146 (12) |
| SVC flow (ml/kg/min) | 174 (54) | 153 (50) | 121(39) | 130 (38) |
| Bias | 95% LOA | RI | COV | |
|---|---|---|---|---|
| Method 1 | ||||
| SVC flow | +42 mL/kg/min | −53/+137 mL/kg/min | 63% | 32% |
| Area | −11 mm2 | −23/+0.4 mm2 | 69% | 35% |
| VTI | 11.1 cm | 4.9/17.3 cm | 51% | 26% |
| Method 2 | ||||
| SVC flow | +23 mL/kg/min | −25/+71 mL/kg/min | 48% | 17% |
| Area | −8 mm2 | −15/−0.5 mm2 | 40% | 20% |
| VTI | 6 cm | 0.7/11 cm | 55% | 28% |
| Method 3 | ||||
| SVC flow | −8 mL/kg/min | −25/+8 mL/kg/min | 13% | 7% |
| Area | −2.7 mm2 | −10/+4.8 mm2 | 35% | 17% |
| Suprasternal VTI | 0.5 cm | −1/+2.1 cm | 23% | 11% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficial, B.; Corsini, I.; Bonafiglia, E.; Petoello, E.; Flore, A.I.; Nogara, S.; Tsatsaris, N.; Groves, A.M. Echocardiographic Quantification of Superior Vena Cava (SVC) Flow in Neonates: Pilot Study of Modified Technique. Diagnostics 2022, 12, 2083. https://doi.org/10.3390/diagnostics12092083
Ficial B, Corsini I, Bonafiglia E, Petoello E, Flore AI, Nogara S, Tsatsaris N, Groves AM. Echocardiographic Quantification of Superior Vena Cava (SVC) Flow in Neonates: Pilot Study of Modified Technique. Diagnostics. 2022; 12(9):2083. https://doi.org/10.3390/diagnostics12092083
Chicago/Turabian StyleFicial, Benjamim, Iuri Corsini, Elena Bonafiglia, Enrico Petoello, Alice Iride Flore, Silvia Nogara, Nicola Tsatsaris, and Alan M. Groves. 2022. "Echocardiographic Quantification of Superior Vena Cava (SVC) Flow in Neonates: Pilot Study of Modified Technique" Diagnostics 12, no. 9: 2083. https://doi.org/10.3390/diagnostics12092083
APA StyleFicial, B., Corsini, I., Bonafiglia, E., Petoello, E., Flore, A. I., Nogara, S., Tsatsaris, N., & Groves, A. M. (2022). Echocardiographic Quantification of Superior Vena Cava (SVC) Flow in Neonates: Pilot Study of Modified Technique. Diagnostics, 12(9), 2083. https://doi.org/10.3390/diagnostics12092083






