A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Studies, Clinical and Ophthalmological Examinations
2.2. Molecular Genetic Study
3. Results
3.1. Patients’ Clinical Reports
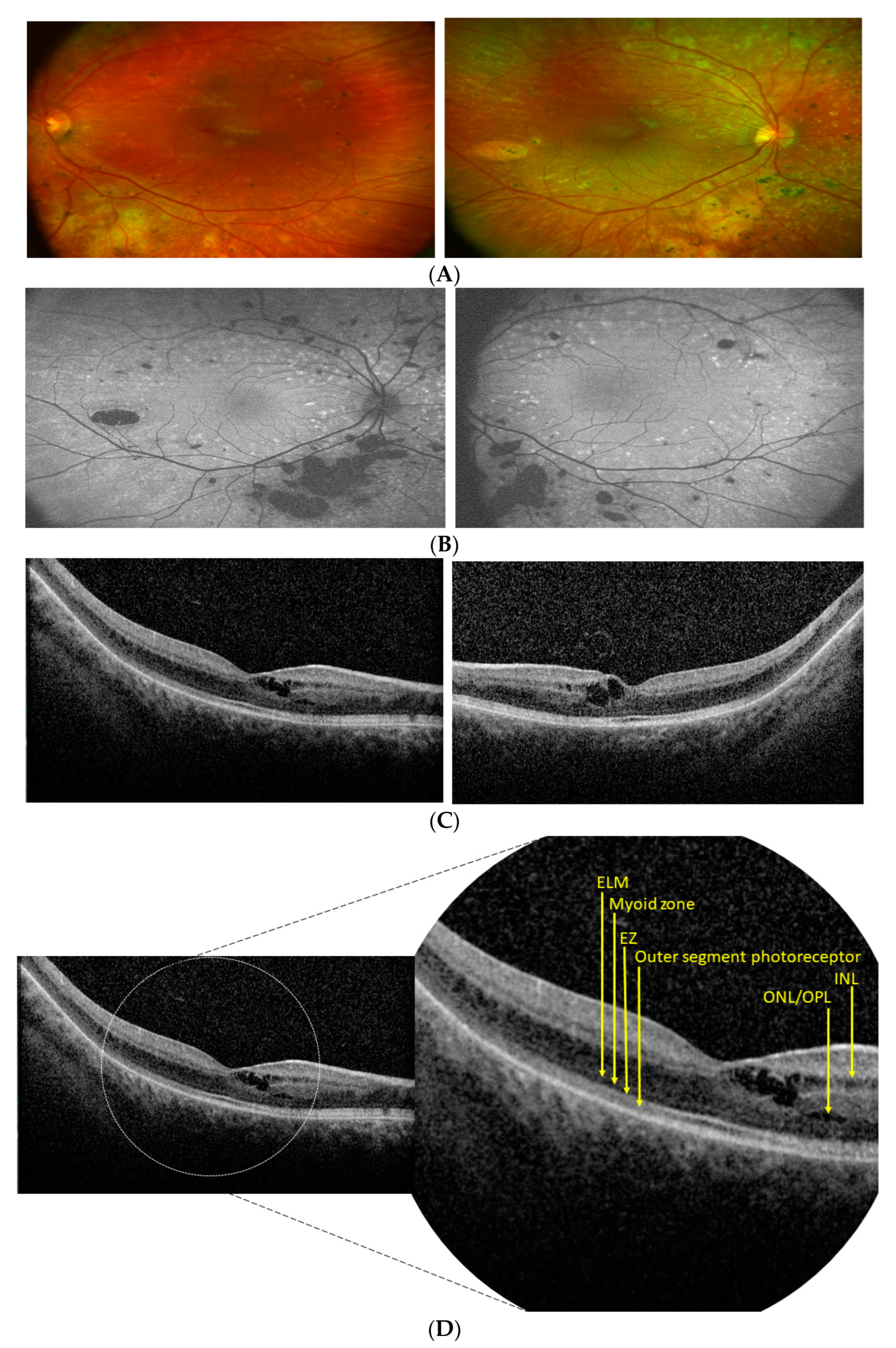

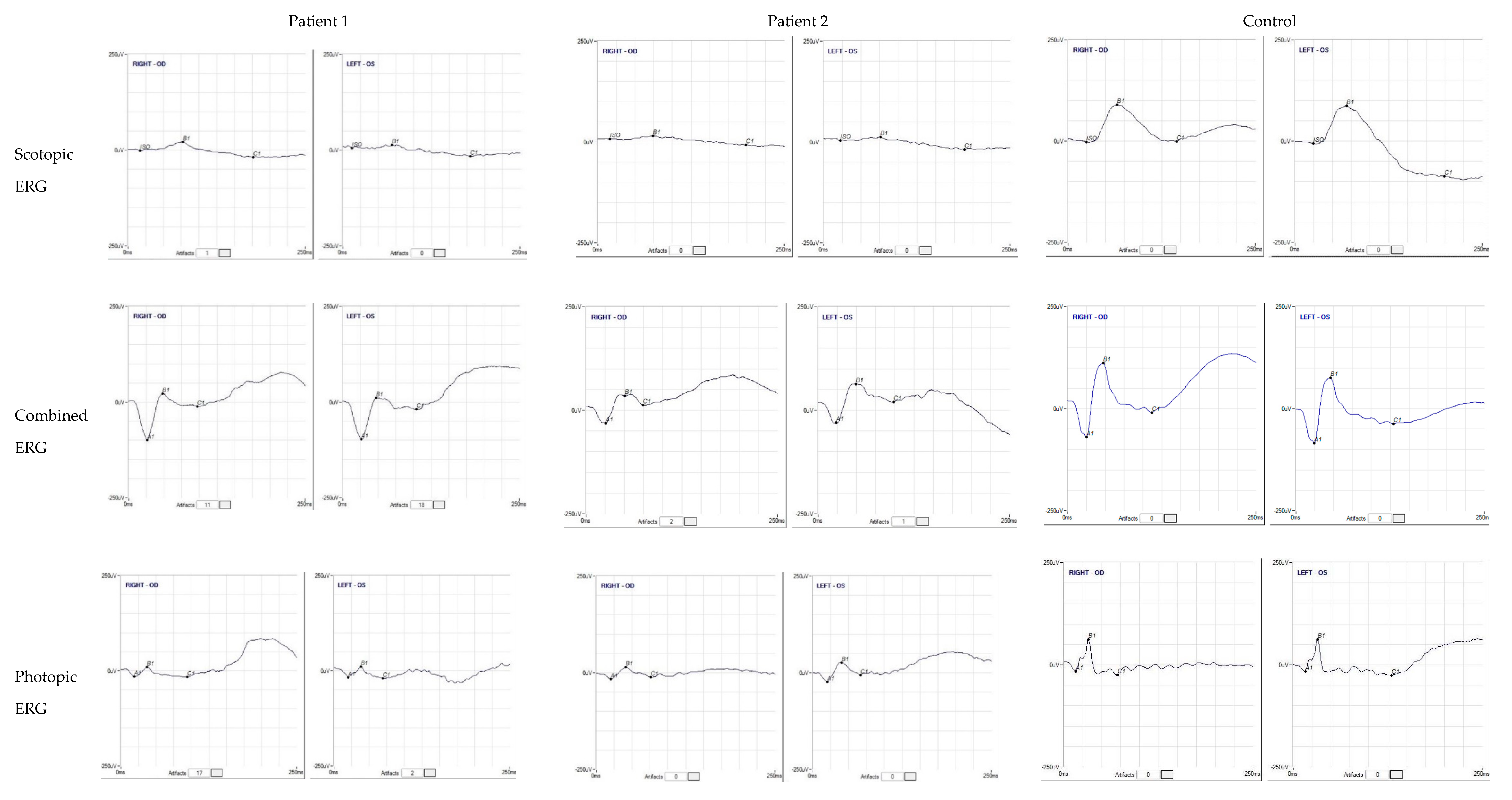

3.2. Genetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, A.; Robson, A.G.; Holder, G.E. Pathognomonic (diagnostic) ERGs A Review and Update. Retina 2013, 33, 5–12. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Marmor, M.F.; Kemp, C.M.; Knighton, R.W. SWS (blue) cone hypersensitivity in a newly identified retinal de-generation. Investig. Ophthalmol. Vis. Sci. 1990, 31, 827–838. [Google Scholar]
- Marmor, M.F.; Jacobson, S.G.; Foerster, M.H.; Kellner, U.; Weleber, R.G. Diagnostic Clinical Findings of a New Syndrome with Night Blindness, Maculopathy, and Enhanced S Cone Sensitivity. Am. J. Ophthalmol. 1990, 110, 124–134. [Google Scholar] [CrossRef]
- Cheng, H.; Khanna, H.; Oh, E.C.; Hicks, D.; Mitton, K.; Swaroop, A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 2004, 13, 1563–1575. [Google Scholar] [CrossRef]
- Kanda, A.; Friedman, J.S.; Nishiguchi, K.M.; Swaroop, A. Retinopathy mutations in the bZIP protein NRL alter phosphoryla-tion and transcriptional activity. Hum. Mutat. 2007, 28, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Farjo, Q.; Jackson, A.; Pieke-Dahl, S.; Scott, K.; Kimberling, W.J.; Sieving, P.A.; Richards, J.E.; Swaroop, A. Human bZIP Transcription Factor GeneNRL:Structure, Genomic Sequence, and Fine Linkage Mapping at 14q11.2 and Negative Mutation Analysis in Patients with Retinal Degeneration. Genomics 1997, 45, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, A.; Xu, J.Z.; Pawar, H.; Jackson, A.; Skolnick, C.; Agarwal, N. A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc. Natl. Acad. Sci. USA 1992, 89, 266–270. [Google Scholar] [CrossRef]
- Mears, A.J.; Kondo, M.; Swain, P.K.; Takada, Y.; Bush, R.A.; Saunders, T.L.; Sieving, P.A.; Swaroop, A. NRL is required for rod photoreceptor development. Nat. Genet. 2001, 29, 447–452. [Google Scholar] [CrossRef]
- Bessant, D.A.; Payne, A.M.; Mitton, K.P.; Wang, Q.-L.; Swain, P.K.; Plant, C.; Bird, A.C.; Zack, D.J.; Swaroop, A.; Bhattacharya, S.S. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat. Genet. 1999, 21, 355–356. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, M.M.; Grimsby, J.L.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. Novel mutations in the NRL gene and associated clinical findings in patients with dominant retinitis pigmentosa. Arch. Ophthalmol. 2002, 120, 369–375. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, S.; Liu, C.; Qin, Y.; Archacki, S.; Jin, L.; Wang, Y.; Liu, F.; Chen, J.; Liu, Y.; et al. Whole exome sequencing identifies a novel NRL mutation in a Chinese family with autosomal dominant retinitis pigmentosa. Mol. Vis. 2016, 22, 234–242. [Google Scholar] [PubMed]
- Hernan, I.; Gamundi, M.J.; Borràs, E.; Maseras, M.; García-Sandoval, B.; Blanco-Kelly, F.; Ayuso, C.; Carballo, M. Novel p.M96T variant of NRL and shRNA-based suppression and replacement of NRL mutants associated with autosomal dominant retinitis pig-mentosa. Clin. Genet. 2012, 82, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.-J.; Li, J.-K.; Chen, H.; Hu, F.-Y.; Zhang, S.-H.; Qi, Y.-H.; Xu, P.; Wang, D.-D.; Wang, L.-S.; Chang, Q.; et al. Genetic and Clinical Findings in a Large Cohort of Chinese Patients with Suspected Retinitis Pigmentosa. Ophthalmology 2019, 126, 1549–1556. [Google Scholar] [CrossRef]
- Nishiguchi, K.M.; Friedman, J.S.; Sandberg, M.A.; Swaroop, A.; Berson, E.L.; Dryja, T.P. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc. Natl. Acad. Sci. USA 2004, 101, 17819–17824. [Google Scholar] [CrossRef] [PubMed]
- Newman, H.; Blumen, S.C.; Braverman, I.; Hanna, R.; Tiosano, B.; Perlman, I.; Ben-Yosef, T. Homozygosity for a Recessive Loss-of-Function Mutation of the NRL Gene Is Associated With a Variant of Enhanced S-Cone Syndrome. Investig. Opthalmology Vis. Sci. 2016, 57, 5361–5371. [Google Scholar] [CrossRef]
- Collin, R.W.J.; Born, L.I.V.D.; Klevering, B.J.; de Castro-Miró, M.; Littink, K.W.; Arimadyo, K.; Azam, M.; Yazar, V.; Zonneveld, M.N.; Paun, C.C.; et al. High-Resolution Homozygosity Mapping Is a Powerful Tool to Detect Novel Mutations Causative of Autosomal Recessive RP in the Dutch Population. Investig. Opthalmology Vis. Sci. 2011, 52, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Neveling, K.; Collin, R.W.; Gilissen, C.; van Huet, R.A.; Visser, L.; Kwint, M.P.; Gijsen, S.J.; Zonneveld, M.N.; Wieskamp, N.; de Ligt, J.; et al. Next-generation genetic testing for retinitis pigmentosa. Hum. Mutat. 2012, 33, 963–972. [Google Scholar] [CrossRef]
- Beryozkin, A.; Shevah, E.; Kimchi, A.; Mizrahi-Meissonnier, L.; Khateb, S.; Ratnapriya, R.; Lazar, C.H.; Blumenfeld, A.; Ben-Yosef, T.; Hemo, Y.; et al. Whole Exome Sequencing Reveals Mutations in Known Retinal Disease Genes in 33 out of 68 Israeli Families with Inherited Retinopathies. Sci. Rep. 2015, 5, srep13187. [Google Scholar] [CrossRef]
- Littink, K.W.; Stappers, P.; Riemslag, F.; Talsma, H.E.; Van Genderen, M.M.; Cremers, F.; Collin, R.; Van den Born, L.I. Autosomal recessive NRL mutations in patients with enhanced Scone syndrome. Genes 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Sustar, M.; Hawlina, M.; Brecelj, J. Electroretinographic evaluation of the retinal S-cone system. Doc. Ophthalmol. 2011, 123, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus rec-ommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Oishi, M.; Oishi, A.; Gotoh, N.; Ogino, K.; Higasa, K.; Iida, K.; Makiyama, Y.; Morooka, S.; Matsuda, F.; Yoshimura, N. Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation se-quencing. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7369–7375. [Google Scholar] [CrossRef]
- Wright, A.F.; Reddick, A.C.; Schwartz, S.B.; Ferguson, J.S.; Aleman, T.S.; Kellner, U.; Jurklies, B.; Schuster, A.; Zrenner, E.; Wissinger, B.; et al. Mutation analysis of NR2E3 and NRL genes in Enhanced S Cone Syndrome. Hum. Mutat. 2004, 24, 439. [Google Scholar] [CrossRef] [PubMed]
- Kallman, A.; Capowski, E.E.; Wang, J.; Kaushik, A.M.; Jansen, A.D.; Edwards, K.L.; Chen, L.; Berlinicke, C.A.; Phillips, M.J.; Pierce, E.A.; et al. Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Commun. Biol. 2020, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Daiger, S.P.; Bowne, S.J.; Sullivan, L.S.; Blanton, S.H.; Weinstock, G.M.; Koboldt, D.C.; Fulton, R.S.; Larsen, D.; Humphries, P.; Humphries, M.M.; et al. Application of next-generation sequencing to identify genes and mutations causing autosomal dominant retinitis pigmentosa (adRP). Single Mol. Single Cell Seq. 2014, 801, 123–129. [Google Scholar] [CrossRef]
- Martinez-Gimeno, M.; Maseras, M.; Baiget, M.; Beneito, M.; Antiñolo, G.; Ayuso, C.; Carballo, M. Mutations P51U and G122E in retinal transcription factor NRL associated with autosomal dominant and sporadic retinitis pigmentosa. Hum. Mutat. 2001, 17, 520. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Kiray, G.; Chiang, J.P.; Vincent, A.L. Molecular and phenotypic investigation of a New Zealand cohort of childhood-onset retinal dystrophy. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 708–717. [Google Scholar] [CrossRef] [PubMed]
- El-Asrag, M.E.; Corton, M.; McKibbin, M.; Avila-Fernandez, A.; Mohamed, M.D.; Blanco-Kelly, F.; Toomes, C.; Inglehearn, C.F.; Ayuso, C.; Ali, M. Novel homozygous mutations in the transcription factor NRL cause non-syndromic retinitis pigmentosa. Mol. Vis. 2022, 28, 48–56. [Google Scholar] [PubMed]
- De Castro-Miró, M.; Tonda, R.; Escudero-Ferruz, P.; Andrés, R.; Mayor-Lorenzo, A.; Castro, J.; Ciccioli, M.; Hidalgo, D.A.; Rodríguez-Ezcurra, J.J.; Farrando, J.; et al. Novel Candidate Genes and a Wide Spectrum of Structural and Point Mutations Responsible for Inherited Retinal Dystrophies Revealed by Exome Se-quencing. PLoS ONE 2016, 11, e0168966. [Google Scholar]
- Porto, F.B.O.; Jones, E.M.; Branch, J.; Soens, Z.T.; Maia, I.M.; Sena, I.F.G.; Sampaio, S.A.M.; Simões, R.T.; Chen, R. Molecular Screening of 43 Brazilian Families Diagnosed with Leber Congenital Amaurosis or Early-Onset Severe Retinal Dystrophy. Genes 2017, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xie, Y.; Sun, T.; Zhang, X.; Chen, C.; Li, Y. Genetic and clinical findings in a Chinese cohort with Leber congenital amaurosis and early onset severe retinal dystrophy. Br. J. Ophthalmol. 2020, 104, 932–937. [Google Scholar] [CrossRef]
- Carrigan, M.; Duignan, E.; Malone, C.; Stephenson, K.; Saad, T.; McDermott, C.; Green, A.; Keegan, D.; Humphries, P.; Kenna, P.F.; et al. Panel-Based Population Next-Generation Sequencing for Inherited Retinal Degenerations. Sci. Rep. 2016, 6, 33248. [Google Scholar] [CrossRef] [PubMed]
- Haer-Wigman, L.; Van Zelst-Stams, W.A.G.; Pfundt, R.; Born, L.I.V.D.; Klaver, C.; Verheij, J.B.G.M.; Hoyng, C.B.; Breuning, M.H.; Boon, C.; Kievit, A.J.; et al. Diagnostic exome sequencing in 266 Dutch patients with visual impairment. Eur. J. Hum. Genet. 2017, 25, 591–599. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, F.; Yu, S.; Yang, L.; Gao, M.; Tang, Z.; Guo, A.Y.; Zhang, M.; Li, P.; Liu, M. Identification of a novel NRL mutation in a Chinese family with retinitis pigmentosa by whole-exome sequencing. Eye 2017, 31, 815–817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Consugar, M.B.; Navarro-Gomez, D.; Place, E.M.; Bujakowska, K.M.; Sousa, M.E.; Fonseca-Kelly, Z.D.; Taub, D.G.; Janessian, M.; Wang, D.Y.; Au, E.D.; et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet. Med. 2014, 17, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ellingford, J.M.; Barton, S.; Bhaskar, S.; O’Sullivan, J.; Williams, S.G.; Lamb, J.A.; Panda, B.; Sergouniotis, P.I.; Gillespie, R.L.; Daiger, S.P.; et al. Molecular findings from 537 individuals with inherited retinal disease. J. Med. Genet. 2016, 53, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Arno, G.; Sergouniotis, P.I.; Tiffin, P.; Borman, A.D.; Chandra, A.; Robson, A.; Holder, G.E.; Webster, A.R.; Moore, A.T. Clinical and Molecular Characterization of Enhanced S-Cone Syndrome in Children. JAMA Ophthalmol. 2014, 132, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Michaelides, M.; Robson, A.G.; Hawlina, M.; Vaclavik, V.; Sandbach, J.M.; Neveu, M.M.; Hogg, C.R.; Hunt, D.M.; Moore, A.T.; et al. Phenotypic Variation in Enhanced S-cone Syndrome. Investig. Opthalmology Vis. Sci. 2008, 49, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
| Known Pathogenic Variations in NRL Gene (NM_006177) | |||
|---|---|---|---|
| Missense/Nonsense Variations | Phenotype | Ref. | |
| DNA Change | Protein Change | ||
| c.91C>T | p.(Arg31Ter) | Night blindness and reduced visual acuity, Autosomal Recessive | [15] |
| c.146C>T | p.(Pro49Leu) | Retinitis pigmentosa, Autosomal Dominant | [11] |
| c.148T>A | p.(Ser50Thr) | Retinitis pigmentosa, Autosomal Dominant | [9] |
| c.148T>C | p.(Ser50Pro) | Retinitis pigmentosa, Autosomal Dominant | [10] |
| c.149C>T | p.(Ser50Leu) | Retinitis pigmentosa, Autosomal Dominant | [10] |
| c.151C>A | p.(Pro51Thr) | Retinitis pigmentosa, Autosomal Dominant | [10] |
| c.151C>G | p.(Pro51Ala) | Retinitis pigmentosa, Autosomal Dominant | [26] |
| c.151C>T | p.(Pro51Ser) | Retinitis pigmentosa, Autosomal Dominant | [14] |
| c.152C>T | p.(Pro51Leu) | Retinitis pigmentosa, Autosomal Dominant | [27] |
| c.152C>G | p.(Pro51Arg) | Rod cone dystrophy, unclear mode of transmission | [28] |
| c.287T>C | p.(Met96Thr) | Retinitis pigmentosa, Autosomal Dominant | [12] |
| c.238C>T | p.(Gln80Ter) | Retinitis pigmentosa, Autosomal Recessive | [29] |
| c.339C>G | p.(Tyr113Ter) | Goldmann-Favre syndrome, Autosomal Recessive | [30] |
| c.365G>A | p.(Gly122Glu) | Retinitis pigmentosa, Autosomal Dominant | [27] |
| c.416C>G | p.(Ser139Trp) | Leber congenital amaurosis, Autosomal Recessive | [31] |
| c.424G>A | p.(Val142Met) | Retinitis pigmentosa, Autosomal Dominant | [13] |
| c.479T>C | p.(Leu160Pro) | Clumped pigmentary retinal degeneration, Autosomal Recessive | [14] |
| c.508C>A | p.(Arg170Ser) | Clumped pigmentary retinal degeneration, Autosomal Recessive | [16,19] |
| c.520C>A | p.(Gln174Lys) | Leber congenital amaurosis/ retinal dystrophy, Autosomal Recessive | [32] |
| c.544C>T | p.(Gln182Ter) | Retinitis pigmentosa, Autosomal Recessive | [29] |
| c.674G>A | p.(Ser225Asn) | Cone dysfunction syndrome, uncertain inheritance pattern | [14] |
| c.713G>T | p.(Ter238Leu) | Retinitis pigmentosa, Autosomal Recessive | [29] |
| Indel Variations | Phenotype | Ref. | |
| DNA Change | Protein Change | ||
| c.16delA | p.(Ser6AlafsTer13) | Leber congenital amaurosis, atypical, Autosomal Recessive | [33] |
| c.23delT | p.(Leu8ArgfsTer11) | Retinitis pigmentosa, Autosomal Dominant | [23] |
| c.104dup | p.(Thr36fs) | Retinitis pigmentosa, Autosomal Recessive | [34] |
| c.147_149delTTC | p.(Ser50del) | Retinitis pigmentosa, Autosomal Dominant | [35] |
| c.223dupC | p.(Leu75ProfsTer19) | Chorioretinal Dystrophy, Autosomal Recessive | [36] |
| c.223insC | p.(Leu75fsProTer18) | Enhanced S-cone syndrome uncertain inheritance pattern | [24] |
| c.224_225insC | p.(Leu75fsTer) | Clumped pigmentary retinal degeneration, Autosomal Recessive | [14,24] |
| c.386delC | p.(Ala129GlufsTer17) | Leber congenital amaurosis, atypical, Autosomal Recessive | [33] |
| c.444_445insGCTGCGGG | p.(Leu149AlafsTer15) | Retinitis pigmentosa, Autosomal Recessive | [18] |
| c.452_459dupGCTGCGGG | p.(Arg154AlafsTer10) | Retinitis pigmentosa, Autosomal Recessive | [18] |
| c.586_627dupGCCCAGCTGGACGCGCTGCGGGCCGAGGTGGCCCGCCTGGCC | p.(Ala196_Ala209dup) | Retinal disease, Autosomal Dominant | [37] |
| c.654delC | p.(Cys219ValfsTer4) | Leber congenital amaurosis, Autosomal Recessive | [14,19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iarossi, G.; Sinibaldi, L.; Passarelli, C.; Coppe’, A.M.; Cappelli, A.; Petrocelli, G.; Catena, G.; Perrone, C.; Falsini, B.; Novelli, A.; et al. A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients. Diagnostics 2022, 12, 2183. https://doi.org/10.3390/diagnostics12092183
Iarossi G, Sinibaldi L, Passarelli C, Coppe’ AM, Cappelli A, Petrocelli G, Catena G, Perrone C, Falsini B, Novelli A, et al. A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients. Diagnostics. 2022; 12(9):2183. https://doi.org/10.3390/diagnostics12092183
Chicago/Turabian StyleIarossi, Giancarlo, Lorenzo Sinibaldi, Chiara Passarelli, Andrea Maria Coppe’, Alessandro Cappelli, Gianni Petrocelli, Gino Catena, Chiara Perrone, Benedetto Falsini, Antonio Novelli, and et al. 2022. "A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients" Diagnostics 12, no. 9: 2183. https://doi.org/10.3390/diagnostics12092183
APA StyleIarossi, G., Sinibaldi, L., Passarelli, C., Coppe’, A. M., Cappelli, A., Petrocelli, G., Catena, G., Perrone, C., Falsini, B., Novelli, A., Bartuli, A., & Buzzonetti, L. (2022). A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients. Diagnostics, 12(9), 2183. https://doi.org/10.3390/diagnostics12092183







