Abstract
Rotator cuff myosteatosis following cuff tears is very common and one of the most important prognostic factors in clinical management. Quantitative ultrasound-based imaging techniques (QUBIT) are frequently used along with magnetic resonance imaging (MRI) to evaluate rotator cuff fatty degeneration. However, the examination of rotator cuff tissue integrity by QUBIT is lacking a standardized imaging protocol and procedural methodologies. In this scoping review, we synthesized the current state of QUBIT against the reference imaging modalities in patients with rotator cuff tears. The literature search was extracted from 963 studies, with 22 studies included in the final review in accordance with the preferred reporting items for systematic reviews and meta-analyses extensions for scoping reviews. The selected studies included human participants and focused on measuring at least one prognostic or diagnostic factor using ultrasonography-based imaging with reference to MRI. The findings suggest both conventional B-mode ultrasound and shear wave elastography imaging were comparable to MRI-based imaging techniques for the evaluation of fatty infiltration and rotator cuff tear characterization. This review establishes guidelines for reporting shoulder-specific QUBIT aimed at developing a standardized imaging protocol. The objective was to enhance the diagnostic and prognostic capabilities of QUBIT in the clinical setting.
1. Introduction
Rotator cuff tears are highly prevalent, and an estimated 272,148 rotator cuff repairs were performed on an ambulatory basis in 2006 [1,2,3]. This amounted to more than $3 billion in direct surgical costs, which did not include expenses from diagnostic tests, office visits, and non-operative treatments such as physical therapy, medications, and injections [4,5]. Fatty infiltration (known as myosteatosis) of the rotator cuff muscles is an ectopic fat deposit that increases with aging and has been found to have a negative effect on tendon healing and on clinical outcomes [6]. The degree of myosteatosis is one of the most important prognostic factors in the operative and non-operative management of rotator cuff tears [7]. The incidence of irreparability of rotator cuff tears is 6.5–30% owing to tear size and fatty infiltration of the muscles [8].
In current clinical practice, risk stratification and surgical decision-making rely on outdated qualitative measurements of myosteatosis [9]. Using valid, reliable, and feasible quantitative imaging technology to evaluate myosteatosis has the potential to fill this void and can have a direct clinical impact at the point-of-care environment. However, the financial and economic impact of the use of diagnostic imaging procedures has substantially increased service demand in the healthcare system [10], due to longer life expectancy and patient expectations and preferences [11]. Magnetic resonance imaging (MRI) is the current standard for the assessment of fatty degeneration. MRI is also one of the most important contributors to healthcare costs in patients with rotator cuff tears [12]. Qualitative rotator cuff muscle atrophy and degenerative assessment using MRI often leads to inter-rater variability and potentially insensitive and inaccurate measurements of rotator cuff abnormalities [13]. Examination of the quantitative imaging at the point-of-care to assess rotator cuff muscle quality and hence its repairability is imperative to develop appropriate prognostic indications, inform care planning, and develop monitoring strategies [14].
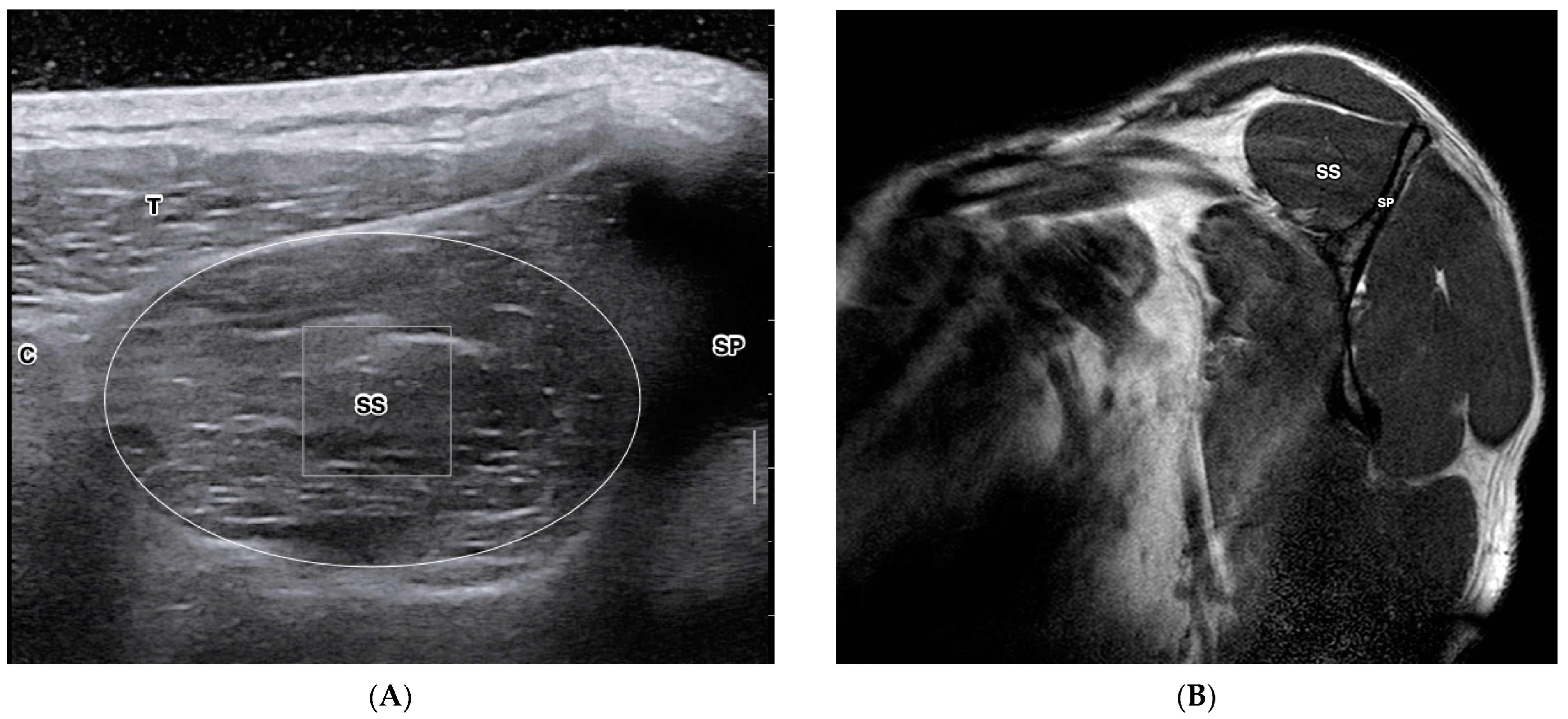
Quantitative ultrasound-based imaging techniques (QUBIT) are ultrasound technologies that derive multiparameter ultrasonographic metrics to quantify tissue properties. QUBIT is clinically accessible to quantify tissue changes induced by trauma, degeneration, healing, or tumors [15,16,17]. Studies on the QUBIT technique have shown promise in preoperative malignancy quantitative assessment for breast cancer [18], liver fibrosis [19], and thyroid lesions [20]. QUBIT has also been used to study musculoskeletal disorders, including rotator cuff tears and fatty degeneration [21,22]. Due to the nature of mechanical anisotropy and heterogeneity in musculoskeletal soft tissues, Cipriano et al. summarized the methodological challenges of establishing normative reference reporting of QUBIT in musculoskeletal soft tissues [23]. This study also suggested QUBIT-specific guidelines to standardize reporting procedures. However, there currently lacks a systematic approach to confirming rotator cuff tissue integrity using QUBIT techniques against the reference measures. Figure 1 depicts a cross-sectional view of the supraspinatus muscle on ultrasound (A) and MRI (B).
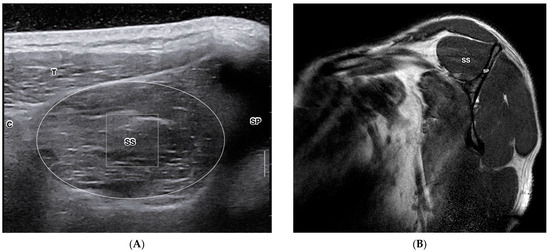
Figure 1.
Cross-sectional view of the supraspinatus muscle of the right shoulder in a healthy 29-year-old female. Short-axis view of supraspinatus muscle in B-mode diagnostic ultrasound (A) and MRI T1 sagittal-oblique view (B). C—Clavicle; SP—Scapular Spine; T—Trapezius; SS—Supraspinatus. B. T1W MRI Y-view. SP—Scapular Spine, SS—Supraspinatus.
The overall purpose of this review is to synthesize imaging methodologies utilized for QUBIT and reference imaging modalities in patients with rotator cuff tears. The objectives are to (1) identify the current knowledge gaps of QUBIT in the literature, (2) compare QUBIT against reference measurements across all articles and within the rotator cuff tissue category, and (3) provide shoulder-specific guidelines for reporting QUBIT to improve the standardized protocol and enhance reproduction and replication, which are essential to advance the diagnostic and prognostic capabilities of this technology not only as a research tool but as point-of-care technology.
2. Materials and Methods
2.1. Search Strategy
A literature search was performed to review studies involving screening of rotator cuff fatty infiltration or tear characterization utilizing QUBIT compared with reference measures. The literature search was conducted according to the guidelines of Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [24]. The literature search was conducted before December 2022 and utilized three databases: PubMed, Scopus, and OVID. The search was conducted using a combination of keywords related to ultrasound-based imaging techniques and rotator cuff fatty infiltration. For conventional ultrasound, the search keywords included those with “ultrasound” or “ultrasonography” and those with “sonography” or “ultrasound imaging”. For ultrasound elastography, the search keywords included “sonoelastography”, “elastography”, and “shear wave elastography”. For fatty infiltration, the search keywords included “fatty infiltration”, “fatty degeneration”, “myosteatosis”, “intramuscular fat”, “intramuscular adipose tissue”, “intermuscular fat”, and “intermuscular adipose tissue”. For rotator cuff, the search keywords included “shoulder”, “rotator cuff”, “supraspinatus”, “infraspinatus”, “teres minor”, “subscapularis”, and excluded “liver” or “cancer”. For searches on PubMed, the search keywords were replaced by the MeSH term.
The inclusion criteria included: (1) screening by ultrasound-based quantitative imaging; (2) diagnostic/screening tests to characterize rotator cuff tears with at least one reference measure; and (3) tests involved with and evaluated by human subject data. Studies were excluded if (1) the study population did not include evaluation of the rotator cuff in human subjects; (2) they were written in a language other than English; (3) they did not include at least one prognostic or diagnostic factor; or (4) they did not compare at least one ultrasound-based quantitative imaging modality to a current reference standard.
2.2. Data Extraction and Screening
The identified references were distributed to two authors (A.J.N. and C.P.), and abstracts were independently reviewed for inclusion. A standardized data extraction form was created by the research team. Two team members then used the pretested data extraction form to extract data from full-text articles. If there was disagreement about the inclusion of a study, a third reviewer (Y.-S.L.) was included for discussion. Finally, two authors (A.J.N. and C.P.) critically reviewed the full-text articles and summarized the findings. The following data were extracted for descriptive analysis and comparison in terms of imaging modality utilized, type of quantitative measurement, comparison to an established reference standard, and the identified prognostic or diagnostic factor explored.
2.3. Methodological Quality Analysis
Methodological quality was assessed for the studies that reported on quantitative metrics between ultrasound-based imaging modalities and the reference measure. The quality of the studies was independently assessed by two review authors (A.J.N. and C.P.). The included studies were systematically analysed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system of rating the quality of evidence [25]. The GRADE framework considers six domains that can influence the quality of the evidence and includes risk of bias, imprecision, inconsistency, indirectness, publication bias, and magnitude of the effect size. The risk of bias assessment was conducted in line with the NHLBI Risk of Bias Assessment Tool [26]. The starting point for the quality of the evidence was “high” for longitudinal studies that sought to confirm independent associations of the quantitative imaging variables between ultrasound and MRI. The evidence could decrease based on five factors: study limitations, inconsistency, indirectness, imprecision, and publication bias. Moreover, study findings with moderate or large effect sizes (i.e., lower limit of 95% confidence interval, odds ratio > 2.0 could lead to an upgrade in the quality of evidence). In total, all items across five categories for quality assessment were assessed: (1) study population, (2) assessment of imaging modality, (3) assessment outcome, (4) study design, and (5) quantitative data analysis. The criteria for each item were scored as “positive”, “negative”, or “not clear”.
3. Results
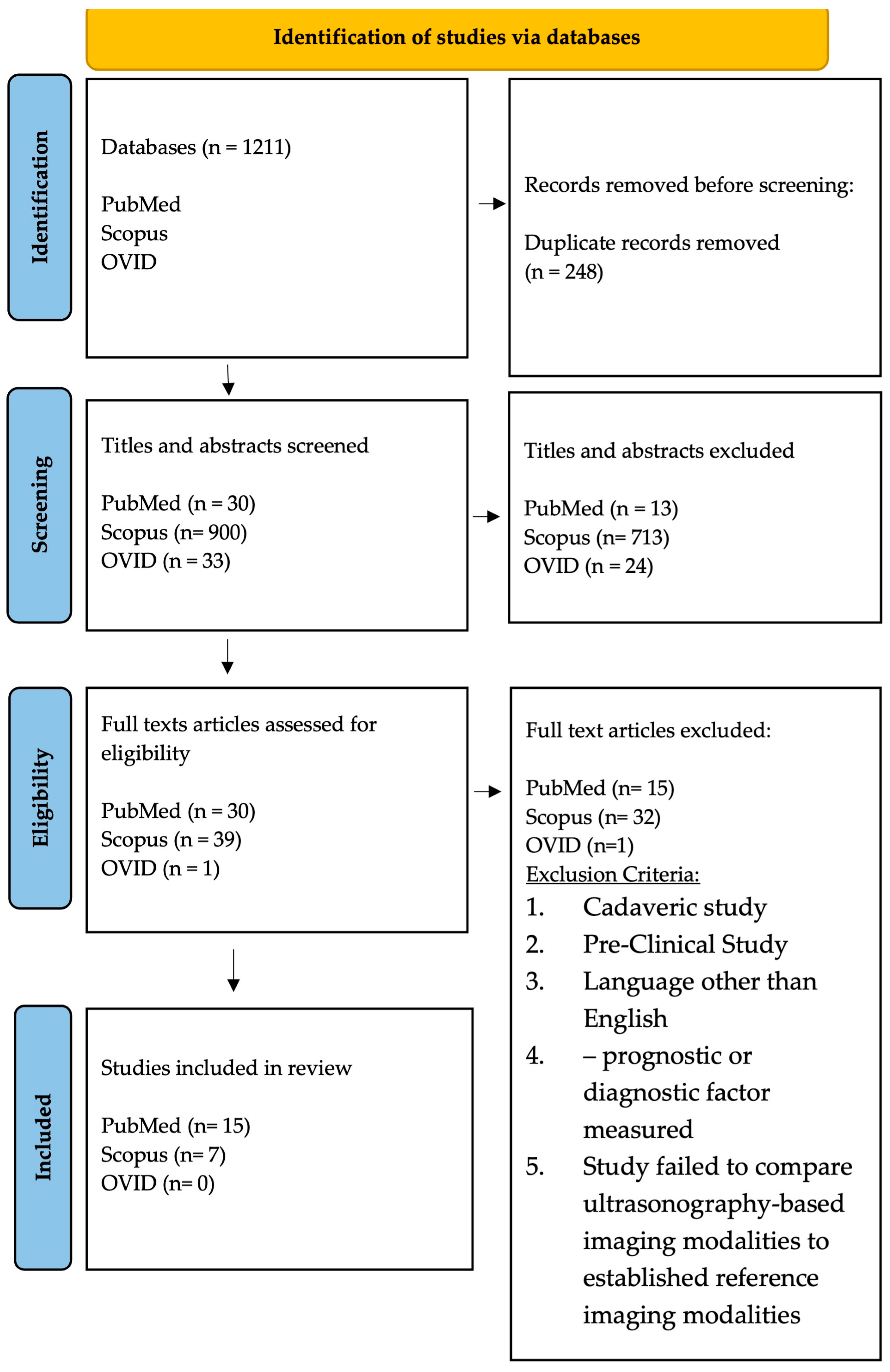
A PRISMA-ScR flowchart diagram of the study selection process is shown in Figure 2. The search identified 963 primary studies without duplication from three databases. 750 articles were excluded after title and abstract reviews. Following 70 potential eligible articles being examined through full-text reviews, a total of 22 studies met inclusion criteria and were included in the final review. In total, six prospective studies, six retrospective studies, three cross-sectional studies, two case-control studies, and five case-series studies were included. Within the 22 articles, four ultrasound-based domains were studied: 8 studies characterized rotator cuff tears using shear wave elastography (SWE), 12 studies employed B-mode ultrasound (US), 1 study used 3D-US, and finally a single study used contrast enhanced ultrasound (CEUS). The characteristics of the rotator cuff tears and imaging modalities are presented in Table 1.
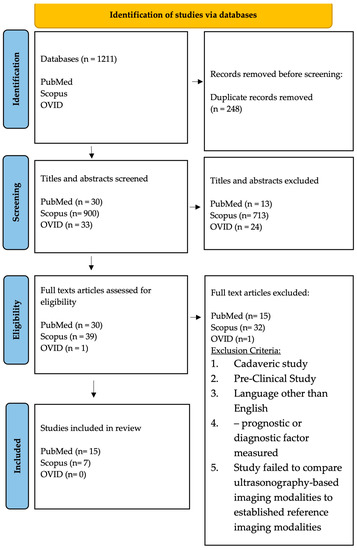
Figure 2.
PRISMA-ScR flow diagram showing the article selection process.

Table 1.
Summary of included articles.
3.1. Participant Characteristics
A total of 1156 adult shoulders were examined with sample sizes ranging from 9 to 133 shoulders. Park et al. did not describe the gender breakdown of the 105 included subjects; otherwise, the collective studies included 564 male and 474 female subjects. Additional participant characteristics are detailed in Table 1.
3.2. Characteristics of Imaging Modalities
A summary of the imaging modalities and protocols can be found in Table 2. The transducers for rotator cuff imaging were linear, with frequencies ranging from 4 MHz to 18 MHz. MRI sequencing varied across all studies, ranging from proton density, T1 and/or T2-weighted sequencing. A more detailed description of ultrasound and MRI sequencing is presented in Table 2.

Table 2.
Imaging Protocols.
3.3. Assessment of Methodological Quality
A summary of the GRADE assessment of methodological quality is presented in Table 3. Overall, 20 of the 22 articles had low to no risk of bias based on the National Heart, Lung, and Blood Institute’s Risk of Bias Assessment Tool. Two articles had a moderate risk of bias due to a dropout rate greater than 30%. For overall quality of grading for the included studies, we adapted the criteria and only utilized five domains of the GRADE framework due to inconsistent and unavailable reports on effect size (Table 3). Three studies were graded 5, eleven studies were graded 4, followed by seven studies graded 3, and one study graded 2. There were disagreements about 20 out of 110 items, all of which were resolved by discussion to assess the total quality rating. The results were presented based on grouping the imaging modalities with diagnostic or prognostic factors.

Table 3.
GRADE ratings for the quality of articles based on the risk of bias, imprecision, inconsistency, indirectness, and publication bias.
3.4. Summary of Results
Fatty Infiltration Grading with Ultrasound and MRI
Park et al. performed a direct comparison of fatty infiltrate on ultrasound to MRI by collapsing the five-point Goutallier classification to a three-point dichotomous scale [27]. The agreement between MRI and US improved in both axes when using a dichotomous scale. However, the compressed imaging data may contribute to bias and inconsistency. Watanabe et al. quantified rotator cuff fatty infiltration and compared ultrasound and MRI to assess rotator cuff muscle using Goutallier’s MRI classification, ultrasound echo intensity of the supraspinatus muscle, and the echo intensity ratio of the supraspinatus muscle related to subcutaneous fat [28]. When compared to the Goutallier MRI classification stage, a significant difference was observed in echo intensities between the subcutaneous fat and the supraspinatus muscle. In addition, the echo intensity ratio of the supraspinatus muscle was significantly lower in Goutallier stage 0/1 than the other stages (stage 0/1 vs. stage 2, p = 0.004; vs. stage 3, p = 0.001; vs. stage 4, p = 0.001). This study suggested a clinically useful, objective, and quantitative assessment of fatty infiltration within the supraspinatus muscle. However, the quantitative assessment of other rotator cuff muscles such as the infraspinatus and teres minor were unclear, particularly in part due to the small sample size and retrospective study design. Seo et al. utilized the sonoelastography technique to quantify fatty degeneration and demonstrated high sensitivity, specificity, and accuracy in grading fatty degeneration [29]. Although sonoelastography showed good correlation with MRI and conventional ultrasound, the diagnostic criteria for fatty degeneration with the use of sonoelastography techniques are unknown. Wall et al. reported the diagnostic performance of ultrasound in grading fatty degeneration of the posterior and superior rotator cuff muscles by collapsing the Goutallier classification [30]. The agreement between the US and MRI scales in supraspinatus and infraspinatus muscles was excellent (0.71 < к < 0.83) but moderate in teres minor muscle (0.47 < к < 0.52). Despite the fact that the predictive values, sensitivity, and specificity showed satisfactory results, the authors acknowledged the rater’s reliability and introduced bias due to the reduction of the rating scale being unknown. Khoury et al. compared sonography with MRI for the evaluation of fatty infiltration within the supraspinatus muscle. This study also investigated the ability of US to quantify muscle atrophy with the evaluation of the occupation ratio of the supraspinatus muscle [31]. Although conventional sonography compared with MRI showed good correlation to assess both supraspinatus muscle atrophy and fatty infiltration, the reproducibility and generalizability of these measures to other rotator cuff muscles were unclear. This review included four studies that investigated the accuracy of ultrasound imaging to detect the rotator cuff tear and compared it with MRI as a reference measure. In these studies, no statistical differences were found in diagnostic accuracy between these two modalities [33,34,35]. While these studies compared the diagnostic accuracies of rotator cuff tears between ultrasound and MRI, the prognostic capacities of quantitative ultrasound-based imaging modalities in the asymptomatic or early stages of the symptomatic phase remain to be investigated.
For the quantitative assessment of the rotator cuff muscle thickness, Ueda et al. demonstrated the agreement between the cross-sectional muscle thickness of the rotator cuff muscles on MRI and ultrasonography [36]. Significant correlations were found for the three rotator cuff muscles: supraspinatus, r = 0.67, p < 0.001; infraspinatus, r = 0.63, p < 0.001; and teres minor, r = 0.61, p < 0.001. However, the cross-sectional area has been shown to be prone to underestimating the muscle atrophy at whole muscle levels [49]. Kretic et al. performed a reliability analysis of muscle thickness in patients with ruptured and intact supraspinatus tendons [37]. For the rupture group, the occupation ratio measured by US and MRI had a Pearson correlation coefficient of r = 0.640, and for the non-rupture group, r = 0.611. When the two groups were combined, r = 0.912. Although US measurements are not as strongly correlated as the cross-sectional area and occupational ratio measured by MRI to quantify muscle atrophy, US is still considered the first imaging modality to describe supraspinatus muscle atrophy and fatty degeneration. Yi et al. investigated the relationship between supraspinatus thickness measured by ultrasound and cross-sectional area measured by MRI in both healthy and hemiplegic patients. The Pearson correlation coefficient when muscle thickness on ultrasound was measured at the scaplar notch and at the Y-view on MRI was r = 0.72. When muscle thickness was measured at the scapular notch in both US and MRI, r = 0.76 [38]. Chen et al. found medium to large effect sizes (r = 0.4–0.8) for correlating the large-to-massive rotator cuff tears and good predictive validity for surgical reparability between B-mode ultrasound and MRI findings [39]. Despite the promising findings on reliability in the examination of these measurements, the small sample size of patient populations and the lack of high quality and comprehensive quantifications of rotator cuff muscle tissue properties were not included.
Eight studies utilized SWE to compare quantitative characteristics of the rotator cuff and correlated the findings with MRI, with conflicting results. Huang et al. found that SWE-derived rotator cuff muscle stiffness is highly correlated with rotator cuff tear size and severity [40]. In addition, shear modulus values of supraspinatus and infraspinatus muscles among affected shoulders were significantly stiffer than the values in the contralateral normal shoulder. However, the shear modulus values within each region of interest were qualitatively determined without quantitative measures. Jeong et al. utilized SWE as a prognostic marker in patients with rotator cuff tears and found that patients with insufficient repair exhibited higher mean Goutallier grade, mean muscle atrophy grade, mean supraspinatus elasticity, mean elasticity ratio, and mean gray-scale fatty infiltration grade and showed a lower mean occupation ratio [42]. While the strong inter- and intra-observer agreement between SWE and MRI measures demonstrated promising prognostic markers for preoperative evaluation of fatty infiltration, the generalizability of the complementary prognostic markers of preoperative evaluation to other patient populations remains to be investigated. Krepkin et al. found a statistically significant negative correlation between shear wave velocities in anteroposterior measurement of tear size in the middle (r = −0.79) and the mean region of interest (r = −0.68) [43]. A significant negative relationship was also found among shear wave velocities, the sum of anterroposterior, and the degree of retraction measurements in the middle region of interest (r = −0.72). However, the matching criteria of regions of interest, sensitivity, and specificity between ultrasound and MRI were not described, nor was it described whether quantitative elastography is able to detect subtle changes of tendon degeneration. Itoigawa et al. found the strongest correlation of stiffness with the shear wave modulus to be the posterior deep muscle of the supraspinatus (r = 0.69) [46]. The correlation of stiffness with the Goutallier stage on MRI was weak (r = 0.48). Although there is a high correlation between ultrasound SWE and MRI measures, the length of the retracted supraspinatus tendon may influence the elasticity measured by SWE.
In terms of controversial findings to interpret the SWE findings with caution, Ruder, Lawrence, and their colleagues reported findings as the lack of statistically significant predictability of the SWE-estimated shear modulus with pre-surgical MRI variables of tear characteristics and repair integrity [41,45]. Rosskopf et al. found decreased shear wave velocities were associated with increased fat content, degrees of muscle atrophy, and tendon retraction, but not tendon integrity [44]. Gilbert et al. found a strong positive correlation (r = 0.82) between SWV measured with SWE and the fat-to-water ratio on MRI analysis [47]. However, the reproducibility of the study findings measured by SWE and spectroscopic measuring techniques was susceptible to observer-dependent bias, inhomogeneous ROIs, and a lack of biological references such as histological quantification. Kunz et al. reported a strong correlation (r = 0.67) between preoperative profusion rate on contrast-enhanced ultrasound and MRI with a 6-month follow-up reassessment of the supraspinatus tendon [48]. Although patients with repaired tendons showed significant preoperative supraspinatus perfusion within the 4th quartile at follow-up, the patients with perfusion within the 1st quartile presented with tendon retears. To establish rigorous quantitative ultrasound-based imaging as a meaningful and valid diagnostic and prognostic tool, standardized protocols, and methodologies need to be employed in future studies. Our group provides recommendations for standardization and reporting of relative methodological practices in Table 4.

Table 4.
Summary of reporting recommendations.
4. Discussion
A prerequisite to quantitative ultrasound disrupting current clinical decision-making patterns is a standardized protocol and methodological reporting. The primary aim of this scoping review was to provide a comprehensive list of methodological reporting recommendations. By employing standardized methodologies in quantitative ultrasound-based imaging, this study serves as the basis for future work involving an umbrella review and meta-analyses for a more comprehensive evaluation of the utility of quantitative imaging tools.
Quantitative ultrasound-based imaging provides objective measures for assessing the characteristics of rotator cuff pathology. Emerging imaging techniques, such as shear wave elastography imaging, are an advanced ultrasound-based modality that has seen increased utilization for better understanding musculoskeletal disorders. Despite the growing interest, guidelines for standardized protocols and clinical assessment of rotator cuff structures to incorporate current clinical practice are lacking. Currently, there is no consensus on the standardization of research methodology for the use of SWE imaging to investigate rotator cuff pathology. Prior studies have shown heterogeneity in the clinical utilization of quantitative ultrasound imaging. However, there is a lack of comparative studies to synthesize characteristic findings between quantitative ultrasound-based imaging and reference measures. To the best of our knowledge, this scoping review is the first to systematically synthesize the use of quantitative ultrasound-based imaging applications to characterize rotator cuff integrity. Future studies based on this interdisciplinary review and discussion will facilitate the development of a standardized ultrasound-based study protocol related to quantitative imaging.
Both conventional B-mode ultrasound and shear wave elastography had strong agreement compared to MRI-based imaging techniques for evaluating the grading of fatty infiltration within rotator cuff muscles. The level of agreement was strongest for the supraspinatus and infraspinatus, and the level of agreement diminished for the teres minor [30]. In addition, strong associations were found when utilizing occupation ratio as the metric for grading muscle atrophy, with a correlation coefficient of 0.90 [31]. Quantitative ultrasound also shows good utility in full-thickness rotator cuff tear detection. Previous studies investigated the accuracy of ultrasound imaging and found no statistically significant difference in diagnostic accuracy compared to MRI [33,34,35]. Studies also found strong associations between B-mode ultrasound and MRI in the measurement of muscle thickness, with correlation values ranging between 0.61 and 0.912 [36,37,38]. SWE is the emerging ultrasound-based quantitative imaging technique and has continued to show promise in the last decade. In six of the eight studies, strong correlations were found between repairability of rotator cuff tears and tear size associated with SWE-derived measures of tissue stiffness. However, Ruder and Lawrence did not find significant associations between SWE-derived measures of stiffness and predictability of post-operative function, tear size, tendon retraction, occupation ratio, or fatty infiltration, respectively. One of the potential explanations for the contradictory findings is attributed to the fundamental difference in medical imaging and technical constructs between SWE and MRI [41,45]. Shear-wave elastography can assess various aspects of tissue properties, whereas MRI-based imaging assesses structural and compositional changes or chemical shifts within the tissues [50,51,52]. Based on findings from an in vitro study, there appears to be clinical promise in combining MRI and SWE in the treatment planning for patients with rotator cuff tears [21]. Contrast-enhanced ultrasound imaging was underrepresented in this scoping review and shows great promise in musculoskeletal applications. Kunz and colleagues found a strong correlation between profusion rate and the healing status of the supraspinatus. Patients with higher profusion rates had a greater likelihood of an intact repair at 6 months. However, the relevance of the findings was hampered by a small sample size and insufficient power, partially due to the higher attrition rate of enrolled patients who completed the follow-up assessments. Future studies using contrast-enhanced ultrasound imaging are needed to increase the clinical utilization and quantitative promises of these imaging measures.
Quantitative ultrasound-based imaging is inherent to conventional B-mode ultrasound measures: rater dependence and steep learning curves. Investigators need to be keenly aware of patient position throughout ultrasound imaging, as any volitional movement or tension on the muscle of interest can skew findings. 21 of the 22 authors reported patient positioning during ultrasound examination; providing a detailed description of patient position is paramount to not only the reproducibility of the methods but also to minimize bias during data collection. Nevertheless, there are advantages to this type of imaging modality, including the absence of ionizing radiation, cost-effectiveness, and real-time application with subsequent interpretation of findings during functional examinations. Standard B-mode, SWE, and CEUS had similar findings when compared to a reference measure. B-mode ultrasound had similar diagnostic accuracy to MRI as well as the ability to measure fatty infiltration and muscle cross-sectional area. SWE, similarly, was comparable for quantifying fatty infiltration, but results were mixed for quantifying stiffness of the rotator cuff muscles and tendons. The CEUS profusion rate had a strong correlation with the integrity of the repaired supraspinatus tendon with MRI findings. Overall, quantitative ultrasound evaluation of rotator cuff tears shows promise, but standardized reporting of methodology will allow for more rigorous assessments of its scientific merit and clinical utilization. As evident from the GRADE Framework, 8 of the 22 studies had overt limitations in study design, data collection, data reporting, and/or other factors leading to a grade of less than or equal to 3 (out of a possible 5).
Rotator cuff tears remain a highly prevalent musculoskeletal condition and account for more than $3 billion in direct surgical costs [1,2,3]. Costs associated with diagnostic imaging are excluded from these cost estimates [4,5]. A 2017 study found the mean cost to the consumer of a shoulder MRI was $1874 (ranging from $500 to $4000) [53]. Risk factors associated with rotator cuff disease remain mostly unmodifiable, including but not limited to age, genetics, and trauma [54]. A previous review concluded that the comprehensive analyses for evaluating fatty infiltration of the rotator cuff involved mostly qualitative or quasi-quantitative methodologies [55]. Given that the presence of fatty infiltration is a strong predictor of poor outcome in patients undergoing rotator cuff repairs, cost-effective diagnostic tools for quantitative evaluation of fatty infiltration at the bedside can greatly improve the management of this patient population. The emergence of quantitative ultrasound-based imaging as a point-of-care tool can improve the decision-making process in the management of patients with rotator cuff disease. Quantitative imaging remains a vital tool in designing more rigorous musculoskeletal research protocols compared to qualitative or quasi-quantitative methods. Establishing ultrasound-based imaging as a reliable and valid tool will reduce the financial burden on the healthcare system in the U.S. To address the limitations within the current literature, this study provides thorough reporting recommendations for the use of QUBIT in the examination of shoulder-related conditions. The focus of these recommendations is to standardize the reporting of participant demographics, study methodologies, imaging protocols, and imaging results with the goal of maximizing the reproducibility and clinical significance of future studies.
This study has several limitations associated with the current state of quantitative ultrasound imaging research. most notably the inconsistent reporting of study methodology. As evident in Table 3, many studies did not provide a detailed imaging protocol for ultrasound or reference imaging. Secondly, the current level of evidence is primarily case-series, case-control, or retrospective studies, each with inherent limitations. Final validation of these new imaging modalities requires more rigorous investigation with large sample sizes and objective metrics. Thirdly, most published data involved studies lacking a sample size justification, and no study has employed the highest level of study design, such as a randomized clinical trial in a longitudinal setting. Finally, given the technical nature and operator dependence of ultrasound imaging, reliability assessments are warranted to inform comparative effectiveness. To date, quantitative ultrasound-based imaging for the assessment of rotator cuff tears remains an early-stage exploratory modality, and the need to validate these tools in the clinical setting remains a glaring gap in the literature.
5. Conclusions
This scoping review synthesizes the current state of the literature on the use of QUBIT for assessing rotator cuff pathology. The review of the literature found that both conventional B-mode ultrasound and SWE imaging were comparable to MRI-based imaging techniques for the evaluation of fatty infiltration and rotator cuff tear characterization. SWE is an emerging form of QUBIT and shows great promise in the quantitative evaluation of rotator cuff characteristics and fatty infiltration. Future work, including emerging radiomic features extracted from multi-parameter MRI, ultrasound-based SWE, and an artificial intelligence approach, is warranted to predict clinically significant rotator cuff lesions and distinguish the rotator cuff pathology phenotypes.
Author Contributions
Writing, A.J.N. and Y.-S.L.; conceptualization, A.J.N., C.J.P. and Y.-S.L.; supervision, Y.-S.L., N.B.J. and M.K.; review and editing N.B.J. and Y.-T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by Research Advisory Committee Interdisciplinary Grant, School of Health Professions, UT Southwestern and Hoffman Endowment Fund, Department of Orthopaedic Surgery, UT Southwestern.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data underlying this article will be shared upon reasonable request to the corresponding authors.
Acknowledgments
The authors would like to thank the funding supports of the Research Advisory Committee Interdisciplinary Grant, School of Health Professions, UT Southwestern and Hoffman Endowment Fund, Department of Orthopaedic Surgery, UT Southwestern.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colvin, A.C.; Egorova, N.; Harrison, A.K.; Moskowitz, A.; Flatow, E.L. National trends in rotator cuff repair. J. Bone Jt. Surg. Am. 2012, 94, 227–233. [Google Scholar] [CrossRef]
- Jain, N.B.; Higgins, L.D.; Losina, E.; Collins, J.; Blazar, P.E.; Katz, J.N. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskelet. Disord. 2014, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; van Doorn, P.; Hegedus, E.; Lewis, J.; van der Windt, D. A systematic review of the global prevalence and incidence of shoulder pain. BMC Musculoskelet. Disord. 2022, 23, 1073. [Google Scholar] [CrossRef] [PubMed]
- Mather, R.C., 3rd; Koenig, L.; Acevedo, D.; Dall, T.M.; Gallo, P.; Romeo, A.; Tongue, J.; Williams, G., Jr. The societal and economic value of rotator cuff repair. J. Bone Jt. Surg. Am. 2013, 95, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.A.; Vitale, M.G.; Zivin, J.G.; Braman, J.P.; Bigliani, L.U.; Flatow, E.L. Rotator cuff repair: An analysis of utility scores and cost-effectiveness. J. Shoulder Elb. Surg. 2007, 16, 181–187. [Google Scholar] [CrossRef]
- Raz, Y.; Henseler, J.F.; Kolk, A.; Riaz, M.; van der Zwaal, P.; Nagels, J.; Nelissen, R.G.; Raz, V. Patterns of Age-Associated Degeneration Differ in Shoulder Muscles. Front. Aging Neurosci. 2015, 7, 236. [Google Scholar] [CrossRef]
- Naimark, M.; Trinh, T.; Robbins, C.; Rodoni, B.; Carpenter, J.; Bedi, A.; Miller, B. Effect of Muscle Quality on Operative and Nonoperative Treatment of Rotator Cuff Tears. Orthop. J. Sport. Med. 2019, 7, 2325967119863010. [Google Scholar] [CrossRef]
- Juhan, T.; Stone, M.; Jalali, O.; Curtis, W.; Prodromo, J.; Weber, A.E.; Hatch, G.F., III; Omid, R. Irreparable rotator cuff tears: Current treatment options. Orthop. Rev. 2019, 11, 8146. [Google Scholar] [CrossRef]
- Miyazaki, A.N.; Santos, P.D.; da Silva, L.A.; Sella Gdo, V.; Miranda, E.R.; Zampieri, R. Fatty Muscle Infiltration in Cuff Tear: Pre and Post Operative Evaluation by Mri. Acta Ortop. Bras. 2015, 23, 251–254. [Google Scholar] [CrossRef]
- European Society of Radiology (ESR). The consequences of the economic crisis in radiology. Insights Imaging 2015, 6, 573–577. [Google Scholar] [CrossRef]
- Ristori, D.; Miele, S.; Rossettini, G.; Monaldi, E.; Arceri, D.; Testa, M. Towards an integrated clinical framework for patient with shoulder pain. Arch. Physiother. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Walker-Bone, K.; van der Windt, D.A.W.M. Shoulder Pain—Where Are We Now? Curr. Treat. Options Rheumatol. 2021, 7, 285–306. [Google Scholar] [CrossRef]
- Shah, S.A.; Kormpakis, I.; Cavinatto, L.; Killian, M.L.; Thomopoulos, S.; Galatz, L.M. Rotator cuff muscle degeneration and tear severity related to myogenic, adipogenic, and atrophy genes in human muscle. J. Orthop. Res. 2017, 35, 2808–2814. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Remer, E.M.; Sakaie, K.; Schneider, E.; Fox, R.J.; Nakamura, K.; Avila, R.; Guimaraes, A. Importance of incorporating quantitative imaging biomarker technical performance characteristics when estimating treatment effects. Clin. Trials 2021, 18, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, Y.; Jiang, W.; Luo, Y.; Peng, J.; Chen, M.; Jing, X. Quantitative Evaluation of Denervated Muscle Atrophy with Shear Wave Ultrasound Elastography and a Comparison with the Histopathologic Parameters in an Animal Model. Ultrasound Med. Biol. 2018, 44, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, C.M.; Baumer, T.G.; Juliano, L.; Sweeney, M.; McGinnis, M.; Sherwood, A.; Moutzouros, V.; Bey, M.J. Shear wave elastography of the healing human patellar tendon following ACL reconstruction. Knee 2019, 26, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Zhao, X.; Shi, J.; Huang, Y. Value of shear wave elastography for diagnosis of primary prostate cancer: A systematic review and meta-analysis. Med. Ultrason. 2019, 21, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Gao, Y.; Chang, C.; Wang, F.; Zeng, W.; Chen, J.J. Ultrasound shear wave elastography of breast lesions: Correlation of anisotropy with clinical and histopathological findings. Cancer Imaging 2018, 18, 11. [Google Scholar] [CrossRef]
- Ding, H.; Ma, J.J.; Wang, W.P.; Zeng, W.J.; Jiang, T.; Huang, B.J.; Chen, S.Y. Assessment of liver fibrosis: The relationship between point shear wave elastography and quantitative histological analysis. J. Gastroenterol. Hepatol. 2015, 30, 553–558. [Google Scholar] [CrossRef]
- Zhao, C.K.; Chen, S.G.; Alizad, A.; He, Y.P.; Wang, Q.; Wang, D.; Yue, W.W.; Zhang, K.; Qu, S.; Wei, Q.; et al. Three-Dimensional Shear Wave Elastography for Differentiating Benign From Malignant Thyroid Nodules. J. Ultrasound Med. 2018, 37, 1777–1788. [Google Scholar] [CrossRef]
- Giambini, H.; Hatta, T.; Rezaei, A.; An, K.N. Extensibility of the supraspinatus muscle can be predicted by combining shear wave elastography and magnetic resonance imaging-measured quantitative metrics of stiffness and volumetric fat infiltration: A cadaveric study. Clin. Biomech. 2018, 57, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hwang, H.J.; Kim, S.G.; Lee, J.H.; Jeong, W.K. Can Shoulder Muscle Activity Be Evaluated With Ultrasound Shear Wave Elastography? Clin. Orthop. Relat. Res. 2018, 476, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, K.J.; Wickstrom, J.; Glicksman, M.; Hirth, L.; Farrell, M.; Livinski, A.A.; Esfahani, S.A.; Maldonado, R.J.; Astrow, J.; Berrigan, W.A.; et al. A scoping review of methods used in musculoskeletal soft tissue and nerve shear wave elastography studies. Clin. Neurophysiol. 2022, 140, 181–195. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 9 October 2022).
- Park, B.K.; Hong, S.H.; Jeong, W.K. Effectiveness of Ultrasound in Evaluation of Fatty Infiltration in Rotator Cuff Muscles. Clin. Orthop. Surg. 2020, 12, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Terabayashi, N.; Fukuoka, D.; Murakami, H.; Ito, H.; Matsuoka, T.; Seishima, M. A pilot study to assess Fatty infiltration of the supraspinatus in patients with rotator cuff tears: Comparison with magnetic resonance imaging. Ultrasound Med. Biol. 2015, 41, 1779–1783. [Google Scholar] [CrossRef]
- Seo, J.B.; Yoo, J.S.; Ryu, J.W. The accuracy of sonoelastography in fatty degeneration of the supraspinatus: A comparison of magnetic resonance imaging and conventional ultrasonography. J. Ultrasound 2014, 17, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wall, L.B.; Teefey, S.A.; Middleton, W.D.; Dahiya, N.; Steger-May, K.; Kim, H.M.; Wessell, D.; Yamaguchi, K. Diagnostic performance and reliability of ultrasonography for fatty degeneration of the rotator cuff muscles. J. Bone Jt. Surg. Am. 2012, 94, e83. [Google Scholar] [CrossRef]
- Khoury, V.; Cardinal, E.; Brassard, P. Atrophy and fatty infiltration of the supraspinatus muscle: Sonography versus MRI. AJR Am. J. Roentgenol. 2008, 190, 1105–1111. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Chen, J.; Zhang, F.; Xu, L.; Yan, X.; Zhu, Y.; Zhang, Q.; Tang, J. Clinical value of three-dimensional ultrasonography in the morphologic evaluation of rotator cuff tear: A prospective study. Eur. Radiol. 2023, 33, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Guerini, H.; Pluot, E.; Pessis, E.; Thevenin, F.; Campagna, R.; Feydy, A.; Gaudin, P.; Drape, J.L. Tears at the myotendinous junction of the infraspinatus: Ultrasound findings. Diagn. Interv. Imaging 2015, 96, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Haw-Chang Lan, H.; Lai, K.-L.; Chen, H.-H.; Chen, Y.-M.; Chen, C.-P. Diagnostic Utility of US for Detecting Rotator Cuff Tears in Rheumatoid Arthritis Patients: Comparison with Magnetic Resonance Imaging. J. Med. Ultrasound 2014, 22, 200–206. [Google Scholar] [CrossRef]
- Rutten, M.J.; Spaargaren, G.J.; van Loon, T.; de Waal Malefijt, M.C.; Kiemeney, L.A.; Jager, G.J. Detection of rotator cuff tears: The value of MRI following ultrasound. Eur. Radiol. 2010, 20, 450–457. [Google Scholar] [CrossRef]
- Ueda, Y.; Tanaka, H.; Takeuchi, Y.; Tachibana, T.; Inui, H.; Nobuhara, K.; Umehara, J.; Ichihashi, N. Agreement in rotator cuff muscles measurement between ultrasonography and magnetic resonance imaging. Asia-Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2022, 28, 13–20. [Google Scholar] [CrossRef]
- Kretic, D.; Turk, T.; Rotim, T.; Saric, G. Reliability of Ultrasound Measurement of Muscle Thickness in Patients with Supraspinatus Tendon Pathology. Acta Clin. Croat. 2018, 57, 335–341. [Google Scholar] [CrossRef]
- Yi, T.I.; Han, I.S.; Kim, J.S.; Jin, J.R.; Han, J.S. Reliability of the supraspinatus muscle thickness measurement by ultrasonography. Ann. Rehabil. Med. 2012, 36, 488–495. [Google Scholar] [CrossRef]
- Chen, P.C.; Wu, K.T.; Chen, Y.C.; Huang, Y.C.; Chang, C.D.; Lin, W.C.; Chou, W.Y. Predicting the surgical reparability of large-to-massive rotator cuff tears by B-mode ultrasonography: A cross-sectional study. Ultrasonography 2022, 41, 177–188. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, L.; Wang, J.; Wu, D.; Huang, W.; Hu, N.; Chen, H. Ultrasound shear wave elastography-derived tissue stiffness is positively correlated with rotator cuff tear size and muscular degeneration. Knee Surg. Sport. Traumatol. Arthrosc. 2022, 30, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Ruder, M.C.; Lawrence, R.L.; Soliman, S.B.; Bey, M.J. Presurgical tear characteristics and estimated shear modulus as predictors of repair integrity and shoulder function one year after rotator cuff repair. JSES Int. 2022, 6, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Khil, E.K.; Kim, A.Y.; Lee, S.A.; Choi, J.A. Utility of Preoperative Shear-Wave Elastography of the Supraspinatus Muscle for Predicting Successful Rotator Cuff Repair: A Prospective Observational Study with MRI Correlation. AJR Am. J. Roentgenol. 2022, 218, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Krepkin, K.; Bruno, M.; Raya, J.G.; Adler, R.S.; Gyftopoulos, S. Quantitative assessment of the supraspinatus tendon on MRI using T2/T2* mapping and shear-wave ultrasound elastography: A pilot study. Skelet. Radiol. 2017, 46, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Rosskopf, A.B.; Ehrmann, C.; Buck, F.M.; Gerber, C.; Fluck, M.; Pfirrmann, C.W. Quantitative Shear-Wave US Elastography of the Supraspinatus Muscle: Reliability of the Method and Relation to Tendon Integrity and Muscle Quality. Radiology 2016, 278, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.L.; Ruder, M.C.; Moutzouros, V.; Makhni, E.C.; Muh, S.J.; Siegal, D.; Soliman, S.B.; van Holsbeeck, M.; Bey, M.J. Ultrasound shear wave elastography and its association with rotator cuff tear characteristics. JSES Int. 2021, 5, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Itoigawa, Y.; Maruyama, Y.; Kawasaki, T.; Wada, T.; Yoshida, K.; An, K.N.; Kaneko, K. Shear Wave Elastography Can Predict Passive Stiffness of Supraspinatus Musculotendinous Unit During Arthroscopic Rotator Cuff Repair for Presurgical Planning. Arthroscopy 2018, 34, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.; Klein, D.; Weng, A.M.; Kostler, H.; Schmitz, B.; Schmalzl, J.; Bohm, D. Supraspinatus muscle elasticity measured with real time shear wave ultrasound elastography correlates with MRI spectroscopic measured amount of fatty degeneration. BMC Musculoskelet. Disord. 2017, 18, 549. [Google Scholar] [CrossRef]
- Kunz, P.; Mick, P.; Gross, S.; Schmidmaier, G.; Zeifang, F.; Weber, M.A.; Fischer, C. Contrast-Enhanced Ultrasound (CEUS) as Predictor for Early Retear and Functional Outcome After Supraspinatus Tendon Repair. J. Orthop. Res. 2020, 38, 1150–1158. [Google Scholar] [CrossRef]
- Frontera, W.R.; Reid, K.F.; Phillips, E.M.; Krivickas, L.S.; Hughes, V.A.; Roubenoff, R.; Fielding, R.A. Muscle fiber size and function in elderly humans: A longitudinal study. J. Appl. Physiol. 2008, 105, 637–642. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Jahanvi, V.; Kelkar, A. Chemical shift imaging: An indispensable tool in diagnosing musculoskeletal pathologies. S. Afr. J. Radiol. 2021, 25, 2061. [Google Scholar] [CrossRef]
- De Mello, R.; Ma, Y.; Ji, Y.; Du, J.; Chang, E.Y. Quantitative MRI Musculoskeletal Techniques: An Update. AJR Am. J. Roentgenol. 2019, 213, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Westermann, R.W.; Schick, C.; Graves, C.M.; Duchman, K.R.; Weinstein, S.L. What Does a Shoulder MRI Cost the Consumer? Clin. Orthop. Relat. Res. 2017, 475, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Bodendorfer, B.M. CORR Insights(R): What Factors Are Associated with Symptomatic Rotator Cuff Tears: A Meta-analysis. Clin. Orthop. Relat. Res. 2022, 480, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Tenbrunsel, T.N.; Whaley, J.D.; Golchian, D.; Malone, D.L.; Lima, D.J.L.; Sabesan, V.J. Efficacy of Imaging Modalities Assessing Fatty Infiltration in Rotator Cuff Tears. JBJS Rev. 2019, 7, e3. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).