Elevated Surgical Pleth Index at the End of Surgery Is Associated with Postoperative Moderate-to-Severe Pain: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
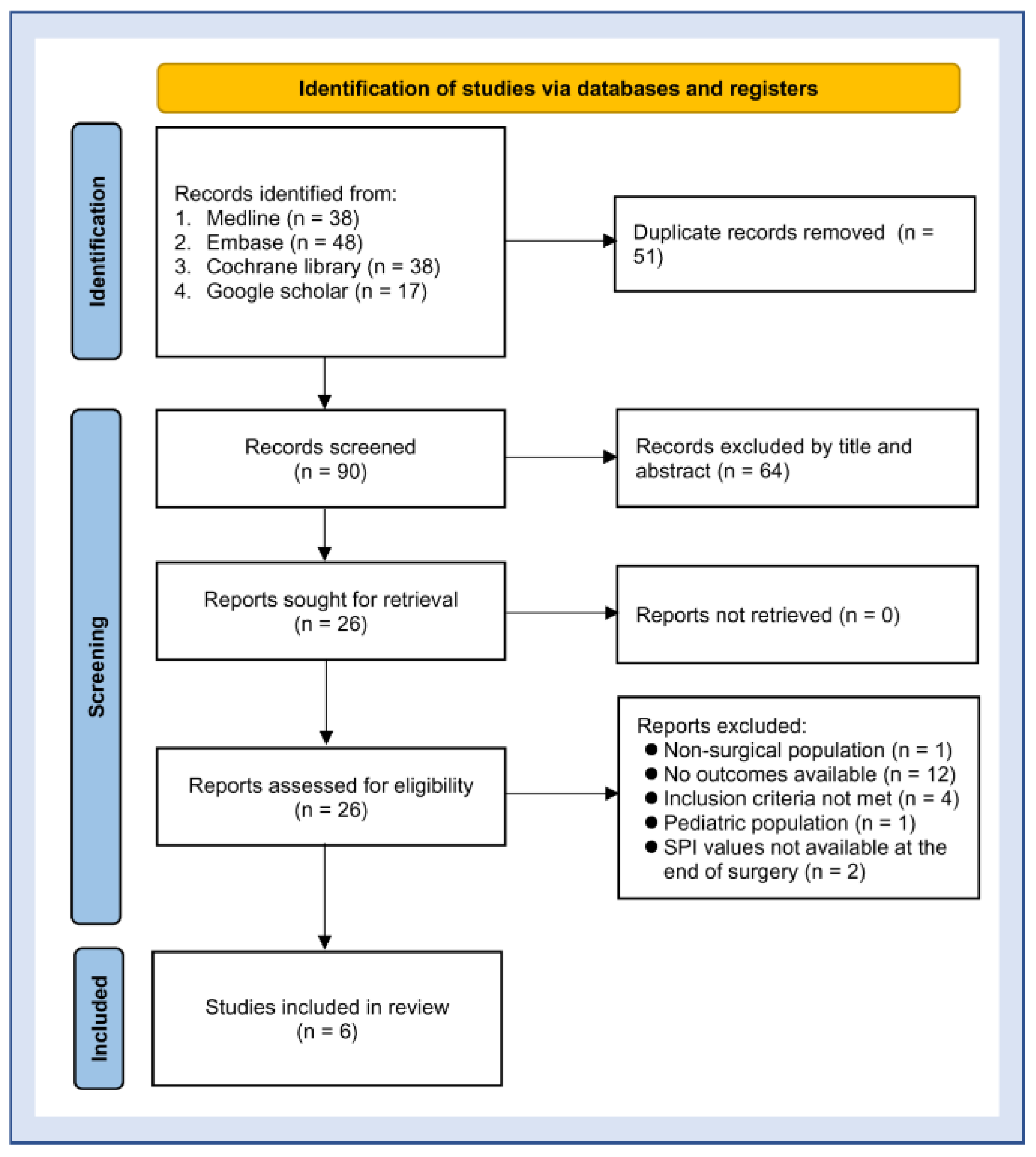
2.1. Protocol, Literature Search, and Study Selection
2.2. Methodologic Quality Assessment
2.3. Data Extraction and Definition
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics of the Studies Included
3.2. Quality of the Enrolled Studies
3.3. Data Analysis
3.3.1. SPI in Patients with or without Moderate-to-Severe Pain
3.3.2. Sensitivity/Specificity, sROC, and Publication Bias
3.3.3. Fagan’s Nomogram Plot Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mischkowski, D.; Palacios-Barrios, E.E.; Banker, L.; Dildine, T.C.; Atlas, L.Y. Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. Pain 2018, 159, 699–711. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Chen, C.; Mehta, S.S.; Gan, T.J. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef]
- Yang, M.M.H.; Hartley, R.L.; Leung, A.A.; Ronksley, P.E.; Jetté, N.; Casha, S.; Riva-Cambrin, J. Preoperative predictors of poor acute postoperative pain control: A systematic review and meta-analysis. BMJ Open 2019, 9, e025091. [Google Scholar] [CrossRef]
- Liu, S.S.; Carpenter, R.L.; Mackey, D.C.; Thirlby, R.C.; Rupp, S.M.; Shine, T.S.; Feinglass, N.G.; Metzger, P.P.; Fulmer, J.T.; Smith, S.L. Effects of perioperative analgesic technique on rate of recovery after colon surgery. Anesthesiology 1995, 83, 757–765. [Google Scholar] [CrossRef]
- Carr, D.B.; Goudas, L.C. Acute pain. Lancet 1999, 353, 2051–2058. [Google Scholar] [CrossRef]
- Breivik, H. Postoperative pain management: Why is it difficult to show that it improves outcome? Eur. J. Anaesthesiol. 1998, 15, 748–751. [Google Scholar] [CrossRef]
- Ready, B.L. Practice guidelines for acute pain management in the perioperative setting. A report by the American Society of Anesthesiologists Task Force on Pain Management, Acute Pain Section. Anesthesiology 1995, 82, 1071–1081. [Google Scholar]
- Tong, D.; Chung, F.; Wong, D. Predictive factors in global and anesthesia satisfaction in ambulatory surgical patients. Anesthesiology 1997, 87, 856–864. [Google Scholar] [CrossRef]
- Perkins, F.M.; Kehlet, H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000, 93, 1123–1133. [Google Scholar] [CrossRef]
- Macrae, W. Chronic pain after surgery. Br. J. Anaesth. 2001, 87, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Bach, A.M.; Forman, A.; Seibaek, L. Postoperative Pain Management: A Bedside Perspective. Pain Manag. Nurs. 2018, 19, 608–618. [Google Scholar] [CrossRef]
- Woldehaimanot, T.E.; Eshetie, T.C.; Kerie, M.W. Postoperative pain management among surgically treated patients in an Ethiopian hospital. PLoS ONE 2014, 9, e102835. [Google Scholar]
- Aubrun, F.; Marmion, F. The elderly patient and postoperative pain treatment. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 109–127. [Google Scholar] [CrossRef]
- Granot, M.; Lowenstein, L.; Yarnitsky, D.; Tamir, A.; Zimmer, E.Z. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiol. J. Am. Soc. Anesthesiol. 2003, 98, 1422–1426. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Somma, J.; Hung, Y.C.; Tsai, P.S.; Yang, C.H.; Chen, C.C. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology 2005, 103, 613–618. [Google Scholar] [CrossRef]
- Huiku, M.; Uutela, K.; van Gils, M.; Korhonen, I.; Kymäläinen, M.; Meriläinen, P.; Paloheimo, M.; Rantanen, M.; Takala, P.; Viertiö-Oja, H.; et al. Assessment of surgical stress during general anaesthesia. Br. J. Anaesth. 2007, 98, 447–455. [Google Scholar] [CrossRef]
- Chen, I.W.; Hung, K.C. Low Body Mass Index may impact the accuracy of Surgical Pleth Index to predict postoperative major pain. Anaesth. Crit. Care Pain Med. 2020, 39, 543–544. [Google Scholar] [CrossRef]
- Ho, C.N.; Fu, P.H.; Chen, J.Y.; Hung, K.C.; Chang, J.H.; Peng, C.K.; Yang, A.C. Heart rate variability and surgical pleth index under anesthesia in poor and normal sleepers. J. Clin. Monit. Comput. 2020, 34, 1311–1319. [Google Scholar] [CrossRef]
- Jiao, Y.; He, B.; Tong, X.; Xia, R.; Zhang, C.; Shi, X. Intraoperative monitoring of nociception for opioid administration: A meta-analysis of randomized controlled trials. Minerva Anestesiol. 2019, 85, 522–530. [Google Scholar] [CrossRef]
- Ledowski, T.; Burke, J.; Hruby, J. Surgical pleth index: Prediction of postoperative pain and influence of arousal. Br. J. Anaesth. 2016, 117, 371–374. [Google Scholar] [CrossRef]
- Ledowski, T.; Sommerfield, D.; Slevin, L.; Conrad, J.; von Ungern-Sternberg, B.S. Surgical pleth index: Prediction of postoperative pain in children? Br. J. Anaesth. 2017, 119, 979–983. [Google Scholar] [CrossRef]
- Choi, B.; Park, C.; Lee, Y.; Shin, H.; Lee, S.; Jeong, S.; Noh, G.J.; Lee, B. Development of a new analgesic index using nasal photoplethysmography. Anaesthesia 2018, 73, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Schneider, M.; Gruenewald, M.; Goyal, R.; Teo, S.; Hruby, J. Surgical pleth index: Prospective validation of the score to predict moderate-to-severe postoperative pain. Br. J. Anaesth. 2019, 123, e328–e332. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.J.; Kim, G.S. Prediction of postoperative pain and analgesic requirements using surgical pleth index: A observational study. J. Clin. Monit. Comput. 2020, 34, 583–587. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Hung, K.-C.; Huang, Y.-T.; Chang, Y.-J.; Yu, C.-H.; Wang, L.-K.; Wu, C.-Y.; Liu, P.-H.; Chiu, S.-F.; Sun, C.-K. Association between Fibrinogen-to-Albumin Ratio and Prognosis of Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1678. [Google Scholar] [CrossRef]
- Hung, K.-C.; Ko, C.-C.; Wang, L.-K.; Liu, P.-H.; Chen, I.-W.; Huang, Y.-T.; Sun, C.-K. Association of Prognostic Nutritional Index with Severity and Mortality of Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1515. [Google Scholar] [CrossRef]
- Takwoingi, Y.; Riley, R.D.; Deeks, J.J. Meta-analysis of diagnostic accuracy studies in mental health. Evid. Based Ment. Health 2015, 18, 103–109. [Google Scholar] [CrossRef]
- Bapteste, L.; Szostek, A.; Chassard, D.; Desgranges, F.; Bouvet, L. Can intraoperative Surgical Pleth Index values be predictive of acute postoperative pain? Anaesth. Crit. Care Pain Med. 2019, 38, 391–392. [Google Scholar] [CrossRef]
- Wang, R.; Deng, Y.; Zhou, S.; Zhang, J. EEG-derived pain threshold index for prediction of postoperative pain in patients undergoing laparoscopic urological surgery: A comparison with surgical pleth index. J. Clin. Monit. Comput. 2021, 35, 1395–1402. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Xiong, J.; Wan, L.; Luo, A.; Wang, X. Continuous analysis of critical incidents for 92,136 postanesthesia care unit patients of a Chinese university hospital. J. PeriAnesthesia Nurs. 2020, 35, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Shajrawi, A.; Henker, R. Predictors of severe postoperative pain after orthopedic surgery in the immediate postoperative period. Int. J. Orthop. Trauma Nurs. 2021, 43, 100864. [Google Scholar] [CrossRef]
- Edgley, C.; Hogg, M.; De Silva, A.; Braat, S.; Bucknill, A.; Leslie, K. Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: A cohort study. Br. J. Anaesth. 2019, 123, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Shahiri, T.S.; Richebé, P.; Richard-Lalonde, M.; Gélinas, C. Description of the validity of the Analgesia Nociception Index (ANI) and Nociception Level Index (NOL) for nociception assessment in anesthetized patients undergoing surgery: A systematized review. J. Clin. Monit. Comput. 2022, 36, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.K.; Chen, I.W.; Tsai, I.T.; Hung, K.C. Association of age with accuracy of surgical pleth index to predict major postoperative pain. Br. J. Anaesth. 2020, 124, e18–e19. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lim, B.G.; Kim, H.; Lee, I.O.; Kong, M.H.; Kim, N.S. Comparison of Surgical Pleth Index-guided Analgesia with Conventional Analgesia Practices in Children: A Randomized Controlled Trial. Anesthesiology 2015, 122, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, M.; Willms, S.; Broch, O.; Kott, M.; Steinfath, M.; Bein, B. Sufentanil administration guided by surgical pleth index vs standard practice during sevoflurane anaesthesia: A randomized controlled pilot study. Br. J. Anaesth. 2014, 112, 898–905. [Google Scholar] [CrossRef]
- Won, Y.J.; Lim, B.G.; Yeo, G.E.; Lee, M.K.; Lee, D.K.; Kim, H.; Lee, I.O.; Kong, M.H. The effect of nicardipine on the surgical pleth index during thyroidectomy under general anesthesia: A prospective double-blind randomized controlled trial. Medicine 2017, 96, e6154. [Google Scholar] [CrossRef]
- Höcker, J.; Broch, O.; Gräsner, J.T.; Gruenewald, M.; Ilies, C.; Steinfath, M.; Bein, B. Surgical stress index in response to pacemaker stimulation or atropine. Br. J. Anaesth. 2010, 105, 150–154. [Google Scholar] [CrossRef]
- Won, Y.J.; Lim, B.G.; Kim, Y.S.; Lee, M.; Kim, H. Usefulness of surgical pleth index-guided analgesia during general anesthesia: A systematic review and meta-analysis of randomized controlled trials. J. Int. Med. Res. 2018, 46, 4386–4398. [Google Scholar] [CrossRef]
- Glasson, J.C.; Sawyer, W.T.; Lindley, C.M.; Ginsberg, B. Patient-specific factors affecting patient-controlled analgesia dosing. J. Pain Palliat. Care Pharmacother. 2002, 16, 5–21. [Google Scholar] [CrossRef]
- De Gregori, M.; Diatchenko, L.; Ingelmo, P.M.; Napolioni, V.; Klepstad, P.; Belfer, I.; Molinaro, V.; Garbin, G.; Ranzani, G.N.; Alberio, G.; et al. Human genetic variability contributes to postoperative morphine consumption. J. Pain 2016, 17, 628–636. [Google Scholar] [CrossRef]
- Yen, C.-R.; Tsou, M.-Y.; Mandell, M.S.; Chan, C.-T.; Chan, K.-H.; Chen, T.H.-H.; Chang, K.-Y. An analysis of patient variables that influence intravenous patient-controlled analgesic use of morphine with quantile regression. Anesthesiol. J. Am. Soc. Anesthesiol. 2010, 112, 688–695. [Google Scholar] [CrossRef]
- Svensson, I.; Sjostrom, B.; Haljamae, H. Influence of expectations and actual pain experiences on satisfaction with postoperative pain management. Eur. J. Pain 2001, 5, 125–133. [Google Scholar] [CrossRef]
- Ledowski, T.; Ang, B.; Schmarbeck, T.; Rhodes, J. Monitoring of sympathetic tone to assess postoperative pain: Skin conductance vs surgical stress index. Anaesthesia 2009, 64, 727–731. [Google Scholar] [CrossRef]
- Thee, C.; Ilies, C.; Gruenewald, M.; Kleinschmidt, A.; Steinfath, M.; Bein, B. Reliability of the surgical Pleth index for assessment of postoperative pain: A pilot study. Eur. J. Anaesthesiol. EJA 2015, 32, 44–48. [Google Scholar] [CrossRef]
- Ledowski, T.; Reimer, M.; Chavez, V.; Kapoor, V.; Wenk, M. Effects of acute postoperative pain on catecholamine plasma levels, hemodynamic parameters, and cardiac autonomic control. Pain 2012, 153, 759–764. [Google Scholar] [CrossRef]
- Abrishami, A.; Chan, J.; Chung, F.; Wong, J. Preoperative pain sensitivity and its correlation with postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2011, 114, 445–457. [Google Scholar] [CrossRef]
- Fillingim, R.B. Sex, gender, and pain: Women and men really are different. Curr. Rev. Pain 2000, 4, 24–30. [Google Scholar] [CrossRef]
- Petrini, L.; Matthiesen, S.T.; Arendt-Nielsen, L. The effect of age and gender on pressure pain thresholds and suprathreshold stimuli. Perception 2015, 44, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Ip, H.Y.V.; Abrishami, A.; Peng, P.W.; Wong, J.; Chung, F. Predictors of postoperative pain and analgesic consumptiona qualitative systematic review. Anesthesiol. J. Am. Soc. Anesthesiol. 2009, 111, 657–677. [Google Scholar]
- Struys, M.M.; Vanpeteghem, C.; Huiku, M.; Uutela, K.; Blyaert, N.B.; Mortier, E.P. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br. J. Anaesth. 2007, 99, 359–367. [Google Scholar] [CrossRef] [Green Version]






| Bapteste (2019) [30] | Choi (2018) [23] | Ledowski (2016) [21] | Ledowski (2019) [24] | Park (2020) [25] | Wang (2021) [31] | |
|---|---|---|---|---|---|---|
| Observational study | Yes | Yes | Yes | Yes | Yes | Yes |
| Patient population | Adult women | Age: 20–80 years | Adult patients | Age: 18–95 years | Age: 20–80 years | Adult patients |
| Age (years) | NA | 54.4 | 43 | 44 | 68 | 58.3 |
| Number of patients | 250 | 120 | 65 | 196 | 49 | 76 |
| Female (%) | 100 | 40 | 70.8 | 42.9 | 30.6 | 22.4 |
| Type of surgery | Gynecologic surgery | Elective surgery | General surgery, n = 48; other surgical specialties n = 17 | General surgery, n = 77; other surgical specialties n = 119 | Liver resection | Urological surgery |
| Risk factors for pain | Female gender | NA | Female gender | NA | Liver resection | NA |
| Exclusion criteria | a,b,c | a,b,c | a,b,c | a,b,c | b,c | a,b,c |
| Outcome parameters (Measure) | Pain severity (VAS) | Pain severity (NRS) | Pain severity (NRS) | Pain severity (NRS) | Pain severity (NRS) | Pain severity (NRS) |
| Definition | VAS > 3 | NRS > 3 | NRS > 3 | NRS > 3 | NRS ≥ 5 | NRS > 3 |
| Incidence (%) | 40 | 74 | 68 | 59 | 63 | 42 |
| Main findings: AUC/Cut-off value | 0.657/53 | 0.703/43.7 | 0.711/30 | 0.601/29 | 0.8419/60 | 0.687/44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, K.-C.; Huang, Y.-T.; Kuo, J.-R.; Hsu, C.-W.; Yew, M.; Chen, J.-Y.; Lin, M.-C.; Chen, I.-W.; Sun, C.-K. Elevated Surgical Pleth Index at the End of Surgery Is Associated with Postoperative Moderate-to-Severe Pain: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2167. https://doi.org/10.3390/diagnostics12092167
Hung K-C, Huang Y-T, Kuo J-R, Hsu C-W, Yew M, Chen J-Y, Lin M-C, Chen I-W, Sun C-K. Elevated Surgical Pleth Index at the End of Surgery Is Associated with Postoperative Moderate-to-Severe Pain: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(9):2167. https://doi.org/10.3390/diagnostics12092167
Chicago/Turabian StyleHung, Kuo-Chuan, Yen-Ta Huang, Jinn-Rung Kuo, Chih-Wei Hsu, Ming Yew, Jen-Yin Chen, Ming-Chung Lin, I-Wen Chen, and Cheuk-Kwan Sun. 2022. "Elevated Surgical Pleth Index at the End of Surgery Is Associated with Postoperative Moderate-to-Severe Pain: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 9: 2167. https://doi.org/10.3390/diagnostics12092167
APA StyleHung, K.-C., Huang, Y.-T., Kuo, J.-R., Hsu, C.-W., Yew, M., Chen, J.-Y., Lin, M.-C., Chen, I.-W., & Sun, C.-K. (2022). Elevated Surgical Pleth Index at the End of Surgery Is Associated with Postoperative Moderate-to-Severe Pain: A Systematic Review and Meta-Analysis. Diagnostics, 12(9), 2167. https://doi.org/10.3390/diagnostics12092167