Is PET/CT Able to Predict Histology in Thymic Epithelial Tumours? A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
Methodology
2. 18F-FDG PET/CT for Predicting Histology in Thymic Epithelial Tumours
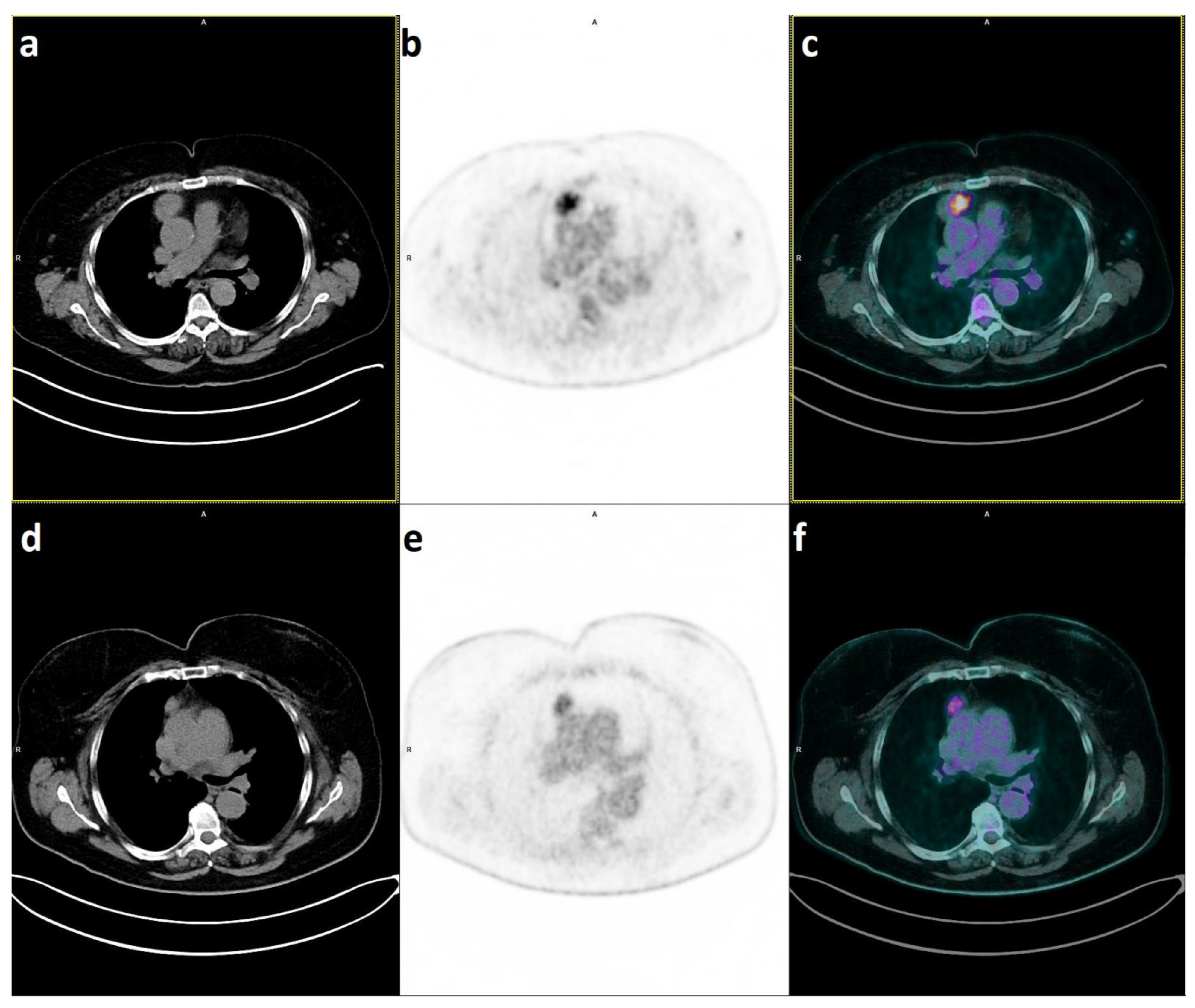
2.1. PET/CT to Distinguish Thymic Hyperplasia from Thymic Epithelial Tumours
2.2. PET/CT Parameters to Distinguish Histology in TETs
3. Future Perspectives
3.1. PET Advanced Analysis in Thymic Epithelial Tumours
3.2. New “Stromal” Tracers and Other Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosai, J.; Sobin, L.H. Histological Typing of Tumors of Thymus. In International Histological Classification of Tumors, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 5–7. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Mueller-Hermelink, H.K.; Harris, C.C. WHO Classification of Tumors. In Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart, 4th ed.; IARC Press: Lyon, France, 2004. [Google Scholar]
- Kondo, K.; Yoshizawa, K.; Tsuyuguchi, M.; Kimura, S.; Sumitomo, M.; Morita, J.; Miyoshi, T.; Sakiyama, S.; Mukai, K.; Monden, Y. WHO histologic classification is a prognostic indicator in thymoma. Ann. Thorac. Surg. 2004, 77, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Cafarotti, S.; Cesario, A.; Dall’Armi, V.; Cusumano, G.; Lauriola, L.; Frederic, M.; Evoli, A.; Margaritora, S.; Granone, P. Okumura resection for thymic malignancies. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2882–2891. [Google Scholar] [PubMed]
- Okumura, M.; Ohta, M.; Tateyama, H.; Nakagawa, K.; Matsumura, A.; Maeda, H.; Tada, H.; Eimoto, T.; Matsuda, H.; Masaoka, A. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: A clinical study of 273 patients. Cancer 2002, 94, 624–632. [Google Scholar] [CrossRef]
- Marchevsky, A.M.; Gupta, R.; McKenna, R.J.; Wick, M.; Moran, C.; Zakowski, M.F.; Suster, S. Evidence-based pathology and the pathologic evaluation of thymomas: The World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 2008, 112, 2780–2788. [Google Scholar] [CrossRef]
- Falkson, C.B.; Bezjak, A.; Darling, G.; Gregg, R.; Malthaner, R.; Maziak, D.E.; Yu, E.; Smith, C.A.; McNair, S.; Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care; et al. The management of thymoma: A systematic review and practice guideline. J. Thorac. Oncol. 2009, 4, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppitz, H.; Rockstroh, J.K.; Schüller, H.; Standop, J.; Skowasch, D.; Müller-Hermelink, H.K.; Schmidt-Wolf, I.G. State-of-the-art classification and multimodality treatment of malignant thymoma. Cancer Treat. Rev. 2012, 38, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.W.; Kang, C.H.; Choi, J.W.; Kim, H.S.; Jeon, J.H.; Park, I.K.; Kim, Y.T. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: Its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur. J. Cardio-Thorac. Surg. 2014, 45, e68–e73. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Al-Janabi, N.J. Computed tomography and magnetic resonance imaging of mediastinal tumors. J. Magn. Reson. Imaging 2010, 32, 1325–1339. [Google Scholar] [CrossRef]
- Restrepo, C.S.; Pandit, M.; Rojas, I.C.; Villamil, M.A.; Gordillo, H.; Lemos, D.; Mastrogiovanni, L.; Diethelm, L. Imaging findings of expansile lesions of the thymus. Curr. Probl. Diagn. Radiol. 2005, 34, 22–34. [Google Scholar] [CrossRef]
- Tomiyama, N.; Johkoh, T.; Mihara, N.; Honda, O.; Kozuka, T.; Koyama, M.; Hamada, S.; Okumura, M.; Ohta, M.; Eimoto, T.; et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. Am. J. Roentgenol. 2002, 179, 881–886. [Google Scholar] [CrossRef]
- Sadohara, J.; Fujimoto, K.; Müller, N.L.; Kato, S.; Takamori, S.; Ohkuma, K.; Terasaki, H.; Hayabuchi, N. Thymic epithelial tumors: Comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur. J. Radiol. 2006, 60, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, M.F.; Moran, C.A.; Mawlawi, O.; Fox, P.S.; Swisher, S.G.; Munden, R.F.; Marom, E.M. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J. Thorac. Oncol. 2013, 8, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, N.; Sakamoto, S.; Karube, Y.; Oyaizu, T.; Ishihama, H.; Chida, M. ¹⁸F-fluorodeoxyglucose positron emission tomography for evaluation of thymic epithelial tumors: Utility for World Health Organization classification and predicting recurrence-free survival. Ann. Nucl. Med. 2014, 28, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Cesario, A.; Okami, J.; Cardillo, G.; Cavuto, S.; Tokunaga, T.; Apolone, G.; Margaritora, S.; Granone, P. Role of combined 18F-FDG-PET/CT for predicting the WHO malignancy grade of thymic epithelial tumors: A multicenter analysis. Lung Cancer 2013, 82, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Takeda, A.; Katsuki, A.; Jinguji, M.; Ohmura, K.; Tani, A.; Sato, M.; Yoshiura, T. The efficacy of 18F-FDG-PET-based radiomic and deep-learning features using a machine-learning approach to predict the pathological risk subtypes of thymic epithelial tumors. Br. J. Radiol. 2022, 95, 20211050. [Google Scholar] [CrossRef] [PubMed]
- Priola, A.M.; Priola, S.M. Imaging of thymus in myasthenia gravis: From thymic hyperplasia to thymic tumor. Clin. Radiol. 2014, 69, e230–e245. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-S.; Yeh, S.-H.; Huang, M.-H.; Wang, L.-S.; Chu, L.-S.; Chang, C.-P.; Chu, Y.-K.; Wu, L.-C. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of thymoma: A preliminary report. Eur. J. Nucl. Med. 1995, 22, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- El-Bawab, H.; Al-Sugair, A.A.; Rafay, M.; Hajjar, W.; Mahdy, M.; Al-Kattan, K. Role of flourine-18 fluorodeoxyglucose positron emission tomography in thymic pathology. Eur. J. Cardio-Thorac. Surg. 2007, 31, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Regmi, S.K.; Dutta, R.; Kumar, R.; Gupta, S.D.; Das, P.; Halanaik, D.; Jindal, T. Characterization of thymic masses using (18)F-FDG PET-CT. Ann. Nucl. Med. 2009, 23, 569–577. [Google Scholar] [CrossRef]
- Watanabe, T.; Shimomura, H.; Mutoh, T.; Saito, R.; Goto, R.; Yamada, T.; Notsuda, H.; Matsuda, Y.; Noda, M.; Sakurada, A.; et al. Positron emission tomography/computed tomography as a clinical diagnostic tool for anterior mediastinal tumors. Surg. Today 2019, 49, 143–149. [Google Scholar] [CrossRef]
- Travaini, L.L.; Petralia, G.; Trifirò, G.; Ravasi, L.; Galetta, D.; Carbone, G.; Falcini, F.; Spaggiari, L.; Bellomi, M.; Paganelli, G. [18F]FDG positron emission tomography/computed tomography and multidetector computed tomography roles in thymic lesion treatment planning. Lung Cancer 2008, 61, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.M.; Lee, K.S.; Kim, B.; Choi, J.Y.; Shim, Y.M.; Yi, C.A. PET/CT of Thymic Epithelial Tumors: Usefulness for Distinguishing and Staging Tumor Subgroups. J. Nucl. Med. 2006, 47, 1628–1634. [Google Scholar] [PubMed]
- Endo, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Kondo, H.; Igawa, S.; Nakamura, Y.; Tsuya, A.; Murakami, H.; Takahashi, T.; et al. Utility of 18 FDG-PET for Differentiating the Grade of Malignancy in Thymic Epithelial Tumors. Lung Cancer 2008, 61, 350–355. [Google Scholar] [CrossRef]
- Fukumoto, K.; Taniguchi, T.; Ishikawa, Y.; Kawaguchi, K.; Fukui, T. The Utility of [18F]-FlUorodeoxyglucose Positron Emission Tomography-Computed Tomography in Thymic Epithelial Tumours. Eur. J. Cardio-Thorac. Surg. 2012, 42, e152–e156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolaccini, L.; Viti, A.; Lanzi, E.; Fortunato, M.; Chauvie, S.; Bianchi, A.; Terzi, A. (18)Fluorine-fluorodeoxyglucose positron emission tomography/computed tomography total glycolytic volume in thymic epithelial neoplasms evaluation: A reproducible image biomarker. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 228–233. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, A.; Bae, M.K.; Lee, C.Y.; Kim, D.J.; Chung, K.Y. Value of 18F-FDG PET/CT for Predicting the World Health Organization Malignant Grade of Thymic Epithelial Tumors: Focused in Volume-Dependent Parameters. Clin. Nucl. Med. 2016, 41, 15–20. [Google Scholar] [CrossRef]
- Purandare, N.; Pramesh, C.; Karimundackal, G.; Jiwnani, S.; Agrawal, A.; Shah, S.; Agarwal, J.; Prabhash, K.; Noronha, V.; Joshi, A.; et al. Thymic epithelial tumors: Can fluorodeoxyglucose positron emission tomography help in predicting histologic type and stage? Indian J. Cancer 2016, 53, 270. [Google Scholar] [CrossRef]
- Shinya, T.; Tanaka, T.; Soh, J.; Matsushita, T.; Sato, S.; Toyooka, S.; Yoshino, T.; Miyoshi, S.; Kanazawa, S. Diagnostic Value of Dual-time-point F-18 FDG PET/CT and Chest CT for the Prediction of Thymic Epithelial Neoplasms. Acta Med. Okayama 2017, 71, 105–112. [Google Scholar] [CrossRef]
- Korst, R.J.; Fernando, S.; Catlin, A.C.; Rutledge, J.R.; Girard, N.; Huang, J. Positron Emission Tomography in Thymic Tumors: Analysis Using a Prospective Research Database. Ann. Thorac. Surg. 2017, 104, 1815–1820. [Google Scholar] [CrossRef] [Green Version]
- Ayabe, T.; Tsuchiya, K.; Nakamura, K.; Tomita, M. Fluorodeoxyglucose Positron Emission Tomography Can Provide Useful Information for Differentiating Thymic Epithelial Tumors. Thorac. Cardiovasc. Surg. 2018, 66, 345–349. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Li, Q. Value of 18 F-FDG PET/Computed Tomography in Predicting the Simplified WHO Grade of Malignancy in Thymic Epithelial Tumors. Nucl. Med. Commun. 2020, 41, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Suzuki, H.; Sakairi, Y.; Wada, H.; Nakajima, T.; Yoshino, I. 18F-FDG-PET/CT Predicts Grade of Malignancy and Invasive Potential of Thymic Epithelial Tumors. Gen. Thorac. Cardiovasc. Surg. 2020, 69, 274–281. [Google Scholar] [CrossRef]
- Han, S.; Jungsu, K.; Seung, S.O.; Seo, Y.; Jae, M.; Geun, P.; Lee, D.; Choi, S.; Ryul, H.; Yong, K.; et al. Diagnostic and Prognostic Values of 2 [18 F] FDG PET/CT in Resectable Thymic Epithelial Tumour. Eur. Radiol. 2021, 32, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S.; ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v40–v55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treglia, G.; Sadeghi, R.; Giovanella, L.; Cafarotti, S.; Filosso, P.L.; Lococo, F. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer 2014, 86, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, E.; Orhan, K.; Soydal, C.; Kahya, Y.; Tunc, S.S.; Celik, O.; Sak, S.D.; Cangir, A.K. Combined clinical and specific positron emission tomography/computed tomography-based radiomic features and machine-learning model in prediction of thymoma risk groups. Nucl. Med. Commun. 2022, 43, 529–539. [Google Scholar] [CrossRef]
- Nakajo, M.; Jinguji, M.; Shinaji, T.; Nakajo, M.; Aoki, M.; Tani, A.; Sato, M.; Yoshiura, T. Texture analysis of 18F-FDG PET/CT for grading thymic epithelial tumours: Usefulness of combining SUV and texture parameters. Br. J. Radiol. 2018, 91, 20170546. [Google Scholar] [CrossRef]
- Lee, H.S.; Oh, J.S.; Park, Y.S.; Jang, S.J.; Choi, I.S.; Ryu, J.-S. Differentiating the grades of thymic epithelial tumor malignancy using textural features of intratumoral heterogeneity via (18)F-FDG PET/CT. Ann. Nucl. Med. 2016, 30, 309–319. [Google Scholar] [CrossRef]
- Lococo, F.; Chiappetta, M.; Triumbari, E.K.A.; Evangelista, J.; Congedo, M.T.; Pizzuto, D.A.; Brascia, D.; Marulli, G.; Annunziata, S.; Margaritora, S. Current Roles of PET/CT in Thymic Epithelial Tumours: Which Evidences and Which Prospects? A Pictorial Review. Cancers 2021, 13, 6091. [Google Scholar] [CrossRef]
- Isik, E.G.; Kuyumcu, S.; Ozkan, Z.G.; Simsek, D.H.; Sanli, Y. Intratumoral Heterogeneity in a Patient With Metastatic Thymic Carcinoma on 18F-FDG, 68Ga-DOTATATE, and 68Ga-FAPI04 PET/CT. Clin. Nucl. Med. 2022, 47, e79–e80. [Google Scholar] [CrossRef]

| Study | Year | Patients | Thymic Pathology and Pet-Findings |
|---|---|---|---|
| Liu [19] | 1995 | 12 | Thymic hyperplasia: TLR 3.4/3.5 Thymoma: TLR 5.7 ±1.7 |
| El-Bawab [20] | 2007 | 25 | Thymic hyperplasia: SUVmax ranging from 0.7 to 2.5 (mean 1.89 ± 0.58) Thymoma: SUVmax ranging from 3.1 to 6.1 (mean 4.75 ± 0.88) |
| Kumar [21] | 2009 | 23 | Thymic hyperplasia: mean SUV max 1.1 (0.7–1.8) Low-risk thymomas: mean SUV max 3 (1.7–3.9), Thymic carcinoma: mean SUVmax 7 (4.3–9.2). |
| Watanabe [22] | 2019 | 70 | Thymic hyperplasia: mean SUVmax 1.4 ± 0.7 Thymoma: mean SUVmax 3.7 ± 1.5 Thymic carcinoid: mean SUVmax 7.0 ± 1.5 Thymic cancer: mean SUVmax 11.4 ± 2.6 |
| Travaini [23] | 2008 | 20 | Thymic hyperplasia: SUVmax ranging from 1.7 to 5 Low-grade thymomas: SUVmax ranging from 2.3 to 15.5 High-grade thymomas and thymic carcinomas: SUVmax ranging from 5 to 9 |
| Author | Year | Patients | Male/Female | Age | Histology (Number) | PET/CT Parameters | Cut-off Value AUC |
|---|---|---|---|---|---|---|---|
| Sung [24] | 2006 | 33 | 15/18 | 54.6 | LR (8) HR (9) CA (16) | SUVmax | NR |
| 4.0 | |||||||
| 5.6 | |||||||
| 10.5 | |||||||
| Endo [25] | 2008 | 36 | 21/15 | 59.1 | LR (15) HR (10) CA (11) | T/M SUV | NR |
| 2.64 | |||||||
| 4.29 | |||||||
| 8.90 | |||||||
| Fukumoto [26] | 2012 | 58 | 31/27 | 62 | LR (23) HR (21) CA (14) | SUVmax | NR |
| 3.6 | |||||||
| 4.1 | |||||||
| 7.2 | |||||||
| Lococo [16] | 2013 | 47 | 25/22 | 60.9 | Thymoma (40) CA (7) | SUVmax | NR |
| 3.63 | 0.955 | ||||||
| 10.3 | |||||||
| SUVmax/T | NR | ||||||
| 0.92 | 0.927 | ||||||
| 1.93 | |||||||
| Bertolaccini [27] | 2014 | 23 | 14/9 | 52 | LR (17) HR (6) | T/M SUV | NR |
| 1.91 ± 0.45 | |||||||
| 3.73 ± 0.95 | |||||||
| MTV | NR | ||||||
| 5.51 ± 2.73 | |||||||
| 9.92 ± 2.23 | |||||||
| TGV | 383 | ||||||
| 99.12 ± 125.98 | |||||||
| 645.83 ± 159.87 | |||||||
| Benveniste [14] | 2014 | 51 | 30/21 | 59.4 | Thymoma (37) CA (12) + Carcinoid (2) | SUVmax | NR |
| 6.27 | |||||||
| 11.09 | |||||||
| SUVpeak | |||||||
| 5.53 | |||||||
| 9.38 | |||||||
| SUVmean | |||||||
| 3.85 | |||||||
| 6.72 | |||||||
| TTV_SUV45% | |||||||
| 176.31 | |||||||
| 153.71 | |||||||
| TTV_SUV3.5 | |||||||
| 139.29 | |||||||
| 203.01 | |||||||
| Park [28] | 2016 | 61 | 24/37 | 50.2 | LR (22) HR (32) CA (7) | SUVmax | 5.05 |
| 3.43 | 0.916 | ||||||
| 4.42 | |||||||
| 8.23 | |||||||
| SUVmax/T | NR | ||||||
| 0.65 | 0.886 | ||||||
| 0.91 | |||||||
| 1.77 | |||||||
| MTV | NR | ||||||
| 90.74 | 0.512 | ||||||
| 80.82 | |||||||
| 90.63 | |||||||
| TLG | NR | ||||||
| 229.36 | 0.521 | ||||||
| 233.93 | |||||||
| 390.94 | |||||||
| Purandare [29] | 2016 | 52 | 37/15 | 49 | LR (28) HR (11) CA (13) | SUVmax | 6.5 |
| 4.2 | 0.96 | ||||||
| 6.0 | |||||||
| 15.2 | |||||||
| Shinja [30] | 2017 | 56 | 32/24 | NR | LR (27) HR (14) CA (15) | ^DTP T/M | 2.39 |
| T/M (early) | |||||||
| 2.20 ± 0.86 | |||||||
| 2.02 ± 0.77 | |||||||
| 3.57 ± 1.23 | |||||||
| T/M (delayed) | |||||||
| 2.29 ± 0.98 | 2.96 | ||||||
| 2.15 ± 0.95 | |||||||
| 3.84 ± 1.55 | |||||||
| Korst [31] | 2017 | 154 | 37/15 | 49 | LR (74) HR (44) CA (23) others (13) | SUVmax | 5.55 |
| NR | 0.79 | ||||||
| Tomita [32] | 2018 | 73 | 37/36 | 63 | LR (41) HR (25) CA (7) | SUVmax | NR |
| NR | |||||||
| SUVmax/T | NR | ||||||
| NR | |||||||
| Zhao [33] | 2020 | 81 | 43/38 | 55.6 | LR (24) HR (29) CA (28) | SUVmax | 5.34 |
| 4.52 | 0.82 | ||||||
| 5.30 | |||||||
| 9.74 | |||||||
| SUVmax/T | NR | ||||||
| 0.11 | 0.691 | ||||||
| 0.13 | |||||||
| 0.17 | |||||||
| Ito [34] | 2021 | 56 | 32/24 | 61.3 | LR (26) HR (18) CA (12) | SUVmax | 7.40 |
| 4.06 | SE 0.84 SP 0.73 | ||||||
| 6.01 | |||||||
| 9.09 | |||||||
| Han [35] | 2022 | 114 | 52/62 | 56.3 | LR (52) HR (33) CA (29) | SUVmax | 6.4 |
| NR | 0.94 | ||||||
| MTV | 81.3 | ||||||
| NR | 0.84 | ||||||
| TLG | 117.7 | ||||||
| NR | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappetta, M.; Mendogni, P.; Cattaneo, M.; Evangelista, J.; Farina, P.; Pizzuto, D.A.; Annunziata, S.; Castello, A.; Congedo, M.T.; Tabacco, D.; et al. Is PET/CT Able to Predict Histology in Thymic Epithelial Tumours? A Narrative Review. Diagnostics 2023, 13, 98. https://doi.org/10.3390/diagnostics13010098
Chiappetta M, Mendogni P, Cattaneo M, Evangelista J, Farina P, Pizzuto DA, Annunziata S, Castello A, Congedo MT, Tabacco D, et al. Is PET/CT Able to Predict Histology in Thymic Epithelial Tumours? A Narrative Review. Diagnostics. 2023; 13(1):98. https://doi.org/10.3390/diagnostics13010098
Chicago/Turabian StyleChiappetta, Marco, Paolo Mendogni, Margherita Cattaneo, Jessica Evangelista, Piero Farina, Daniele Antonio Pizzuto, Salvatore Annunziata, Angelo Castello, Maria Teresa Congedo, Diomira Tabacco, and et al. 2023. "Is PET/CT Able to Predict Histology in Thymic Epithelial Tumours? A Narrative Review" Diagnostics 13, no. 1: 98. https://doi.org/10.3390/diagnostics13010098






