Abstract
Objectives: The aim of this work was to determine hand joint inflammation in systemic sclerosis (SSc); patients with rheumatoid arthritis (RA) with hand joint involvement were used as controls. Our investigation also aimed at examining the relationship between these subclinical inflammatory changes in the hands, verified by low-frequency MRI, and clinical (especially cardiopulmonary) manifestations, disease activity, and functional capacity in patients with diffuse cutaneous (dcSSc) and limited cutaneous SSc (lcSSc). Methods: Out of 250 SSc patients, the selection included 82 patients with signs and symptoms of joint involvement, and 35 consecutive RA patients. These patients underwent clinical and laboratory investigations, and hand X-ray and MRI of the dominant hand. Synovitis/tenosynovitis, bone edema, and erosions were investigated, and the bone changes were quantified and scored using the RAMRIS method. HAQ index, modified Rodnan skin score, examination of internal organ involvement, and serological markers for SSc, as well as rheumatoid factor (RF) and cyclic citrullinated peptides antibodies (ACPA), were performed on all experimental group subjects. Results: MRI of the dominant hand showed a significantly higher number of cases with synovitis (78%) than the number of patients with clinically swollen joints (17.1%; p < 0.001); bone edema was found in 62 (75.6%) SSc patients. MRI also showed a higher number of erosions (52; 63.4%) compared to those (22; 27.5%) detected with X-ray (p < 0.001). The average values of the total MRI score of synovitis/edema and erosions in the wrist (p < 0.001) and MCP joints (p < 0.001) were statistically higher in RA than in SSc patients (p < 0.001). The probability of the MRI-detected inflammatory changes was considerably higher in SSc patients who had vascular complications (digital ulceration, OR = 4.68; 95% IP: 1.002–22.25; p < 0.05), in patients with more severe functional impairment (OR = 8.22; 95% IP: 1.74–38.89; p < 0.01), and in patients with active disease (OR = 3.132; 95% IP: 1.027–9.551; p < 0.05). In our investigation, patients with a limited form of the disease and with inflammatory changes on MR more often had higher functional impairment compared to the other group without MRI inflammation. Conclusions: Our data show that in SSc MRI can detect a significant subclinical joint inflammation. RAMRIS confirmed the high degree of joint inflammation in RA, but also revealed a great deal of joint inflammation in SSc. That inflammation is associated with systemic inflammation (disease activity), vascular complications, and more severe forms of the disease, as synovitis cannot be precisely diagnosed by the clinical examination of joints. These results suggest that a careful joint investigation is necessary in SSc, and that in symptomatic patients, MRI may identify joint inflammation. In clinical practice, this evidence might drive to an early targeted therapy, thus preventing joint erosions.
1. Introduction
Systemic sclerosis (SSc) is a complex autoimmune disease characterized by vasculopathy, multiorgan fibrosis, and immune dysregulation [1].
In SSc, musculoskeletal manifestations (MSM) are frequently observed; in particular, arthralgias, arthritis, contractures, tendon friction rubs (TFRs), tenosynovitis, as well as myalgias, muscle tenderness, and myositis [2]. In this context, the assessment of arthritis may be challenging due to the presence of skin edema, thickening, or tethering, as well as the presence of digital ulcers [3], subcutaneous calcinosis [4], and atrophic contractures [2,5]. Earlier in the disease, clinical examination and laboratory and X-ray findings are neither sensitive nor specific enough to provide relevant information of joint inflammation. In practice, X-rays may detect late erosions as a consequence of synovitis, but cannot show the synovial inflammation or the early-stage degeneration of the cartilage and bones [5,6]. Today, we know that magnetic resonance imaging (MRI) is more sensitive and precise for assessing inflammatory joint changes to the hand when compared to clinical examination and X-ray findings in patients with rheumatoid arthritis (RA) [7]. A study on a small number of SSc patients has suggested the role of MRI in identifying and quantifying joint inflammation. Therefore, the use of MRI in SSc may be meaningful for the early detection of inflammatory changes in hand joints in order to provide early treatment. In fact, MRI may detect much earlier bone modifications that cannot be identified with X-rays, visualizing the inflammation in the synovial membrane, bursae, tendons, and ligaments. Furthermore, this method can be used for quantifying either cartilage loss and synovial membrane and the volume of synovial fluid, which are useful for monitoring the disease progression and therapeutic efficacy [7,8,9,10].
Our aim was to evaluate the presence of subclinical joint inflammation (synovitis, tenosynovitis, bone edema), as well as bone erosions and acro-osteolysis, on the dominant hand of symptomatic SSc patients. Furthermore, we wanted to establish the relationship between these subclinical inflammatory changes in the hands, verified by low-frequency MRI, and clinical disease activity (especially cardiopulmonary manifestations), as well as functional capacity, in patients with diffuse cutaneous (dcSSc) and limited cutaneous SSc (lSSc).
2. Patients and Methods
Out of 250 SSc patients followed up at the Rheumatology Clinic, Institute Niška Banja, Serbia, classified according to ACR/EULAR criteria [11], without clinical features of overlap syndromes or mixed connective tissue disease (MCTD), 82 patients (77 females, average age 54.4 ± 12.6, and disease duration 7.41 ± 6.12 years) complaining of hand arthralgia and/or tendon friction rub, swollen joints, and contractures were selected and divided into subsets [12] (59 limited cutaneous SSc and 23 diffuse cutaneous SSc). The patients positive for cyclic citrullinated peptides antibodies (ACPA) and rheumatoid factor (RF) were separated from the other negative SSc patients. A group of 35 RA patients (28 females, average age 54.28 ± 9.6, and average disease duration 6.8 ± 5.8 years) who met the 2010 RA ACR/EULAR criteria [13] were used as control. All patients provided a signed informed consent, and the study was approved by the Local Medical Ethical Committee of the Institute for Treatment and Rehabilitation, Niška Banja, and Faculty of Medicine, University of Niš, Serbia.
All SSc patients were scored with the Revised 2017 EUSTAR disease activity index (EScSG-AI criteria) [14] to detect systemic disease activity. Functional status was defined based on the Health Assessment Questionnaire (HAQ). Internal organ involvement was defined based on specific criteria [15], whereas muscle involvement was evaluated based on the presence of muscle tenderness and/or increased value of creatine phosphokinase (CPK). Skin involvement was measured with the modified Rodnan skin score [16], and the presence of digital ulcers and pitting scars was also recorded. Antinuclear (ANA) and anticentromere (ACA) antibodies were determined by means of indirect immunofluorescence on HEP-2 cells (Immunoconcepts, Sacramento, CA, USA). Titer 1:40, i.e., titer which was in practice recommended as “screening”, was taken as limit ANA titer (“cut off” titer, i.e., titer representing the lowest value accepted as positive test result). Anti-topoisomerase antibodies (ATA) were determined using the CIE method (Counter Immunoelectrophoresis), Imtec, Immunodiagnostics, Berlin, Germany. All patients were tested for the presence of rheumatoid factor (RF) for values > 20 IU/L, which were considered positive. The ELISA (enzyme-linked immunosorbent assay) method, Imtec, was used to determine the presence of anti-CCP antibodies, considered positive when exceeding > 25 U/mL. All RA patients were scored for disease activity with the DAS28SE.
Standard hand and wrist X-ray was performed in all patients with SSc and RA. The changes were evaluated by a radiologist and rheumatologist blinded to clinical and serological patient data. The following features were investigated: focal or diffuse joint space narrowing, erosions (cortical bony surface discontinuity), juxta-articular or generalized osteoporosis and acro-osteolysis, tissue calcifications, and flexion contractures (in these cases, joint space narrowing at proximal interphalangeal (PIP) joints and distal interphalangeal (DIP) joints were not analyzed).
In SSc and RA patients, MRI joint visualization was conducted with a 0.2T Artroscan MRI Unit (Esaote Biomedica, Genoa, Italy) with special surface coil. Aside from an axial and coronal view of standard T1W and T2W sequences, a specially created fat-suppression sequence, STIR (Short Time Inversion Recovery), was used. The stated MRI sequences on dominant hand [17] were used to scan metacarpophalangeal (MCP) joints, i.e., from the second to the fifth, as well as the metacarpal base of all carpal bones, distal radius, and ulna. A contrast agent (gadolinium) IV bolus injection 0.1 mmol/kg (Gadopentate Dimeglumine-Magnevist®, Gd DTPA, Schering) was used for differentiating the intensity of the synovial signal. MRI visualization results were analyzed by the OMERACT (Outcome Measures in Rheumatology) RAMRIS (Rheumatoid Arthritis Magnetic Resonance Imaging Scoring System) method [7,18]. Two radiologists, blinded to disease diagnosis, interpreted the results, and gave their professional opinion on the presence of synovitis, tenosynovitis, and bone lesions.
2.1. Inflammation Assessment Using MRI
The assessment of inflammation with the MRI scan allowed the scoring of synovitis, bone lesions (bone erosions and bone edema), and the presence of tenosynovitis (Supplementary Figures S1–S4).
2.1.1. Synovitis Score
Synovitis detected on MRI was scored after contrast-enhanced signal intensity in the synovial tissue at T1W SE (spin echo) and STIR sequence: the score range was from 0 to 3, with 0 being normal synovial tissue, whereas score 1, 2, and 3 indicated mild, moderate, and severe synovial thickening (thicker than joint capsule), respectively. The total score was monitored for each of the 4 MCP joints (excluding MCP 1), the total sum being from 0 to 12.
The total score for the wrist was monitored in three areas: radiocarpal, radioulnar, and carpometacarpal joints (range 0–9).
2.1.2. Bone Lesion Score
Bone erosions were assessed on a two-plane MRI scan. Erosions implied cortical bone interruptions at minimum on one plane, and were scored from 0 to 10 depending on the size and volume of trabecular bone loss. Erosion assessment was also conducted on the wrist bones and MCP joints, i.e., from the second to the fifth joint.
Bone edema was assessed either as an isolated condition or as a condition defined by gradually blurring edges that surrounded the erosion with a high signal intensity at the T2W STIR MRI sequence.
Bone erosion and edema were assessed at 15 locations (distal end of the radius; the head of the ulna; wrist bones: scaphoid bone, lunate bone, triquetral bone, pisiform bone, trapezium, trapezoid bone, capitate bone, hamate bone; at bases; from the first to the fifth metacarpal bone; and at eight locations (metacarpal heads and phalanx bases from the second to the fifth MCP joint). The total score of carpal bone erosions ranged from 0 to 150, whereas the score for MCP joints was 0–80. The total edema score was 0–24 for MCP joints and 0–45 for carpal bones.
2.1.3. Assessment of Tenosynovitis
The MRI scan was evaluated based on the presence of a high-intensity signal at the T2W STIR sequence followed by appropriate localization. After administration of the gadolinium-based contrast agent, tenosynovitis was confirmed in T1W SE sequences: 5 tendon groups (extensor pollicis longus and brevis, extensor carpi radialis longus and brevis, extensor digitorum, extensor digiti minimi, and extensor carpi ulnaris) in the wrist, i.e., analyzed at dorsal side of the joint; and 2 tendon groups (flexor carpi radialis, flexor digitorum profundus, and superficialis) analyzed at the palmar side. The extensor and flexor tendon groups in MCP joints, i.e., from the first to the fifth MCP joint at the dorsal and palmar side of the joint, were assessed.
2.2. Statistical Analysis
Statistical parameters used for data overview included arithmetic means ± standard deviation (S.D.), medians, and index structure (%). The average values of continuous variables in the two tested groups were compared by means of Student’s t-test or Mann–Whitney test (depending on data distribution normality for continuous variables). Fisher’s exact test was used for comparing the frequency of category features. Mutual dependence between the values of different characteristics was assessed by applying Pearson’s correlation coefficient (r). Student’s t-test was used for testing the statistical significance of correlation coefficients. The assessment of the association between the examined factors and the probability of the detection of inflammatory changes, synovitis, bone edema, erosions, and tenosynovitis in the subjects of the experimental group was performed by logistic regression analysis. The approximate values of relative risk were calculated (odds ratio—OR), as well as their 95% confidence interval. The estimation of the statistical significance of the OR value was performed by calculating the Wald value. Significance was set at p < 0.05.
The results of statistical analysis were shown in tables and graphs. Calculations were performed using SPSS software, V 23.0.
3. Results
According to the EUSTAR disease activity index, 47/82 (57.3%) SSc patients had an active disease, 12 had a positive RF (14.6%), and 11 had a positive ACPA. The characteristics of SSc patients are shown in Table 1.

Table 1.
Characteristics of patients with systemic sclerosis (SSc).
Out of 82 SSc patients, swollen joints were detected in 14 (17.1%), arthralgia in 66 (80.5%), tendon friction rubs in 12 (14.6%), and joint contractures in 28 (34.1%) patients.
A positive correlation was found between ACPA-positive patients and swollen joints (p < 0.0001) and also bone erosions (p < 0.0002).
Out of 35 RA patients, 26 (74.3%) were RF-positive, whereas 27 (77.1%) were ACPA-positive. In the same group, 20 (57.1%) patients had C-reactive protein (CRP) > 6 mg/dL. The average DAS 28-SE was 5.2 ± 1.34 (range: 2.1 to 7.2).
Overall, the clinical and MRI findings were compatible in 14 (17.1%) patients with positive and in 18 (22.0%) patients with negative findings (total of 32 (39.1%) patients), whereas 50 (61.0%) patients had a clearly positive MRI, but negative clinical findings (Table 2).

Table 2.
Prevalence of synovitis in clinical findings compared to MRI findings.
Comparing MRI Scan Results with Clinical and Radiographic Musculoskeletal Manifestations in Patients with Systemic Sclerosis
Among 82 SSc patients, MRI showed the presence of synovitis in a very high number of patients (78.0%), which was much more significant than what was observed with the clinical analysis (17.1%; chi-squared test: p < 0.001). On the other hand, tendon friction rubs, clinically detected in 14.6% of patients, were confirmed by MRI in 14.1% of patients (p = 0.375). The number of clinically detected joint contractures (34.1%) was very close to that seen at X-ray (32.1%, p = 0.617). On X-rays, the erosions were observed in 27.5% of patients, whereas with MRI, they were more frequently detected (in 63.4% patients, p < 0.001). Similarly, on the wrist (51.2 vs. 11.5%, respectively; p < 0.001) and on MCP joints (45.1 vs. 9.1%, respectively, p < 0.001), MRI was able to more frequently detect erosions than X-ray.
On X-ray, acro-osteolysis was found in 17 (21.3%) patients, and with MRI, in 22 (27.5%) patients (p = 0.357) (Table 3).

Table 3.
Musculoskeletal manifestations in systemic sclerosis assessed by clinical findings, radiographic findings, and magnetic resonance imaging (MRI).
No statistical difference was found between the total MRI score of synovitis, edema, and erosions in the wrist and MCP joints of the dominant hand between lcSSc and dcSSc (Supplementary Table S1).
The average value of the total MRI score of synovitis, edema, and erosions in the wrist of the dominant hand was statistically more significant in RA than in SSc patients (p < 0.001). Similarly, the average values of the total MRI score of synovitis, edema, and erosions in MCP joints of the dominant hand were statistically much higher in RA than in SSc patients (p < 0.001) (Table 4).

Table 4.
Average values of total MRI score of synovitis, edema, and erosions in the wrist and MCP joints of dominant hand in SSc and RA.
In the wrist, the localization of the highest total score of synovitis, either in SSc or RA, was detected in the distal radio-ulnar joints (DRUJ), and as expected, edema was significantly higher in RA than in SSc patients (p < 0.001). In SSc, the highest total score of erosions and edema was localized in the capitate bone area, whereas in RA patients, it was localized in the lunate bone, still statistically more numerous than in SSc patients (p < 0.001). In MCP2/3/5 joints, the highest total score of synovitis (p < 0.01) and erosion score were higher in RA patients (p < 0.001) (Table 5).

Table 5.
Localization of the highest total MRI score in the wrist and MCP joints of dominant hand in patients with SSc and RA.
The number of patients with synovitis (100.0 vs. 63.4%), erosions (94.3 vs. 51.2%), and bone edema (100.0 vs. 61%) in the dominant hand wrist was significantly higher in RA (p < 0.001), as well as the number of patients with inflammatory changes (synovitis 100.0 vs. 58.5%: erosion 91.4 vs. 45.1%; bone edema 97.1 vs. 56.1%, at MCP joints (p < 0.001)).
In SSc patients, MRI showed erosions in 52 (63.4%) patients, whereas X-ray showed erosions in 22 (27.5%) patients only (chi-squared test: p < 0.001): 18 (22.5%) patients had both positive X-ray and MRI findings, whereas 26 (32.5%) patients had both of these findings as negative. In four patients (5.1%), erosions were detected by X-ray only, whereas in 32 (40.0%) patients, erosions were found only by MRI. In RA, MRI confirmed erosions in 34 (97.1%) patients, whereas upon X-ray, erosions were found in six (17.1%) patients only (chi-squared test: p < 0.001) (Table 6).

Table 6.
Prevalence of erosions in MRI compared to radiography in SSc and control RA groups.
Out of 65 patients with SSc in whom inflammation was detected by MRI, univariate logistic regression analysis showed digital ulcerations in 25 (38.5%) cases, which was a significantly higher frequency compared to that in patients without inflammation (2 out of 17 patients; 11.8%; OR = 4.68; 95% IP: 1.002–22.25; p < 0.05). Patients with inflammation were significantly more likely to have HAQ values greater than 1.5 (52.3 vs. 11.8%; OR = 8.22; 95% IP: 1.74–38.89; p < 0.01), but also an active disease (OR = 3.132; 95% IP: 1.027–9.551; p < 0.05), as compared to the group without inflammation (Table 7).

Table 7.
Assessment of the association between the examined factors and probability of MRI-detected inflammatory changes in subjects with SSc; results of univariate logistic regression analysis.
Patients with acro-osteolysis had a statistically significant higher erosion score compared to those without acro-osteolysis (20.68 ± 34.17 vs. 6.85 ± 10.24, p < 0.05), and also had a statistically significant higher disease activity (5.23 ± 1.74 vs. 3.43 ± 1.77 p < 0.0001) (Supplementary Table S2).
Out of the 46 patients with lSSc in whom inflammation was verified by MRI, 21 (45.7%) patients had HAQ values higher than 1.5, which was significantly more often than in patients without inflammation (1 out of 13 patients; 7.7%; OR = 10.08; 95% IP: 1.20–84.05; p < 0.05). The differences in values and frequency of all examined characteristics in subjects with dSSc with and without MRI-detected inflammation were not statistically significant. The results were obtained by multivariate logistic regression analysis (Table 8).

Table 8.
Characteristics of the disease in SSC patients depending on the presence of MRI-detected inflammation.
A significant positive correlation between synovitis score (r = 0.371, p < 0.001), erosion score (r = 0.229, p < 0.001), and edema score (r = 0.279, p = 0.016) was found on MRI and EScSG-AI (Figure 1) in all SSc patients. However, no significant correlation between RAMRIS score and Rodnan skin score, disease duration, disease subset, and antibodies was found in SSc.
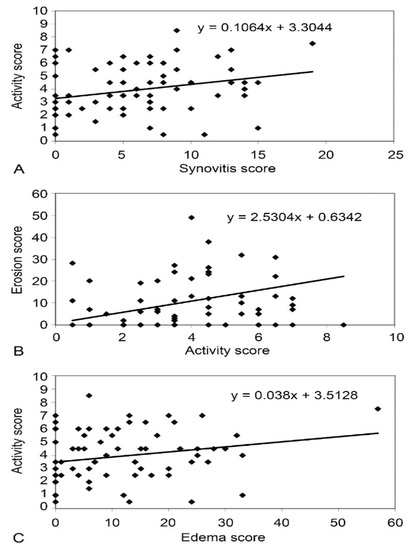
Figure 1.
Correlation between synovitis score and EScSG-AI in patients with SSc (A), between EScSG-AI and erosion score in SSc group (B), and between edema score and EScSG-AI in patients with SSc (C).
In RA, a significant correlation was confirmed between synovitis score, erosion score (r = 0.698 and p < 0.001), and edema (r = 0.622 and p < 0.001) on MRI and disease activity score, DAS 28SE (r = 0.344 and p < 0.05).
4. Discussion
Our data show that in SSc, inflammatory joint involvement is a rather frequent subclinical manifestation in symptomatic patients [19,20,21,22]. In our patients, the clinical and X-ray evaluation was not sensitive enough to identify the whole range of inflammatory changes either at the articular or periarticular level. In the literature, MRI was found to accurately detect soft tissue inflammation, proving it to be more sensitive than ultrasonography for identifying inflammation in patients with arthralgia without evident signs of synovitis [23,24]. In our patients, the contrast (gadolinium) provided significant visualization of inflamed synovial membrane, and it also showed evidence of the edema of the bone and soft tissue structures.
For assessing erosions in extremities, low-frequency MRI is considered equivalent to high-frequency MRI, but it is less sensitive in assessing bone edema, and requires the use of a contrast agent for the adequate detection of synovitis [7,17]. Unlike other methods, which include full-body scan, low-frequency MRI has numerous advantages, such as flexible patient positioning, affordable price, and reduced claustrophobia. The disadvantages of this method are reduced spatial resolution and a limited number of possible imaging techniques compared to full-body scan [25,26,27].
Our aim was to determine the capacity of low-frequency MRI to identify inflammatory hand changes in SSc patients, correlating the results with clinical and X-ray findings, and comparing the results with those obtained in RA patients. Furthermore, we wanted to establish the relationship between these subclinical inflammatory changes and clinical disease activity, as well as the functional capacity in patients with dcSSc and lSSc.
The results of this study have shown a surprisingly significant number of inflammatory changes in the hands of SSc patients. Based on clinical examination, synovitis was suspected in 14/82 patients (17.1%) patients, but MRI was able to detect synovitis in 64/82 (78%) patients that were apparently clinically negative despite being symptomatic. The fact that 61% of our patients with positive MRI findings did not have positive clinical findings of synovitis definitively stresses the important role of MRI for diagnosing synovitis in SSc. Moreover, MRI could detect erosions in a significant number of patients (52/82 patients (63.4%)), compared to 22/82 (27.5%) detected by X-ray. Moreover, 40% of SSc-positive patients detected by MRI did not have any positive X-ray finding.
It is clear that MRI is an added imaging tool which may show inflammatory joint modifications when the clinical and X-ray findings are still negative. In practice, this evidence may help to design a targeted disease-modifying therapy to prevent the progression of joint damage and functional impairment in SSc patients as well [28,29,30,31,32,33].
Previously, the presence of MRI-detected erosive arthritis was confirmed in 41% of SSc patients [34], showing that MRI was more sensitive in assessing erosions than X-ray. The authors concluded that it was not clear if the erosive changes could be related to the overlap between SSc and RA or if they were merely the result of inflammatory changes in SSc. However, the selected group consisted of patients with clinical findings of arthritis, accompanied by serology which pointed to RA (RF+, ACPA+) [35,36]. Allanore et al. [37] examined hand vascular involvement by MRI angiography in 38 SSc patients, and confirmed that erosions were present in 16% of patients, whereas synovitis and tenosynovitis were recorded in 50% and 11% of patients, respectively. The possible explanation of the lower frequency of erosions in the French study could be due to the shorter duration of the disease in the SSc group (in 42% of cases, the disease lasted less than 5 years). Using a high-frequency MRI, Akbayrak et al. [25] found a significantly higher percentage of erosions (87%), similar to Chitale et al. (75%) [23] and to our study (63.4%).
Acro-osteolysis is the most common bone disorder in SSc, which occurs in 20–25% of patients [38]. Acro-osteolysis may be diagnosed by physical examination and X-ray (the gold standard for the detection), and recent studies have confirmed a comparable sensitivity of X-ray and ultrasonography (US) [39,40]. Our study showed a similar sensitivity of X-ray (17, 21.3%) and MRI (22, 27.5%) in detecting acro-osteolysis. Research on the prevalence of acro-osteolysis and its association with vascular changes and other systemic manifestations pointed out the link with a severe systemic disease, pulmonary arterial hypertension, digital ulcers, and late capillaroscopic pattern [41,42,43]. In agreement with the literature, our data confirm that SSc patients with acro-osteolysis had a significantly higher disease activity.
In our patients, MRI was significantly more sensitive for diagnosing synovitis, joint effusion, and tenosynovitis than clinical examination. Previously, low-frequency MRI of dominant hand has shown synovitis in all eight (100%) investigated SSc patients, whereas tenosynovitis was confirmed in 88% of patients [23]. An extremely high percentage of synovitis (87.5%), tenosynovitis (81.3%), and erosions (62.5%) was also detected [24], which is almost identical to our study. In our patients, MRI identified synovitis in 78% of the cases, and this result is close to that obtained by Akbayrak al (68%) [25]. The frequency of tenosynovitis seen in our study (14%) was similar to previous results (11%) [37].
It is well known that MRI is the only tool for detecting bone edema, which, as proved in RA, represents a pre-erosive change characterized by osteitis (cellular inflammatory infiltrate in subchondral bone, which can be a precursor of pre-erosive lesions) [44,45]. In our cases, we could quantify inflammatory changes and compare the degree of hand inflammation in SSc vs. RA patients with the RAMRIS, thus scoring the MRI-detected inflammatory changes in wrists and MCP joints (bone edema score, synovitis score, and erosion score) [46,47]. In our RA patients, bone edema occurred more frequently (p < 0.001) and had a higher statistically significant score (p < 0.001) in wrists and MCP joints than in SSc patients. Moreover, bone edema (seen 75.6% of patients) correlated with CRP in SSc patients, which might also be regarded as a marker of joint involvement in SSc. It still remains to be determined whether bone edema could be a marker pointing to a more aggressive disease requiring prompt treatment also, because MRI detected a significant percentage of synovitis in those patients that were clinically/radiologically negative. This evidence strongly suggests that MRI might be promptly used as a screening tool for subclinical hand inflammation in symptomatic patients only, in order to achieve an early diagnosis and treatment.
The localization of inflammatory changes found in the wrist and MCP joints of SSc patients is in agreement with previous studies [48]. Low et al. [34] confirmed the most frequent localization of synovitis in the radioulnar joint in SSc patients, whereas the study by Akbayrak et al. [25] showed the presence of synovitis in intercarpal joints. In our SSc patients, we recorded the highest percentage of erosions and bone edema in the capitate bone, whereas Akbayrak et al. [25] documented the highest percentage in the lunate bone.
Our research has shown that the probability of the MRI-detected inflammatory changes was considerably higher (over four times) in SSc patients who had vascular complications (digital ulcerations), in patients with more severe functional impairment (HAQ values > 1.5), and in patients with active disease, which was one of the most important findings. In our study, patients with a limited form of the disease, with inflammatory changes on MR, more often had higher functional impairment compared to the other group without MR inflammation.
The data from EUSTAR Database (2010) also show that synovitis, joint contracture, and tendon friction rubs are associated with a more severe disease and with systemic inflammation [20].
Concerning the prevalence of synovial involvement in SSc determined by power Doppler ultra-sonography, a recent study has shown that 32% of 103 patients had ultrasonographic tenosynovial involvement. Inflammatory synovitis was more frequent in the wrist and MCP joints, which is in line with our results. Sclerosing tenosynovitis (TS) was more frequent in men, and was associated with anti-RNA polymerase III antibodies, diffuse SSc, interstitial lung disease, and inflammatory arthralgia [49]. The data from an interesting, prospective, cross-sectional study, using grayscale and Doppler musculoskeletal ultrasound, show that the Scleroderma Health Assessment Questionnaire–Disability Index correlated with musculoskeletal ultrasound erosions. The skin score correlated with tendinitis grayscale [50].
In a prospective follow-up of 9165 EUSTAR patients, using univariate analysis, synovitis and tendon friction were identified as precursors of the progression of skin changes. Multivariate analysis showed that, with regard to disease subsets and the status of antibodies, synovitis and tendon friction were also the precursors of the progression of skin changes and cardiovascular manifestations, as well as the occurrence of ischemic digital ulcers, left chamber dysfunction, and renal crisis [51].
Our study has a number of limitations. Only symptomatic patients were included in the study. We recommend that further studies involve a group of patients without joint symptoms. The major limitation is that, being a cross-sectional study, it included patients at various stages of the disease. There is no possibility for the prospective monitoring of disease activity or therapeutic effect, which should be our goal in the coming period. The lack of a control group of healthy people is another limitation. A comparison with a control group of healthy people would be more adequate and would give more effective conclusions.
5. Conclusions
Our data show that there is a high subclinical joint inflammation in SSc, and that MRI was more sensitive than clinical and radiographic examinations in detecting the severity and degree of synovitis, bone edema, erosions, and tenosynovitis.
The importance of our research for the detection of MRI-verified inflammation of the dominant hand joints in SSc patients lies in the finding that inflammation is associated with systemic inflammation (disease activity), vascular complications, and more severe forms of the disease accompanied by interstitial lung disease, as synovitis cannot be precisely diagnosed by clinical examination of the joints. A significant correlation between MRI-detected synovitis, bone edema and erosion scores, and disease activity in SSc may point to the mechanisms of inflammatory changes in joints and their mutual dependence in terms of joint involvement and functional disability. The detection of the subclinical inflammation of hands in SSc using MRI scan has highlighted the need to apply this method in patients with specific symptomatology, all with the aim of initiating early contemporary treatment.
In conclusion, the problem of joint involvement in SSc should be more frequently addressed, and MRI should be employed as a standard of care in symptomatic patients to guide the therapy to target this neglected manifestation of the disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12092165/s1, Figure S1: Bone edema in wrist presented as the zone of high signal values at T2w STIR cor SE sequence (A); erosive lesions at left hand MCP IV and bone edema in PIP II with perivascular component at T2w STIR cor SE sequence in patient with SSc (B); Figure S2: Large volume of synovial fluid in the tendon sheaths of the flexor tendons seen when passing through the carpal tunnel in patient with SSc: coronal plane STIR (A); Axial plane STIR (B); Figure S3: CMC I subluxation with erosion on the articular facets, small amount of fluid inside the articular capsule and edema in the subchondral bone of both articular processes in patient with SSc; Figure S4: Deep chondro-subchondral cyst on the articular facet of the head of the IV metacarpal bone in patient with SSc; STIR and T1 coronal plane; Table S1: Average values of total MRI score of synovitis, edema and erosions in wrist and MCP joints of dominant hand in SSc and SSc subtypes; Table S2: Association of acro-osteolysis in hand MRI with erosion score and disease activity index (EScSG-AI) in patients with SSc.
Author Contributions
B.S.—study design, wrote the manuscript, corresponding author. S.S.—methodology. V.Z. and N.R.—formed data bases for analysis. D.D.—statistical analysis, checked the reference list. M.B.—preparation of tables, figures, and supplementary materials. N.D.—internal revision of the whole manuscript. A.S.—evaluation of the whole manuscript. M.M.C.—final approval of the manuscript version to submit. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Medical Ethical Committee of the Institute for Treatment and Rehabilitation Niška Banja and Medical Faculty, University of Niš, Serbia (decision dated 21 March 2019).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations
| ACA | Anticentromere antibodies; |
| ACPA | Anti-citrullinated protein antibodies; |
| ANA | Antinuclear antibodies; |
| ATA | Anti-topoisomerase antibodies; |
| CPK | Creatine phosphokinase; |
| DIP | Distal interphalangeal; |
| DRUJ | Distal radio-ulnar joints; |
| EScSG-AI | European Scleroderma Study Group Activity Index; |
| MCP | Metacarpophalangeal; |
| MRI | Magnetic resonance imaging; |
| MSM | Musculoskeletal manifestations; |
| OMERACT | Outcome Measures in Rheumatology; |
| PIP | Proximal interphalangeal; |
| RA | Rheumatoid arthritis; |
| RAMRIS | Rheumatoid Arthritis Magnetic Resonance Imaging Scoring System; |
| RF | Rheumatoid factor; |
| SSc | Systemic sclerosis; |
| MCTD | Mixed connective tissue disease; |
| STIR | Short Time Inversion Recovery. |
References
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Sandler, R.D.; Matucci-Cerinic, M.; Hughes, M. Musculoskeletal hand involvement in systemic sclerosis. Semin. Arthritis Rheum. 2020, 50, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Amanzi, L.; Braschi, F.; Fiori, G.; Galluccioz, F.; Miniati, I.; Guiducci, S.; Conforti, M.-L.; Kaloudi, O.; Nacci, F.; Sacu, O.; et al. Digital ulcers in scleroderma: Staging, characteristics and sub-setting through observation of 1614 digital lesions. Rheumatology 2010, 49, 1374–1382. [Google Scholar] [CrossRef]
- Herrick, A.L.; Gallas, A. Systemic sclerosis-related calcinosis. J. Scleroderma Relat. Disord. 2016, 1, 194–203. [Google Scholar] [CrossRef]
- Avouac, J.; Guerini, H.; Wipff, J.; Assous, N.; Chevrot, A.; Kahan, A.; Allanore, Y. Radiological hand involvement in systemic sclerosis. Ann. Rheum. Dis. 2006, 65, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Mogavero, G.; Guerini, H.; Drapé, J.L.; Mathieu, A.; Kahan, A.; Allanore, Y. Predictive factors of hand radiographic lesions in systemic sclerosis: A prospective study. Ann. Rheum. Dis. 2011, 70, 630–633. [Google Scholar] [CrossRef]
- Østergaard, M.; Edmonds, J.; McQueen, F.; Peterfy, C.; Lassere, M.; Ejbjerg, B.; Bird, P.; Emery, P.; Genant, H.; Conaghan, P. An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann. Rheum. Dis. 2005, 64, i3–i7. [Google Scholar] [CrossRef]
- Schirmer, C.; Scheel, A.K.; Althoff, C.E.; Schink, T.; Eshed, I.; Lembcke, A.; Burmester, G.-R.; Backhaus, M.; Hamm, B.; Hermann, K.-G.A. Diagnostic quality and scoring of synovitis, tenosynovitis and erosions in low-field MRI of patients with rheumatoid arthritis: A comparison with conventional MRI. Ann. Rheum. Dis. 2007, 66, 522–529. [Google Scholar] [CrossRef]
- Eshed, I.; Althoff, C.E.; Schink, T.; Scheel, A.K.; Schirmer, C.; Backhaus, M.; Lembcke, A.; Bollow, M.; Hamm, B.; Hermann, K.-G.A. Low-field MRI for assessing synovitis in patients with rheumatoid arthritis. Impact of Gd-DTPA dose on synovitis scoring. Scand. J. Rheumatol. 2006, 35, 277–282. [Google Scholar] [CrossRef]
- Duer-Jensen, A.; Ejbjerg, B.; Albrecht-Beste, E.; Vestergaard, A.; Døhn, U.M.; Hetland, M.L.; Østergaard, M. Does low-field dedicated extremity MRI (E-MRI) reliably detect bone erosions in rheumatoid arthritis? A comparison of two different E-MRI units and conventional radiography with high-resolution CT scanning. Ann. Rheum. Dis. 2009, 68, 1296–1302. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Neal, S.B.; Gerd, R.B.; Vivian, P.B.; Marc, D.C.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Valentini, G.; Iudici, M.; Walker, U.A. The European Scleroderma Trials and Research Group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: Derivation and validation of a preliminarily revised EUSTAR activity index. Ann. Rheum. Dis. 2017, 76, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Black, C.M.; Matucci-Cerinic, M.; Guillevin, L. Progress in systemic sclerosis: A 10-year perspective. Rheumatology 2009, 48, iii1–iii2. [Google Scholar] [CrossRef][Green Version]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Dis. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Ejbjerg, B.J.; Narvestad, E.; Jacobsen, S.; Thomsen, H.S.; Ostergaard, M. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: Comparison with conventional high field MRI and radiography. Ann. Rheum. Dis. 2005, 64, 1280–1287. [Google Scholar] [CrossRef]
- Ejbjerg, B.; McQueen, F.; Lassere, M.; Haavardsholm, E.; Conaghan, P.; O’Connor, P.; Bird, P.; Peterfy, C.; Edmonds, J.; Szkudlarek, M.; et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: The wrist joint. Ann. Rheum. Dis. 2005, 64, i23–i47. [Google Scholar] [CrossRef]
- Avouac, J.; Clements, P.J.; Khanna, D.; Furst, D.E.; Allanore, Y. Articular involvement in systemic sclerosis. Rheumatology 2012, 51, 1347–1356. [Google Scholar] [CrossRef]
- Avouac, J.; Walker, U.; Tyndall, A.; Kahan, A.; Matucci-Cerinic, M.; Allanore, Y. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: Results from the EULAR Scleroderma Trial and Research Group (EUSTAR) database. J. Rheumatol. 2010, 37, 1488–1501. [Google Scholar] [CrossRef]
- Bellando-Randone, S.; Guiducci, S.; Matucci-Cerinic, M. Musculoskeletal involvement in systemic sclerosis. Best Pract. Res. Clin. Rheumatol. 2008, 22, 339–350. [Google Scholar] [CrossRef]
- Molina-Rios, S.; Ordonez, C.E.; Quintana-Lopez, G. Osteoarticular manifestations of systemic sclerosis: A systematic review of the literature. Rev. Colomb. Reumatol. 2020, 27, 85–110. [Google Scholar] [CrossRef]
- Chitale, S.; Ciapetti, A.; Hodgson, R.; Grainger, A.; O’Connor, P.; Goodson, N.J.; Thompson, R.N.; Estrach, C.; Moots, R.J.; Grassi, W.; et al. Magnetic resonance imaging and musculoskeletal ultrasonography detect and characterize covert inflammatory arthropathy in systemic sclerosis patients with arthralgia. Rheumatology 2010, 49, 2357–2361. [Google Scholar] [CrossRef]
- Abdel-Magiedt, R.A.; Lotfi, A.; Abdelgawad, E.A. Magnetic resonance imaging versus musculoskeletal ultrasonography in detecting inflammatory arthropathy in systemic sclerosis patients with hand arthralgia. Rheumatol. Int. 2013, 33, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Akbayrak, E.; Dinser, R.; Müller-Ladner, U.; Tarner, I.H. Low-field magnetic resonance imaging study on carpal arthritis in systemic sclerosis-low-grade erosive arthritis of carpal bones is an unexpected and frequent disease manifestation. Arthritis Res. Ther. 2013, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Ito, S.; Handa, S.; Tomiha, S.; Kose, K.; Haishi, T.; Tsutsumi, A.; Sumida, T. Low-field compact magnetic resonance imaging system for the hand and wrist in rheumatoid arthritis. J. Magn. Reson. Imaging 2006, 23, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Hagiwara, A.; Goto, M.; Wada, A.; Aoki, S. Low-Field Magnetic Resonance Imaging: Its History and Renaissance. Investig. Radiol. 2021, 56, 669–679. [Google Scholar] [CrossRef]
- Denton, C.P. Therapeutic targets in systemic sclerosis. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Distrel, O. Tyrosine kinase inhibitors for the treatment of fibrotic diseases such as systemic sclerosis: Towards molecular targeted therapies. Ann. Rheum. Dis. 2010, 69, i48–i51. [Google Scholar] [CrossRef]
- Bruni, C.; Praino, E.; Guiducci, S.; Bellando-Randone, S.; Furst, D.E.; Matucci-Cerinic, M. Hydroxychloroquine and joint involvement in systemic sclerosis: Preliminary beneficial results from a retrospective case-control series of an EUSTAR center. Jt. Bone Spine 2017, 84, 747–748. [Google Scholar] [CrossRef]
- Elhai, M.; Meunier, M.; Matucci-Cerinic, M.; Maurer, B.; Riemekasten, G.; Leturcq, T.; Pellerito, R.; Von Mühlen, C.A.; Vacca, A.; Airo, P.; et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: A EUSTAR observational study. Ann. Rheum. Dis. 2013, 72, 1217–1220. [Google Scholar] [CrossRef]
- Daoussis, D.; Melissaropoulos, K.; Sakellaropoulos, G.; Antonopoulos, I.; Markatseli, T.E.; Simopoulou, T.; Georgiou, T.; Andonopoulos, A.P.; Drosos, A.A.; Sakkas, L.; et al. A multicenter, open-label, comparative study of B-cell depletion therapy with rituximab for systemic sclerosis-associated interstitial lung disease. Semin. Arthritis Rheum. 2017, 46, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.K.; Martyanov, V.; Franks, J.M.; Bernstein, E.J.; Szymonifka, J.; Magro, C.; Wildman, H.F.; Wood, T.A.; Whitfield, M.L.; Spiera, R.F. Belimumab for the treatment of early diffuse systemic sclerosis: Results of a randomized, double-blind, placebo-controlled, pilot trial. Arthritis Rheumatol. 2018, 70, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Low, A.H.; Lax, M.; Johnson, S.R.; Lee, P. Magnetic resonance imaging of the hand in systemic sclerosis. J. Rheumatol. 2009, 36, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, B.; Stanković, A.; Dimić, A.; Damjanov, N.; Nedović, J.; Stojanović, S.; Savić, V.; Đorđević, D. The clinical significance of antibody determination to cyclic citrullinated peptides in systemic sclerosis. Srp. Arh. Celok. Lek. 2012, 140, 350–354. [Google Scholar] [CrossRef]
- Laustriat, G.; Ruyssen-Witrand, A.; Constantin, A.; Barnetche, T.; Adoue, D.; Cantagrel, A.; Degboé, Y. Anti-citrullinated peptides antibodies in systemic sclerosis: Meta-analysis of frequency and meaning. Jt. Bone Spine 2018, 85, 147–153. [Google Scholar] [CrossRef]
- Allanore, Y.; Seror, R.; Chevrot, A.; Kahan, A.; Drapé, J.L. Hand vascular involvement assessed by magnetic resonance angiography in systemic sclerosis. Arthritis Rheum. 2007, 56, 2747–2754. [Google Scholar] [CrossRef]
- Morrisroe, K.B.; Nikpour, M.; Proudman, S.M. Musculoskeletal manifestations of systemic sclerosis. Rheum. Dis. Clin. N. Am. 2015, 41, 507–518. [Google Scholar] [CrossRef]
- Lorand, V.; Czirjak, L.; Minier, T. Musculoskeletal involvement in systemic sclerosis. Presse Méd. 2014, 43, e315–e328. [Google Scholar] [CrossRef]
- Freire, V.; Bazeli, R.; Elhai, M.; Campagna, R.; Pessis, É.; Avouac, J.; Allanore, Y.; Drapé, J.L.; Guérini, H. Hand and wrist involvement in systemic sclerosis: US features. Radiology 2013, 269, 824–830. [Google Scholar] [CrossRef]
- Johnstone, E.M.; Hutchinson, A.V.; Chevance, A.; Herrick, A.L. Acro-osteolysis in systemic sclerosis is associated with digital ischaemia and severe calcinosis. Rheumatology 2012, 51, 2234–2238. [Google Scholar] [CrossRef]
- Koutaissoffz, S.; Vanthuyne, M.; Smith, V.; De Langhe, E.; Depresseux, G.; Westhovens, R.; De Keyser, F.; Malghem, J.; Houssiau, F.A. Hand radiological damage in systemic sclerosis: Comparison with a control group and clinical and functional correlations. Semin. Arthritis Rheum. 2011, 40, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Morardet, L.; Avouac, J.; Sammour, M.; Baron, M.; Kahan, A.; Feydy, A.; Allanore, Y. Late Nailfold Videocapillaroscopy Pattern Associated with Hand Calcinosis and Acro-Osteolysis in Systemic Sclerosis. Arthritis Care Res. 2016, 68, 366–373. [Google Scholar] [CrossRef] [PubMed]
- McQueen, F.M. A vital clue to deciphering bone pathology: MRI bone oedema in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Freeston, J.E.; Bird, P.; Conaghan, P.G. The role of MRI in rheumatoid arthritis: Research and clinical issues. Curr. Opin. Rheum. 2009, 21, 95–101. [Google Scholar] [CrossRef]
- Ostergaard, M.; Peterfy, C.; Conaghan, P.; McQueen, F.; Bird, P.; Ejbjerg, B.; Shnier, R.; O’Connor, P.; Klarlund, M.; Emery, P.; et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies: Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J. Rheumatol. 2003, 30, 1385–1386, Erratum in J. Rheumatol. 2004, 31, 198. [Google Scholar]
- Hodgson, R.J.; O’Connor, P.; Moots, R. MRI of Rheumatoid arthritis: Scoring systems. Rheumatology 2008, 47, 13–21. [Google Scholar] [CrossRef]
- Boutry, N.; Lardé, A.; Lapègue, F.; Solau-Gervais, E.; Flipo, R.M.; Cotton, A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J. Rheumatol. 2003, 30, 671–679. [Google Scholar]
- Lescoat, A.; Ballerie, A.; Belhomme, N.; Cazalets, C.; de Carlan, M.; Droitcourt, C.; Perdriger, A.; Jégo, P.; Coiffier, G. Synovial involvement assessed by power Doppler ultra-sonography in systemic sclerosis: Results of a cross-sectional study. Rheumatology 2018, 57, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Scheiman-Elazary, A.; Ranganath, V.K.; Ben-Artzi, A.; Kafaja, S.; Borazan, H.; Woodworth, T.; Duon, L.; Elashoff, D.; Clements, P.; Furst, D. Validation of Sonography findings of synovitis and tenosynovitis of hands and wrists in patients with systemic sclerosis. J. Scleroderma Rel. Dis. 2018, 3, 228–236. [Google Scholar] [CrossRef]
- Avouac, J.; Walker, U.A.; Hachulla, E.; Riemekasten, G.; Cuomo, G.; Carreira, P.E.; Caramaschi, P.; Ananieva, L.P.; Matucci-Cerinic, M.; Czirjak, L.; et al. Joint and tendon involvement predict disease progression in systemic sclerosis: A EUSTAR prospective study. Ann. Rheum. Dis. 2016, 75, 103–109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).