PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Classification of Cases
2.2. PRAME Immunostaining
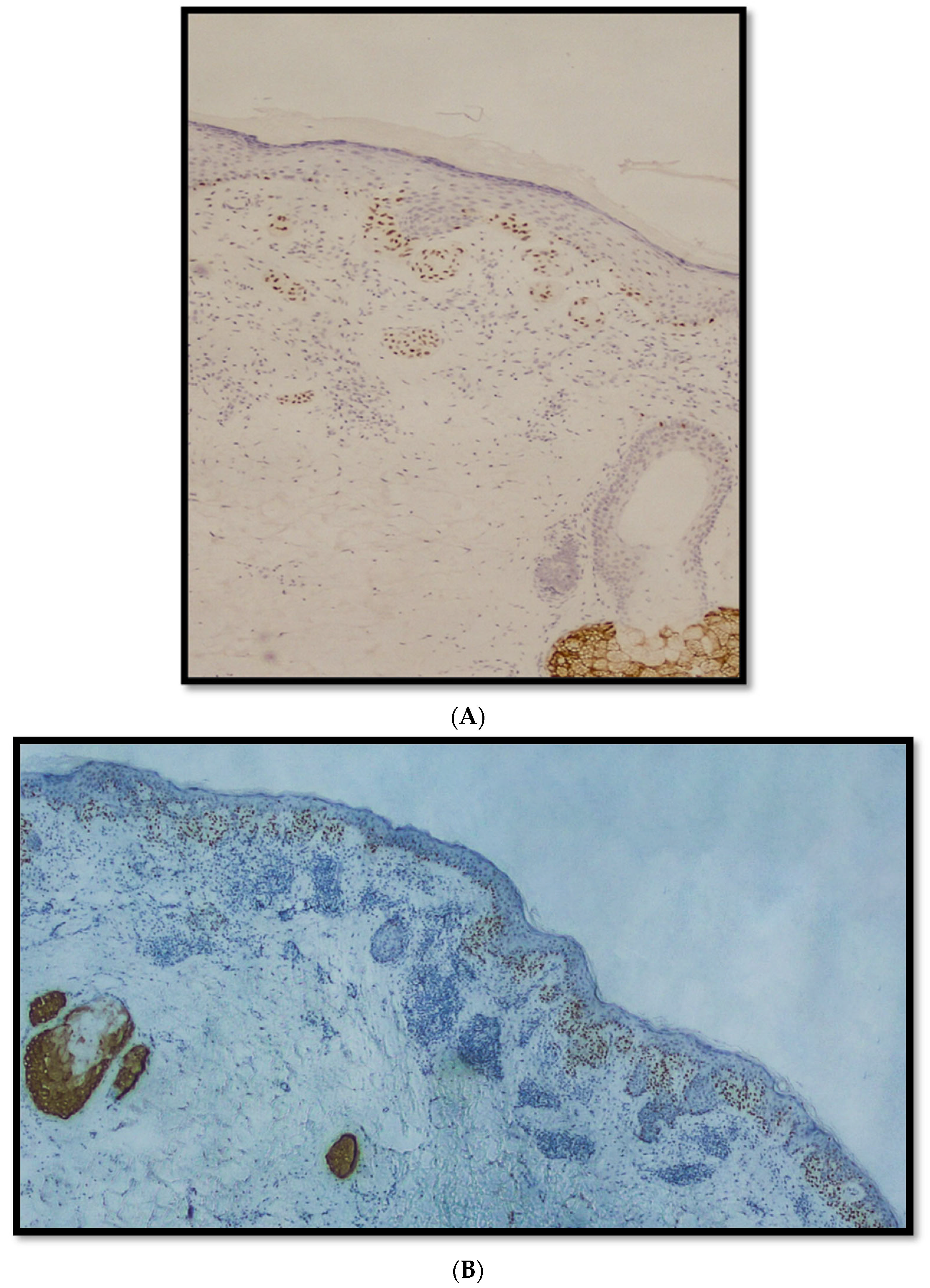
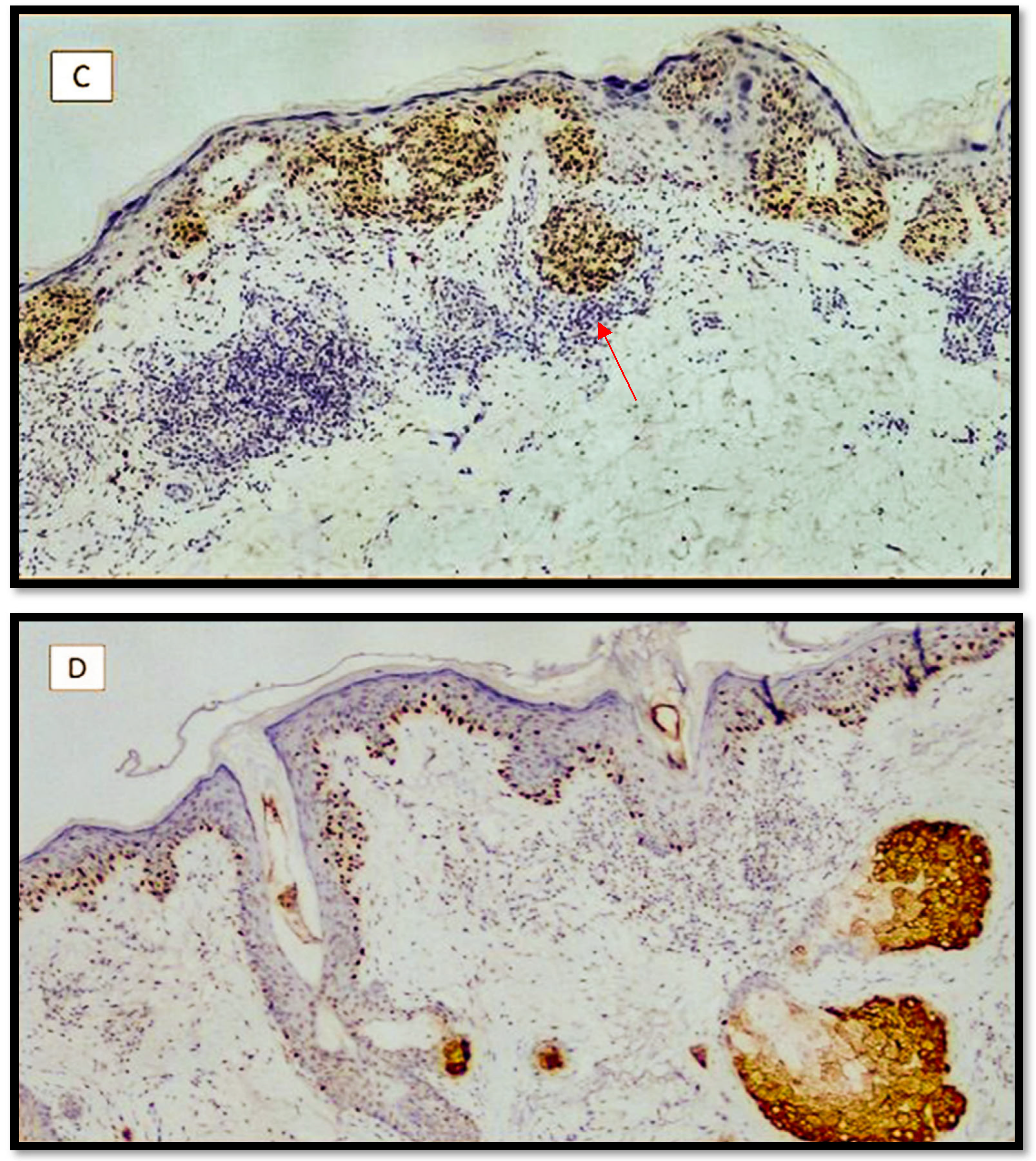
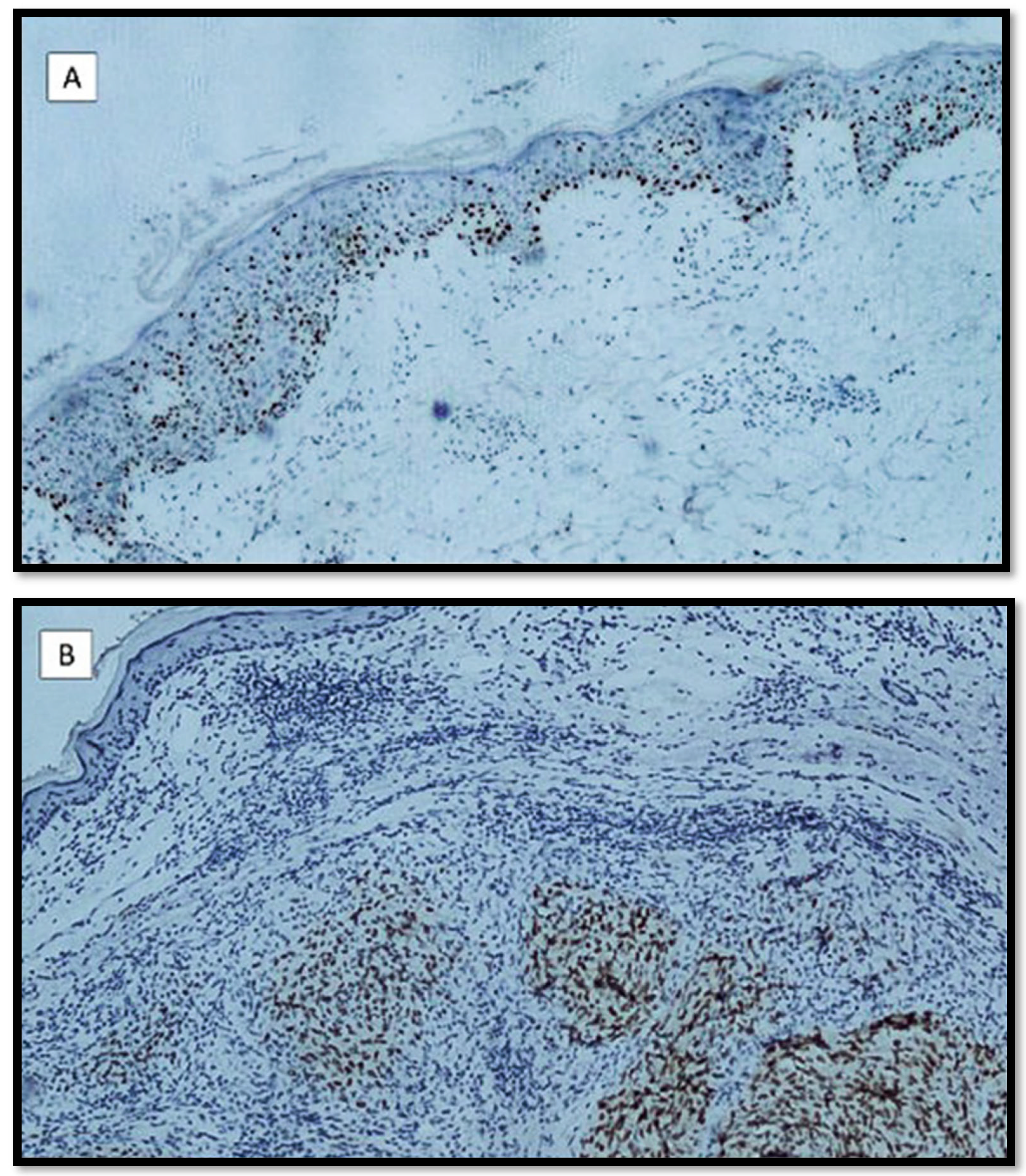
3. Results
Features of the Examined Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, O.; Miller, D.D.; Bhawan, J. Cutaneous malignant melanoma: Update on diagnostic and prognostic biomarkers. Am. J. Dermatopathol. 2014, 36, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Mihm, M.C., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Luca, R. Diagnostic, Prognostic and Predictive Immunohistochemistry in Malignant Melanoma of the Skin. Klin. Onkol. 2018, 31, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J.; Jungbluth, A.A. Melan-A, a new melanocytic differentiation marker. Adv. Anat. Pathol. 1999, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Smoller, B.R.; McNutt, N.S.; Hsu, A. HMB-45 recognizes stimulated melanocytes. J. Cutan. Pathol. 1989, 16, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cabala, C.; Li-Ning-Tapia, E.; Hwu, W.J. Pathology-based Biomarkers Useful for Clinical Decisions in Melanoma. Arch. Med. Res. 2020, 51, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Abrahamian, C.; Grimm, C. Endolysosomal Cation Channels and MITF in Melanocytes and Melanoma. Biomolecules 2021, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Mangialardi, K.; Falcicchio, G.; Colagrande, A.; Ingravallo, G.; Arezzo, F.; Giliberti, G.; Trilli, I.; Loizzi, V.; Lettini, T.; et al. Preferentially Expressed Antigen in Melanoma (PRAME) and Human Malignant Melanoma: A Retrospective Study. Genes 2022, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.A.; Toland, A.E.; Scharschmidt, T.J.; Mayerson, J.L.; Guttridge, D.C.; Iwenofu, O.H. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod. Pathol. 2014, 27, 1238–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iura, K.; Maekawa, A.; Kohashi, K.; Ishii, T.; Bekki, H.; Otsuka, H.; Yamada, Y.; Yamamoto, H.; Harimaya, K.; Iwamoto, Y.; et al. Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum. Pathol. 2017, 61, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Engelsberg, A.; Decker, J.; Störkel, S.; Jaeger, E.; Huber, C.; Seliger, B. Heterogeneous expression of the tumor-associated antigens RAGE-1, PRAME, and glycoprotein 75 in human renal cell carcinoma: Candidates for T-cell-based immunotherapies? Cancer Res. 1998, 58, 4090–4095. [Google Scholar] [PubMed]
- Pujol, J.L.; De Pas, T.; Rittmeyer, A.; Vallières, E.; Kubisa, B.; Levchenko, E.; Wiesemann, S.; Masters, G.A.; Shen, R.; Tjulandin, S.A.; et al. Safety and Immunogenicity of the PRAME Cancer Immunotherapeutic in Patients with Resected Non-Small Cell Lung Cancer: A Phase I Dose Escalation Study. J. Thorac. Oncol. 2016, 11, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Skin Tumours. WHO Classification of Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; IARC Publications: Geneve, Switzerland, 2018; Volume 11. [Google Scholar]
- Nosrati, M.; Kashani-Sabet, M. Immunohistochemical diagnostic and prognostic markers for melanoma. Methods Mol. Biol. 2014, 1102, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Lethé, B.; Lehmann, F.; van Baren, N.; Baurain, J.F.; de Smet, C.; Chambost, H.; Vitale, M.; Moretta, A.; Boon, T.; et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 1997, 6, 199–208. [Google Scholar] [CrossRef]
- Gutzmer, R.; Rivoltini, L.; Levchenko, E.; Testori, A.; Utikal, J.; Ascierto, P.A.; Demidov, L.; Grob, J.J.; Ridolfi, R.; Schadendorf, D.; et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: Results of a phase I dose escalation study. ESMO Open 2016, 1, e000068. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME Expression in Melanocytic Tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, M.; Hahn, M.; Metzler, G.; Bauer, J.; Yazdi, A.S.; Keim, U.; Garbe, C.; Wagner, N.B.; Forchhammer, S. Diffuse PRAME Expression Is Highly Specific for Thin Melanomas in the Distinction from Severely Dysplastic Nevi but Does Not Distinguish Metastasizing from Non-Metastasizing Thin Melanomas. Cancers 2021, 13, 3864. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, W.J.; Guo, R.P.; Su, J. [Application of immunohistochemical staining of PRAME in differential diagnosis between melanoma and melanocytic nevus]. Zhonghua Bing Li Xue Za Zhi 2022, 51, 621–626. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.S.; Wang, J.Y.; Kwok, S.; Rieger, K.E.; Novoa, R.A.; Brown, R.A. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J. Cutan. Pathol. 2020, 47, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Cascardi, E.; Colagrande, A.; Belsito, V.; Lospalluti, L.; Foti, C.; Arezzo, F.; Dellino, M.; Casatta, N.; Lupo, C.; et al. PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience. Diagnostics 2022, 12, 2197. https://doi.org/10.3390/diagnostics12092197
Cazzato G, Cascardi E, Colagrande A, Belsito V, Lospalluti L, Foti C, Arezzo F, Dellino M, Casatta N, Lupo C, et al. PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience. Diagnostics. 2022; 12(9):2197. https://doi.org/10.3390/diagnostics12092197
Chicago/Turabian StyleCazzato, Gerardo, Eliano Cascardi, Anna Colagrande, Vincenzo Belsito, Lucia Lospalluti, Caterina Foti, Francesca Arezzo, Miriam Dellino, Nadia Casatta, Carmelo Lupo, and et al. 2022. "PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience" Diagnostics 12, no. 9: 2197. https://doi.org/10.3390/diagnostics12092197








