Reducing Visceral-Motion-Related Artifacts on the Liver with Dual-Energy CT: A Comparison of Four Different CT Scanner Techniques
Abstract
:1. Introduction
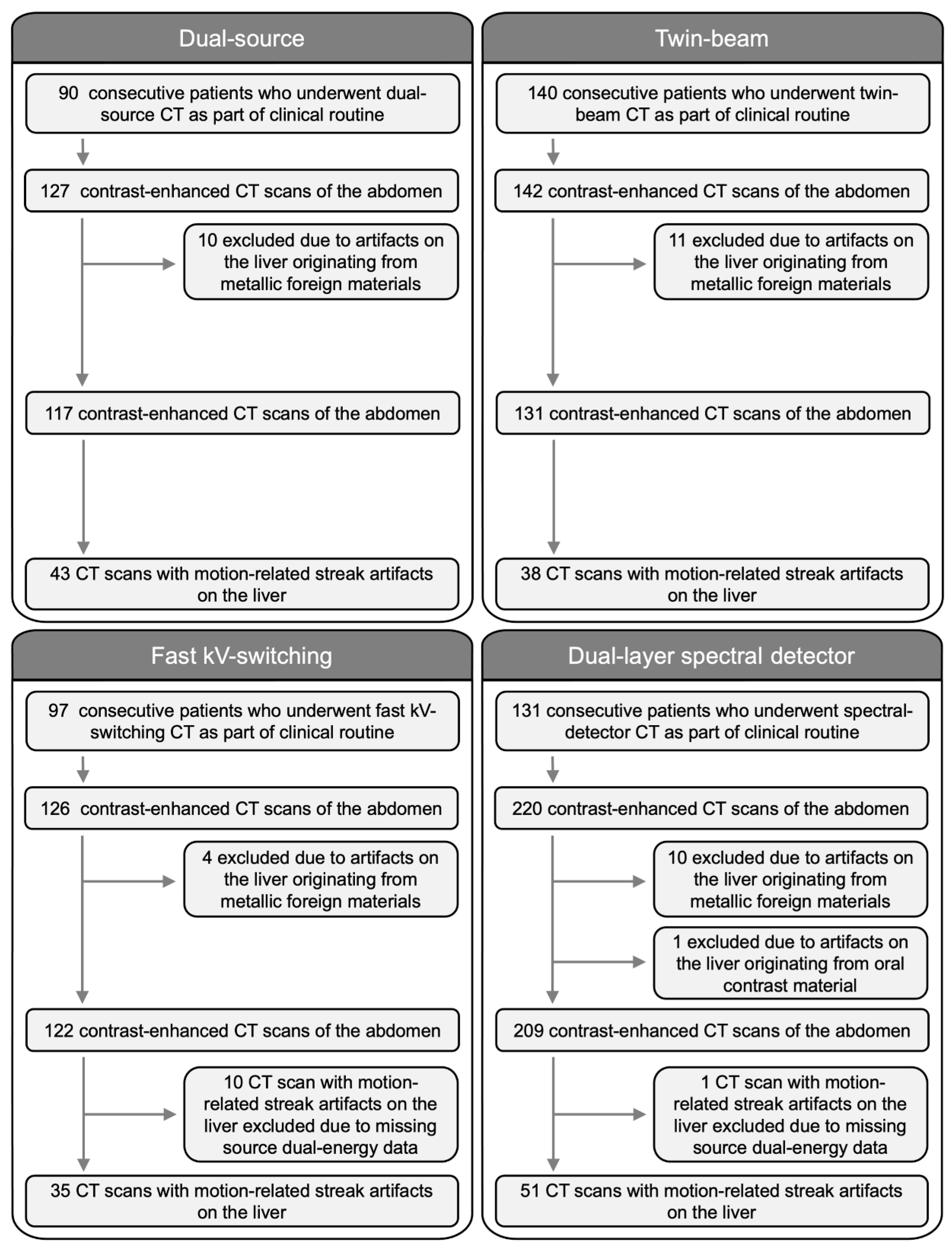
2. Materials and Methods
2.1. Study Population
2.2. CT Image Acquisition
2.3. Image Analysis
2.4. Quantitative Visceral-Motion-Related Artifact Evaluation
2.5. Qualitative Visceral-Motion-Related Artifact Evaluation
2.6. Statistical Analysis
3. Results
3.1. Study Population
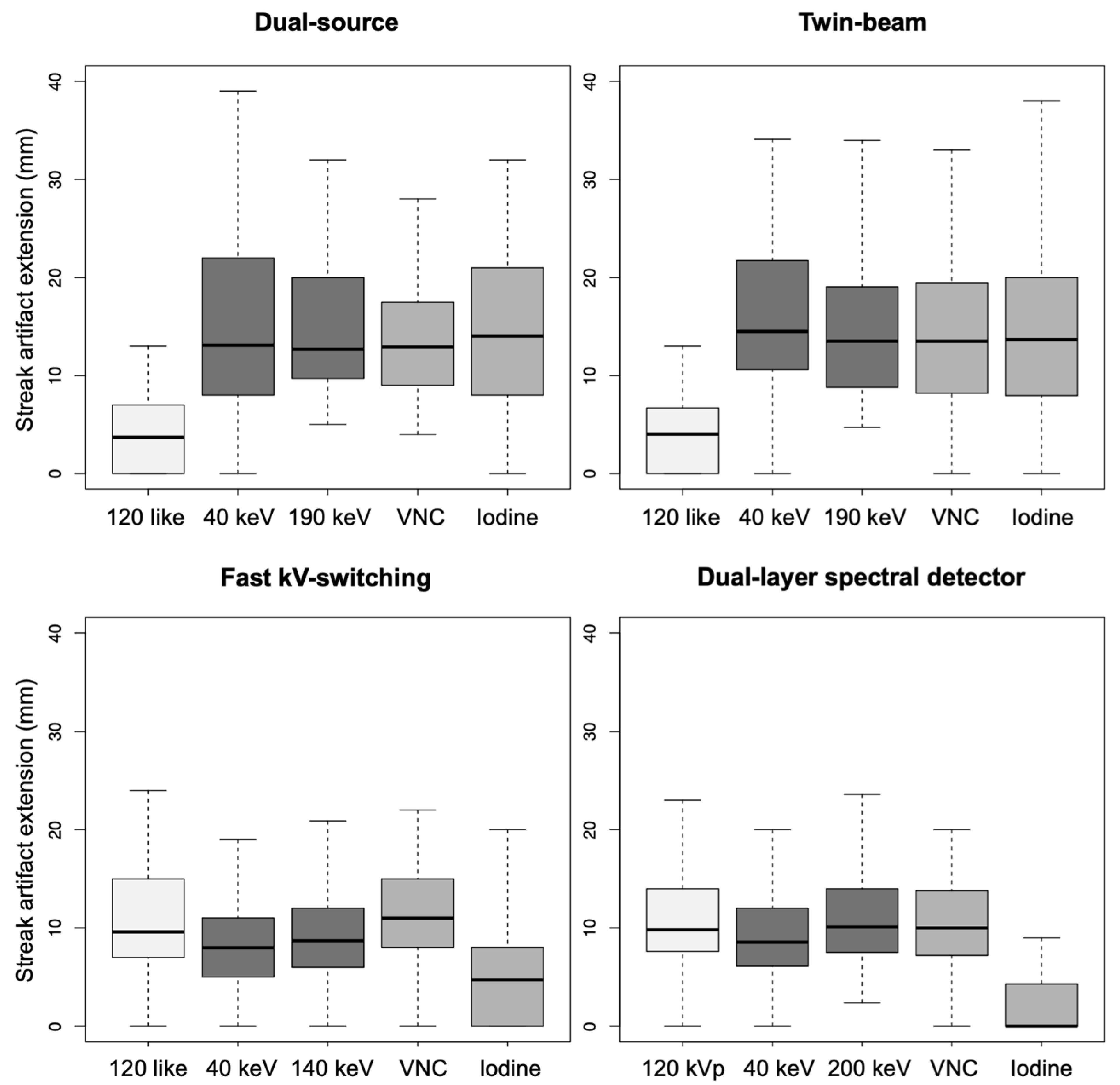
3.2. Quantitative Visceral-Motion-Related Artifact Evaluation
- (1)
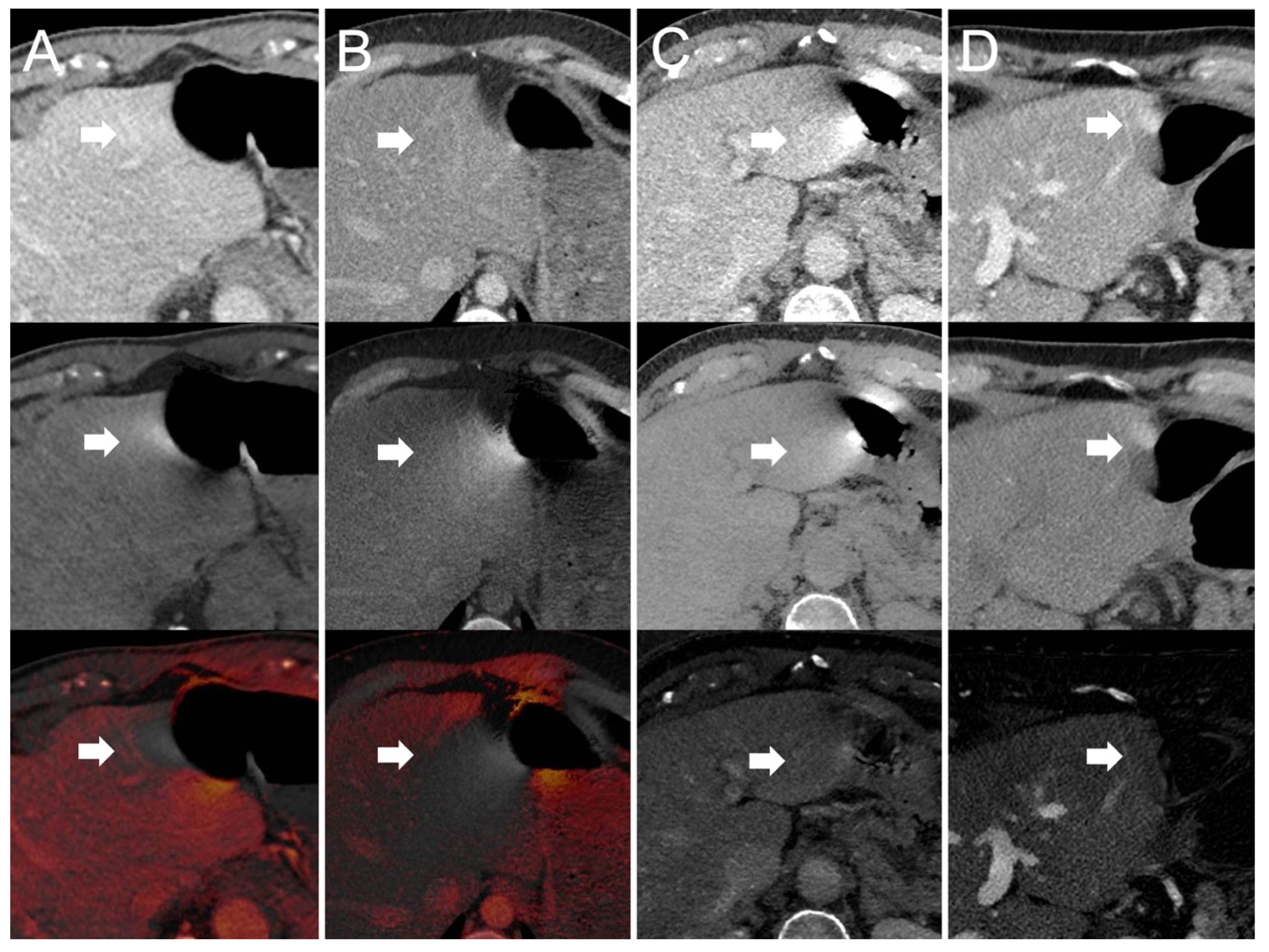
- Dual-source scanner: Depth of extension of visceral-motion-related artifacts into the liver (see Figure 2 and Figure 3) was significantly shorter (p < 0.001, each) for 120-kVp-like images (mean length: 5 ± 6 mm) compared with 40-keV (mean length: 16 ± 11 mm), 190-keV (mean length: 16 ± 9 mm), VNC (mean length: 15 ± 10 mm), and iodine (mean length: 16 ± 11 mm) images. Mean ROI measurements (HU or iodine concentration, respectively) were significantly different in the bright and dark artifact components compared to the neighboring liver parenchyma not affected by artifacts in 40-keV (p < 0.001, each), 190-keV (p < 0.001, each), VNC (p < 0.001, each), and iodine images (p < 0.001, each). However, in 120-kVp-like images, mean ROI measurements were not significantly different in bright (ROImin) artifact components compared to unaffected liver parenchyma (p = 0.32), as opposed to dark (ROImax) artifact components (p < 0.001).
- (2)
- Twin-beam scanner: Depth of extension of visceral-motion-related artifacts into the liver was significantly shorter (p < 0.001, each) for 120-kVp-like images (mean length: 4 ± 5 mm) compared with 40-keV (mean length: 18 ± 12 mm), 190-keV (mean length: 16 ± 11 mm), VNC (mean length: 15 ± 11 mm), and iodine (mean length: 16 ± 11 mm) images. Mean ROI measurements were significantly different in the bright and dark artifact components compared to unaffected liver parenchyma in 120-kVp-like (p = 0.006, p = 0.03), 40-keV (p < 0.001, each), 190-keV (p < 0.001, each), VNC (p < 0.001, each), and iodine images (p < 0.001, each).
- (3)
- Fast kV-switching scanner: Depth of extension of visceral-motion-related artifacts into the liver was significantly shorter (p < 0.001, each) for iodine (mean length: 6 ± 7 mm) images compared with 120-kVp-like (mean length: 11 ± 7 mm), 40-keV (mean length: 9 ± 8 mm), 140-keV (mean length: 10 ± 8 mm), and VNC (mean length: 13 ± 8 mm) images. Mean ROI measurements were significantly different in the bright and dark artifact components compared to unaffected liver parenchyma in 120-kVp-like (p < 0.001, each), 40-keV (p < 0.001, each), 140-keV (p < 0.001, each), VNC (p < 0.001, each), and iodine images (p < 0.001, each).
- (4)
- Dual-layer spectral detector scanner: Depth of extension of visceral-motion-related artifacts into the liver was significantly shorter (p < 0.001, each) for iodine (mean length: 2 ± 5 mm) images compared with 120-kVp (mean length: 11 ± 5 mm), 40-keV (mean length: 10 ± 6 mm), 200-keV (mean length: 11 ± 5 mm), and VNC (mean length: 11 ± 5 mm) images. Mean ROI measurements were significantly different in the bright and dark artifact components compared to unaffected liver parenchyma in 120-kVp (p < 0.001, each), 40-keV (p < 0.001, each), 200-keV (p < 0.001, each), and VNC (p < 0.001, each) images. However, in iodine images mean ROI measurements were not significantly different in bright (ROImax) artifact components compared to unaffected liver parenchyma (p = 0.15), as opposed to dark (ROImin) artifact components (p < 0.001). Further details on quantitative artifact measurements are provided in Table 2 and the Supplementary Material.
3.3. Qualitative Visceral-Motion-Related Artifact Evaluation
- (1)
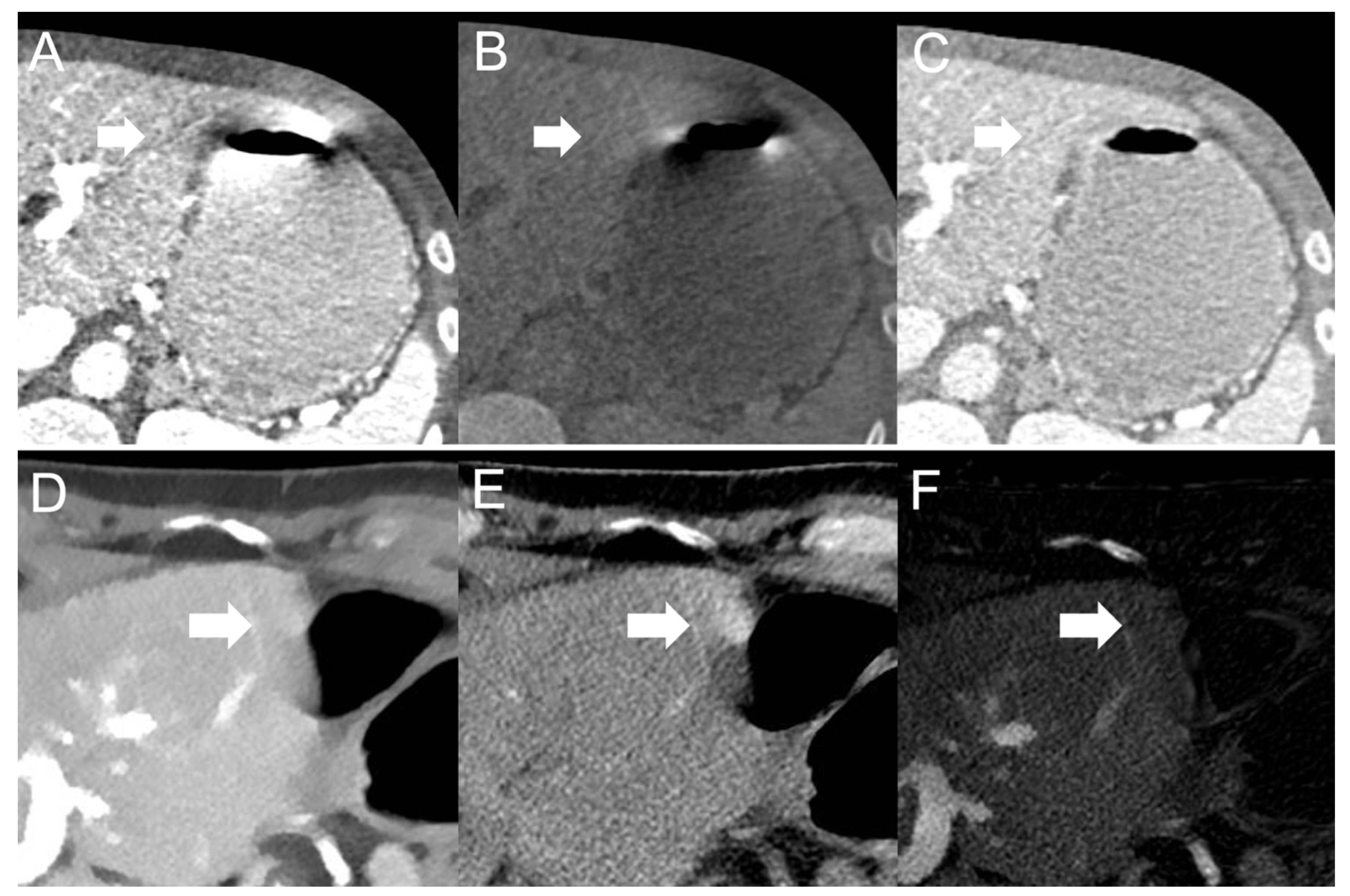
- Dual-source scanner: Qualitative artifact scores (see Figure 4) were significantly lower (p < 0.001, each) for 120-kVp-like images (median score: 2, range: 1–5) compared with 40-keV (median score: 4, range: 1–5), 190-keV (median score: 3, range: 2–5), VNC (median score: 3, range: 2–5), and iodine (median score: 4, range: 1–5) images.
- (2)
- Twin-bean scanner: Qualitative artifact scores were significantly lower (p < 0.001, each) for 120-kVp-like images (median score: 2, range: 1–4) compared with 40-keV (median score: 5, range: 1–5), 190-keV (median score: 3, range: 2–5), VNC (median score: 3, range: 1–5), and iodine (median score: 4, range: 1–5) images.
- (3)
- Fast kV-switching scanner: Qualitative artifact scores were significantly lower (p < 0.001, each) for iodine images (median score: 2, range: 1–5) compared with 120-kVp-like (median score: 3, range: 1–5), 40-keV (median score: 3, range: 1–5), 140-keV (median score: 3, range: 1–5), and VNC (median score: 3, range: 1–5) images.
- (4)
- Dual-layer spectral detector scanner: Qualitative artifact scores were significantly lower (p < 0.001, each) for iodine images (median score: 1, range: 1–3) compared with 120-kVp (median score: 3, range: 1–5), 40-keV (median score: 3, range: 1–5), 200-keV (median score: 3, range: 2–5), and VNC (median score: 3, range: 1–5) images. Further details on qualitative artifact scores are provided in the Supplementary Material.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Khoram, R.; Lambert, J.W.; Sun, Y.; Wang, Z.J.; Webb, E.M.; Yeh, B.M. Effect of gantry rotation speed and scan mode on peristalsis motion artifact frequency and severity at abdominal CT. Abdom. Radiol. 2018, 43, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Wilting, J.E.; Timmer, J. Artefacts in spiral-CT images and their relation to pitch and subject morphology. Eur. Radiol. 1999, 9, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J. Computed Tomography: Principles, Design, Artifacts, and Recent Advances; SPIE Press: Bellingham, WA, USA, 2003; Volume 114. [Google Scholar]
- Bamberg, F.; Dierks, A.; Nikolaou, K.; Reiser, M.F.; Becker, C.R.; Johnson, T.R. Metal artifact reduction by dual energy computed tomography using monoenergetic extrapolation. Eur. Radiol. 2011, 21, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cuevas, C.; Moss, A.A.; Kolokythas, O.; Dubinsky, T.J.; Kinahan, P.E. Gas bubble motion artifact in MDCT. AJR Am. J. Roentgenol. 2008, 190, 294–299. [Google Scholar] [CrossRef]
- Winklhofer, S.; Lambert, J.W.; Wang, Z.J.; Sun, Y.; Gould, R.G.; Zagoria, R.J.; Yeh, B.M. Reduction of peristalsis-related gastrointestinal streak artifacts with dual-energy CT: A patient and phantom study. Abdom. Radiol. 2016, 41, 1456–1465. [Google Scholar] [CrossRef]
- Partanen, K.; Kormano, M. Streak artifacts in dynamic CT. A phantom study of the anterior upper abdomen. Acta Radiol. Diagn. 1984, 25, 285–288. [Google Scholar] [CrossRef]
- Grosu, S.; Wang, Z.J.; Obmann, M.M.; Sugi, M.D.; Sun, Y.; Yeh, B.M. Reduction of Peristalsis-Related Streak Artifacts on the Liver with Dual-Layer Spectral CT. Diagnostics 2022, 12, 782. [Google Scholar] [CrossRef]
- Takayanagi, T.; Suzuki, S.; Katada, Y.; Ishikawa, T.; Fukui, R.; Yamamoto, Y.; Abe, O. Comparison of Motion Artifacts on CT Images Obtained in the Ultrafast Scan Mode and Conventional Scan Mode for Unconscious Patients in the Emergency Department. AJR Am. J. Roentgenol. 2019, 213, W153–W161. [Google Scholar] [CrossRef]
- Fleischmann, D.; Boas, F.E. Computed tomography—Old ideas and new technology. Eur. Radiol. 2011, 21, 510–517. [Google Scholar] [CrossRef] [Green Version]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Yeh, B.M.; Obmann, M.M.; Westphalen, A.C.; Ohliger, M.A.; Yee, J.; Sun, Y.; Wang, Z.J. Dual Energy Computed Tomography Scans of the Bowel: Benefits, Pitfalls, and Future Directions. Radiol. Clin. N. Am. 2018, 56, 805–819. [Google Scholar] [CrossRef]
- Mileto, A.; Ananthakrishnan, L.; Morgan, D.E.; Yeh, B.M.; Marin, D.; Kambadakone, A.R. Clinical Implementation of Dual-Energy CT for Gastrointestinal Imaging. AJR Am. J. Roentgenol. 2021, 217, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Megibow, A.J.; Sahani, D. Best practice: Implementation and use of abdominal dual-energy CT in routine patient care. AJR Am. J. Roentgenol. 2012, 199, S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Große Hokamp, N.; Salem, J.; Hesse, A.; Holz, J.A.; Ritter, M.; Heidenreich, A.; Maintz, D.; Haneder, S. Low-Dose Characterization of Kidney Stones Using Spectral Detector Computed Tomography: An Ex Vivo Study. Investig. Radiol. 2018, 53, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.F.; Xie, X.Q.; Cheng, S.; Yang, Y.; Yan, J.; Zhang, H.; Chai, W.M.; Schmidt, B.; Yan, F.H. Dual-Energy CT for Patients Suspected of Having Liver Iron Overload: Can Virtual Iron Content Imaging Accurately Quantify Liver Iron Content? Radiology 2015, 277, 95–103. [Google Scholar] [CrossRef]
- McCollough, C.H.; Boedeker, K.; Cody, D.; Duan, X.; Flohr, T.; Halliburton, S.S.; Hsieh, J.; Layman, R.R.; Pelc, N.J. Principles and applications of multienergy CT: Report of AAPM Task Group 291. Med. Phys. 2020, 47, e881–e912. [Google Scholar] [CrossRef]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef]
- Große Hokamp, N.; Höink, A.J.; Doerner, J.; Jordan, D.W.; Pahn, G.; Persigehl, T.; Maintz, D.; Haneder, S. Assessment of arterially hyper-enhancing liver lesions using virtual monoenergetic images from spectral detector CT: Phantom and patient experience. Abdom. Radiol. 2018, 43, 2066–2074. [Google Scholar] [CrossRef]
- Yoon, J.H.; Chang, W.; Lee, E.S.; Lee, S.M.; Lee, J.M. Double Low-Dose Dual-Energy Liver CT in Patients at High-Risk of HCC: A Prospective, Randomized, Single-Center Study. Investig. Radiol. 2020, 55, 340–348. [Google Scholar] [CrossRef]
- Caruso, D.; De Cecco, C.N.; Schoepf, U.J.; Schaefer, A.R.; Leland, P.W.; Johnson, D.; Laghi, A.; Hardie, A.D. Can dual-energy computed tomography improve visualization of hypoenhancing liver lesions in portal venous phase? Assessment of advanced image-based virtual monoenergetic images. Clin. Imaging 2017, 41, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Muenzel, D.; Lo, G.C.; Yu, H.S.; Parakh, A.; Patino, M.; Kambadakone, A.; Rummeny, E.J.; Sahani, D.V. Material density iodine images in dual-energy CT: Detection and characterization of hypervascular liver lesions compared to magnetic resonance imaging. Eur. J. Radiol. 2017, 95, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Rosenberg, M.; Vernuccio, F.; Ramirez-Giraldo, J.C.; Nelson, R.; Farjat, A.; Marin, D. Characterization of Small Incidental Indeterminate Hypoattenuating Hepatic Lesions: Added Value of Single-Phase Contrast-Enhanced Dual-Energy CT Material Attenuation Analysis. AJR Am. J. Roentgenol. 2018, 211, 571–579. [Google Scholar] [CrossRef]
- Yang, C.B.; Zhang, S.; Jia, Y.J.; Yu, Y.; Duan, H.F.; Zhang, X.R.; Ma, G.M.; Ren, C.; Yu, N. Dual energy spectral CT imaging for the evaluation of small hepatocellular carcinoma microvascular invasion. Eur. J. Radiol. 2017, 95, 222–227. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver and Boyd: Edinburgh, UK, 1954. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Obmann, M.M.; Sun, Y.; An, C.; Ohliger, M.A.; Wang, Z.J.; Yeh, B.M. Bowel Peristalsis Artifact on Dual-Energy CT: In-Vitro Study of the Influence of Different Dual-Energy CT Platforms and Enteric Contrast Agents. AJR Am. J. Roentgenol. 2022, 218, 290–299. [Google Scholar] [CrossRef]

| Dual-Source | Twin-Beam | Fast kV-Switching | Dual-Layer Spectral Detector | |
|---|---|---|---|---|
| Tube voltage | Source A: 100 kVp Source B: 140 kVp | 120 kVp | 80 and 140 kVp (0.25 millisecond kV-switching) | 120 kVp |
| Filtration | Tin filter (Source B) | Tin/gold split-filter | none | none |
| Tube current–time product reference values | 70 mAs (automatic tube current adaption) | 70 mAs (automatic tube current adaption) | 70 mAs (automatic tube current adaption) | 70 mAs (automatic tube current adaption) |
| Collimation | 2 × 64 × 0.6 mm | 64 × 0.6 mm | 128 × 0.625 mm | 64 × 0.625 mm |
| Reconstruction algorithm | ADMIRE strength 3 | ADMIRE strength 3 | 30% ASIR- V | Spectral level 3 |
| Slice thickness | 2.5 mm | 2.5 mm | 2.5 mm | 2.5 mm |
| 120-kVp-like images | Mix of 100 kVp and 140 kVp (tin filter) | Mix of 120 kVp (gold filter) and 120 kVp (tin filter) | 70 keV | 120 kVp |
| Dual-source | |||||
| Image reconstructions | Quantitative artifact measurements | p-values | |||
| mean ROImax | mean ROImin | mean ROIref | mean ROImax compared with mean ROIref | mean ROImin compared with mean ROIref | |
| 120-like | 97 HU | 105 HU | 108 HU | p < 0.001 | p = 0.32 |
| 40 keV | 345 HU | 39 HU | 277 HU | p < 0.001 | p < 0.001 |
| 190 keV | 36 HU | 126 HU | 68 HU | p < 0.001 | p < 0.001 |
| VNC | 35 HU | 107 HU | 62 HU | p < 0.001 | p < 0.001 |
| Iodine | 4.0 mg/mL | −2.7 mg/mL | 2.5 mg/mL | p < 0.001 | p < 0.001 |
| Twin-beam | |||||
| Image reconstructions | Quantitative artifact measurements | p-values | |||
| mean ROImax | mean ROImin | mean ROIref | mean ROImax compared with mean ROIref | mean ROImin compared with mean ROIref | |
| 120-like | 98 HU | 101 HU | 106 HU | p = 0.006 | p = 0.03 |
| 40 keV | 513 HU | −79 HU | 269 HU | p < 0.001 | p < 0.001 |
| 190 keV | 21 HU | 142 HU | 70 HU | p < 0.001 | p < 0.001 |
| VNC | 25 HU | 107 HU | 67 HU | p < 0.001 | p < 0.001 |
| Iodine | 4.8 mg/mL | −3.1 mg/mL | 2.3 mg/mL | p < 0.001 | p < 0.001 |
| Fast kV-switching | |||||
| Image reconstructions | Quantitative artifact measurements | p-values | |||
| mean ROImax | mean ROImin | mean ROIref | mean ROImax compared with mean ROIref | mean ROImin compared with mean ROIref | |
| 120-like | 183 HU | 55 HU | 115 HU | p < 0.001 | p < 0.001 |
| 40 keV | 379 HU | 151 HU | 255 HU | p < 0.001 | p < 0.001 |
| 140 keV | 106 HU | 19 HU | 62 HU | p < 0.001 | p < 0.001 |
| VNC | 94 HU | 21 HU | 59 HU | p < 0.001 | p < 0.001 |
| Iodine | 3.8 mg/mL | 1.8 mg/mL | 2.6 mg/mL | p < 0.001 | p < 0.001 |
| Dual-layer spectral detector | |||||
| Image reconstructions | Quantitative artifact measurements | p-values | |||
| mean ROImax | mean ROImin | mean ROIref | mean ROImax compared with mean ROIref | mean ROImin compared with mean ROIref | |
| 120 kVp | 134 HU | 86 HU | 102 HU | p < 0.001 | p < 0.001 |
| 40 keV | 242 HU | 173 HU | 216 HU | p < 0.001 | p < 0.001 |
| 200 keV | 94 HU | 54 HU | 62 HU | p < 0.001 | p < 0.001 |
| VNC | 89 HU | 50 HU | 74 HU | p < 0.001 | p < 0.001 |
| Iodine | 1.9 mg/mL | 1.7 mg/mL | 2.1 mg/mL | p = 0.15 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, S.; Vijittrakarnrung, K.; Wang, Z.J.; Obmann, M.M.; Sun, Y.; Sugi, M.D.; Yeh, B.M. Reducing Visceral-Motion-Related Artifacts on the Liver with Dual-Energy CT: A Comparison of Four Different CT Scanner Techniques. Diagnostics 2022, 12, 2155. https://doi.org/10.3390/diagnostics12092155
Grosu S, Vijittrakarnrung K, Wang ZJ, Obmann MM, Sun Y, Sugi MD, Yeh BM. Reducing Visceral-Motion-Related Artifacts on the Liver with Dual-Energy CT: A Comparison of Four Different CT Scanner Techniques. Diagnostics. 2022; 12(9):2155. https://doi.org/10.3390/diagnostics12092155
Chicago/Turabian StyleGrosu, Sergio, Korawan Vijittrakarnrung, Zhen J. Wang, Markus M. Obmann, Yuxin Sun, Mark D. Sugi, and Benjamin M. Yeh. 2022. "Reducing Visceral-Motion-Related Artifacts on the Liver with Dual-Energy CT: A Comparison of Four Different CT Scanner Techniques" Diagnostics 12, no. 9: 2155. https://doi.org/10.3390/diagnostics12092155
APA StyleGrosu, S., Vijittrakarnrung, K., Wang, Z. J., Obmann, M. M., Sun, Y., Sugi, M. D., & Yeh, B. M. (2022). Reducing Visceral-Motion-Related Artifacts on the Liver with Dual-Energy CT: A Comparison of Four Different CT Scanner Techniques. Diagnostics, 12(9), 2155. https://doi.org/10.3390/diagnostics12092155






