Vascular Abnormalities of the Parotid Region: An Uncommon Presentation of a Common Condition—A Case Series
Abstract
1. Introduction
2. Case Presentations
2.1. Case 1
2.2. Case 2
2.3. Case 3
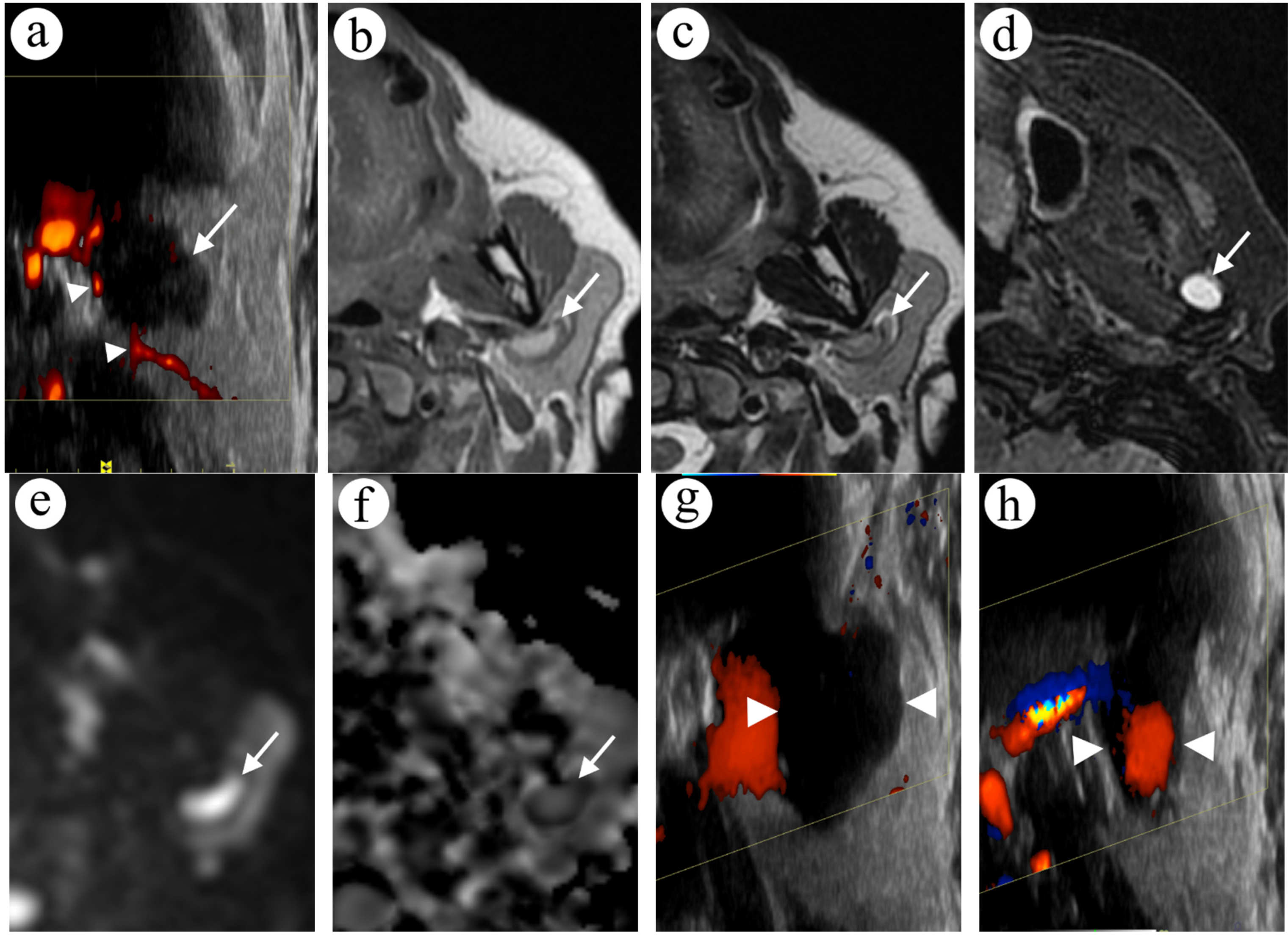
2.4. Case 4
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McCuaig, C.C. Update on classification and diagnosis of vascular malformations. Curr. Opin. Pediatr. 2017, 29, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Bartlett, E.; Lee, E.I. Vascular Malformations: A Review. Semin. Plast. Surg. 2014, 28, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Carqueja, I.M.; Sousa, J.; Mansilha, A. Vascular malformations: Classification, diagnosis and treatment. Int. Angiol. 2018, 37, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Bonner, M.Y.; Berrios, R.L. Hemangiomas, Angiosarcomas, and Vascular Malformations Rep-Resent the Signaling Abnormalities of Pathogenic Angiogenesis Pathogenesis of Malignant and Benign Endothelial Neoplasms. Curr. Mol. Med. 2009, 9, 929–934. [Google Scholar] [CrossRef]
- Behravesh, S.; Yakes, W.; Gupta, N.; Naidu, S.; Chong, B.W.; Khademhosseini, A.; Oklu, R. Venous malformations: Clinical diagnosis and treatment. Cardiovasc. Diagn. Ther. 2016, 6, 557–569. [Google Scholar] [CrossRef]
- Eifert, S.; Villavicencio, J.; Kao, T.-C.; Taute, B.M.; Rich, N.M. Prevalence of deep venous anomalies in congenital vascular malformations of venous predominance. J. Vasc. Surg. 2000, 31, 462–471. [Google Scholar] [CrossRef]
- Tang, C.Y.; Wijnen, M.; van Sambeeck, S.J.; Halbertsma, F.J.J. Acute neonatal presentation of a lymphatic malformation. BMJ Case Rep. 2013, 2013, bcr2012006784. [Google Scholar] [CrossRef]
- Ahmad, F.I.; Clayburgh, D.R. Venous thromboembolism in head and neck cancer surgery. Cancers Head Neck 2016, 1, 13. [Google Scholar] [CrossRef]
- Soudet, S.; Dakpe, S.; Le Gloan, S.; Carmi, E.; Arnault, J.P.; Testelin, S.; Plancq, M.-C.; Devauchelle, B.; Sevestre, M.A. Thrombotic Complications in Venous Malformations: Are There Differences Between Facial and Other Localizations? Clin. Appl. Thromb. 2020, 26, 1–4. [Google Scholar] [CrossRef]
- Gupta, M.; Nijhawan, V.S.; Kaur, C.; Kaur, S.; Gupta, A. The Rare Cases of Parotid Gland Arteriovenous Malformations. Case Rep. Otolaryngol. 2021, 2021, 6072155. [Google Scholar] [CrossRef]
- Achache, M.; Fakhry, N.; Varoquaux, A.; Coulibaly, B.; Michel, J.; Lagier, A.; Antonini, F.; Turner, F.; Dessi, P.; Giovanni, A. Management of vascular malformations of the parotid area. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 55–60. [Google Scholar] [CrossRef][Green Version]
- Dompmartin, A.; Acher, A.; Thibon, P.; Tourbach, S.; Hermans, C.; Deneys, V.; Pocock, B.; Lequerrec, A.; Labbé, D.; Barrellier, M.-T.; et al. Association of Localized Intravascular Coagulopathy with Venous Malformations. Arch. Dermatol. 2008, 144, 873–877. [Google Scholar] [CrossRef]
- Matsumura, Y.; Inui, M.; Nomura, J.; Yanase, S.; Nagai, K.; Tagawa, T. A case of thrombosis mimicking a buccal tumour: Usefulness of MRI. Dentomaxillofacial Radiol. 2004, 33, 202–205. [Google Scholar] [CrossRef]
- Kala, C.; Kala, S.; Khan, L. Milan system for reporting salivary gland cytopathology: An experience with the implication for risk of malignancy. J. Cytol. 2019, 36, 160–164. [Google Scholar] [CrossRef]
- Minami, M.; Tanioka, H.; Oyama, K.; Itai, Y.; Eguchi, M.; Yoshikawa, K.; Murakami, T.; Sasaki, Y. Warthin tumor of the parotid gland: MR-pathologic correlation. AJNR Am. J. Neuroradiol. 1993, 14, 209–214. [Google Scholar]
- Sąsiadek, M.J.; Zimny, A.; Zińska, L.; Bladowska, J.; Neska-Matuszewska, M. Intracranial lesions with high signal intensity on T1-weighted MR images—review of pathologies. Pol. J. Radiol. 2013, 78, 36–46. [Google Scholar] [CrossRef]
- Bradley, W.G., Jr. MR appearance of hemorrhage in the brain. Radiology 1993, 189, 15–26. [Google Scholar] [CrossRef]
- Unal, O.; Koparan, H.I.; Avcu, S.; Kalender, A.M.; Kisli, E. The diagnostic value of diffusion-weighted magnetic resonance imaging in soft tissue abscesses. Eur. J. Radiol. 2011, 77, 490–494. [Google Scholar] [CrossRef]
- Jeremy Daniel, D.O.; Yoon Cho, D.O.; Donald von Borstel, D.O.; Kyle Summers, D.O. Overview of Restricted Diffusion on Brain MRI. J. Am. Osteopath. Coll. Radiol. 2020, 9, 20–31. [Google Scholar]
- Garry, S.; Wauchope, J.; Moran, T.; Kieran, S.M. Phleboliths in a vascular malformation within the parotid gland. J. Pediatr. Surg. Case Rep. 2022, 83, 102327. [Google Scholar] [CrossRef]
- Braswell, L.; Richter, G.T. Management of Venous Malformations. Facial Plast. Surg. 2012, 28, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ardillon, L.; Lambert, C.; Eeckhoudt, S.; Boon, L.M.; Hermans, C. Dabigatran etexilate versus low-molecular weight heparin to control consumptive coagulopathy secondary to diffuse venous vascular malformations. Blood Coagul. Fibrinolysis 2016, 27, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.M.; Richter, G.T.; Crary, S. Effectiveness and Safety of Treatment with Direct Oral Anticoagulant Rivaroxaban in Patients with Slow-Flow Vascular Malformations: A Case Series. Lymphat. Res. Biol. 2018, 16, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.A.; Zeinati, C. Venous Thromboembolism in Pediatric Vascular Anomalies. Front. Pediatr. 2017, 5, 158. [Google Scholar] [CrossRef]
- Cramer, J.D.; Shuman, A.G.; Brenner, M.J. Antithrombotic Therapy for Venous Thromboembolism and Prevention of Thrombosis in Otolaryngology–Head and Neck Surgery: State of the Art Review. Otolaryngol. Neck Surg. 2018, 158, 627–636. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicki, T.K.; Joskowski, M.; Garsta, E.; Mikaszewski, B. Vascular Abnormalities of the Parotid Region: An Uncommon Presentation of a Common Condition—A Case Series. Diagnostics 2022, 12, 2236. https://doi.org/10.3390/diagnostics12092236
Nowicki TK, Joskowski M, Garsta E, Mikaszewski B. Vascular Abnormalities of the Parotid Region: An Uncommon Presentation of a Common Condition—A Case Series. Diagnostics. 2022; 12(9):2236. https://doi.org/10.3390/diagnostics12092236
Chicago/Turabian StyleNowicki, Tomasz K., Michał Joskowski, Ewa Garsta, and Bogusław Mikaszewski. 2022. "Vascular Abnormalities of the Parotid Region: An Uncommon Presentation of a Common Condition—A Case Series" Diagnostics 12, no. 9: 2236. https://doi.org/10.3390/diagnostics12092236
APA StyleNowicki, T. K., Joskowski, M., Garsta, E., & Mikaszewski, B. (2022). Vascular Abnormalities of the Parotid Region: An Uncommon Presentation of a Common Condition—A Case Series. Diagnostics, 12(9), 2236. https://doi.org/10.3390/diagnostics12092236






