What Imaging Modality Is More Effective in Predicting Early Recurrence of Hepatocellular Carcinoma after Hepatectomy Using Radiomics Analysis: CT or MRI or Both?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Follow-Up and Study Endpoint
2.3. Imaging Techniques
2.4. Image Analysis
2.5. Radiomics Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Information
3.2. Radiologic Characteristics
3.3. Radiomics Feature Selection
3.4. Comparison Performance of Different Models for Discriminating ER vs. Non-ER
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Lee, Y.-S.; Kim, B.K.; Jung, Y.K.; Kim, S.U.; Park, J.Y.; Kim, J.H.; An, H.; Kim, D.Y.; Yim, H.J.; et al. Change in the Recurrence Pattern and Predictors over Time after Complete Cure of Hepatocellular Carcinoma. Gut Liver 2021, 15, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Yong, C.-C.; Lin, C.-C.; Wang, C.-C.; Chen, C.-L.; Cheng, Y.-F.; Wang, J.-H.; Yen, Y.-H. Six months as a cutoff time point to define early recurrence after liver resection of hepatocellular carcinoma based on post-recurrence survival. Updat. Surg. 2021, 73, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.L.; Zhu, J.K.; Yang, Y.; Wang, Y.D.; Liu, F.Y.; Zhu, M.; Liu, C.Z. Repeated postoperative adjuvant TACE after curative hepatectomy improves outcomes of patients with HCC. Minim. Invasive Ther. Allied Technol. 2021, 30, 163–168. [Google Scholar] [CrossRef]
- Harding-Theobald, E.; Louissaint, J.; Maraj, B.; Cuaresma, E.; Townsend, W.; Mendiratta-Lala, M.; Singal, A.G.; Su, G.L.; Lok, A.S.; Parikh, N.D. Systematic review: Radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 54, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Hectors, S.; Taouli, B. Radiomics of hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 111–123. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ouhmich, F.; Gonzalez-Cabrera, C.; Felli, E.; Saviano, A.; Agnus, V.; Savadjiev, P.; Baumert, T.F.; Pessaux, P.; Marescaux, J.; et al. Radiomics in hepatocellular carcinoma: A quantitative review. Hepatol. Int. 2019, 13, 546–559. [Google Scholar] [CrossRef]
- Zhou, Y.; He, L.; Huang, Y.; Chen, S.; Wu, P.; Ye, W.; Liu, Z.; Liang, C. CT-based radiomics signature: A potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom. Radiol. 2017, 42, 1695–1704. [Google Scholar] [CrossRef]
- Lee, I.-C.; Huang, J.-Y.; Chen, T.-C.; Yen, C.-H.; Chiu, N.-C.; Hwang, H.-E.; Huang, J.-G.; Liu, C.-A.; Chau, G.-Y.; Lee, R.-C.; et al. Evolutionary Learning-Derived Clinical-Radiomic Models for Predicting Early Recurrence of Hepatocellular Carcinoma after Resection. Liver Cancer 2021, 10, 572–582. [Google Scholar] [CrossRef]
- Ji, G.-W.; Zhu, F.-P.; Xu, Q.; Wang, K.; Wu, M.-Y.; Tang, W.-W.; Li, X.-C.; Wang, X.-H. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology 2020, 294, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-B.; Zheng, Z.-Y.; Zhao, H.; Zhang, J.; Li, Y.-H.; Dong, Z.-Y.; Xiao, L.-S.; Kuang, J.-J.; Zhang, X.-L.; Liu, L. Radiomics-based nomogram using CT imaging for noninvasive preoperative prediction of early recurrence in patients with hepatocellular carcinoma. Diagn. Interv. Radiol. 2020, 26, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.-W.; Zhu, F.-P.; Xu, Q.; Wang, K.; Wu, M.-Y.; Tang, W.-W.; Li, X.-C.; Wang, X.-H. Machine-learning analysis of contrast-enhanced CT radiomics predicts recurrence of hepatocellular carcinoma after resection: A multi-institutional study. Ebiomedicine 2019, 50, 156–165. [Google Scholar] [CrossRef]
- Shan, Q.-Y.; Hu, H.-T.; Feng, S.-T.; Peng, Z.-P.; Chen, S.-L.; Zhou, Q.; Li, X.; Xie, X.-Y.; Lu, M.-D.; Wang, W.; et al. CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Long, L.-H.; Cui, Y.; Jia, A.Y.; Zhu, X.-G.; Wang, H.-Z.; Wang, Z.; Zhan, C.-M.; Wang, Z.-H.; Wang, W.-H. MRI-based radiomics model for preoperative prediction of 5-year survival in patients with hepatocellular carcinoma. Br. J. Cancer 2020, 122, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Zhang, Q.; Hua, Z.; Qi, W.; Wang, N.; Lin, T.; Sheng, L.; Cui, D.; Liu, J.; et al. Radiomics Analysis Based on Multiparametric MRI for Predicting Early Recurrence in Hepatocellular Carcinoma After Partial Hepatectomy. J. Magn. Reson. Imaging 2021, 53, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, J.; Kim, D.-Y.; Choi, G.H.; Kim, M.-J.; Choi, J.-Y. Radiomics on Gadoxetic Acid–Enhanced Magnetic Resonance Imaging for Prediction of Postoperative Early and Late Recurrence of Single Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 3847–3855. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, H.; Chen, J.; Wei, Y.; Cao, L.; Ye, Z.; Li, X.; Ma, L.; Song, B. Hepatocellular carcinoma: Radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging 2019, 19, 22. [Google Scholar] [CrossRef]
- Chong, H.; Gong, Y.; Pan, X.; Liu, A.; Chen, L.; Yang, C.; Zeng, M. Peritumoral Dilation Radiomics of Gadoxetate Disodium-Enhanced MRI Excellently Predicts Early Recurrence of Hepatocellular Carcinoma without Macrovascular Invasion After Hepatectomy. J. Hepatocell. Carcinoma 2021, 8, 545–563. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Hou, J.; Jiang, X.; Guo, L.; Tian, L. Radiomics-based model using gadoxetic acid disodium-enhanced MR images: Associations with recurrence-free survival of patients with hepatocellular carcinoma treated by surgical resection. Abdom. Radiol. 2021, 46, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Guo, R.; Liu, W.; Li, J.; Zong, X.; Chen, Q.; Wang, J. The diagnostic performance of contrast-enhanced CT versus extracellular contrast agent-enhanced MRI in detecting hepatocellular carcinoma: Direct comparison and a meta-analysis. Abdom. Radiol. 2022, 47, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, S.; Li, X. Comparison of values of CT and MRI imaging in the diagnosis of hepatocellular carcinoma and analysis of prognostic factors. Oncol. Lett. 2019, 17, 1184–1188. [Google Scholar] [CrossRef]
- Cannella, R.; Ronot, M.; Sartoris, R.; Cauchy, F.; Hobeika, C.; Beaufrere, A.; Trapani, L.; Paradis, V.; Bouattour, M.; Bonvalet, F.; et al. Enhancing capsule in hepatocellular carcinoma: Intra-individual comparison between CT and MRI with extracellular contrast agent. Diagn. Interv. Imaging 2021, 102, 735–742. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Y.; Zhou, J.; Yu, Q.; Lu, C.; Xia, C.; Tang, T.; Xu, J.; Sun, K.; Xiao, W.; et al. Comparison of MRI and CT for the Prediction of Microvascular Invasion in Solitary Hepatocellular Carcinoma Based on a Non-Radiomics and Radiomics Method: Which Imaging Modality Is Better? J. Magn. Reson. Imaging 2021, 54, 526–536. [Google Scholar] [CrossRef]
- He, Y.; Hu, B.; Zhu, C.; Xu, W.; Ge, Y.; Hao, X.; Dong, B.; Chen, X.; Dong, Q.; Zhou, X. A Novel Multimodal Radiomics Model for Predicting Prognosis of Resected Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 745258. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Zhang, Y.-D.; Hou, Y.; Yan, X.; Wang, Y.; Zhou, M.; Yao, Y.-F.; Yang, G. FeAture Explorer (FAE): A tool for developing and comparing radiomics models. PLoS ONE 2020, 15, e0237587. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, S.; Wei, J.; Liu, F.; Li, B.; Li, X.; Hou, Y.; Gu, D.; Tang, M.; Xiao, H.; et al. Pretreatment prediction of immunoscore in hepatocellular cancer: A radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur. Radiol. 2019, 29, 4177–4187. [Google Scholar] [CrossRef]
- Zech, C.J.; Reiser, M.F.; Herrmann, K.A. Imaging of Hepatocellular Carcinoma by Computed Tomography and Magnetic Resonance Imaging: State of the Art. Dig. Dis. 2009, 27, 114–124. [Google Scholar] [CrossRef]
- Kiryu, S.; Akai, H.; Nojima, M.; Hasegawa, K.; Shinkawa, H.; Kokudo, N.; Yasaka, K.; Ohtomo, K. Impact of hepatocellular carcinoma heterogeneity on computed tomography as a prognostic indicator. Sci. Rep. 2017, 7, 12689. [Google Scholar] [CrossRef]
- Sheng, R.; Zeng, M.; Jin, K.; Zhang, Y.; Wu, D.; Sun, H. MRI-based Nomogram Predicts the Risk of Progression of Unresectable Hepatocellular Carcinoma After Combined Lenvatinib and anti-PD-1 Antibody Therapy. Acad. Radiol. 2022, 29, 819–829. [Google Scholar] [CrossRef]
- Lee, C.-M.; Choi, S.H.; Byun, J.H.; Lee, S.J.; Kim, S.Y.; Won, H.J.; Shin, Y.M.; Kim, P.-N. Combined computed tomography and magnetic resonance imaging improves diagnosis of hepatocellular carcinoma ≤ 3.0 cm. Hepatol. Int. 2021, 15, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Beumer, B.R.; Takagi, K.; Vervoort, B.; Buettner, S.; Umeda, Y.; Yagi, T.; Fujiwara, T.; Steyerberg, E.W.; Ijzermans, J.N.M. Prediction of Early Recurrence After Surgery for Liver Tumor (ERASL): An International Validation of the ERASL Risk Models. Ann. Surg. Oncol. 2021, 28, 8211–8220. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, W.; Song, D.; Yang, C.; Zhu, K.; Zeng, M.; Rao, S.-X.; Wang, M. A predictive model integrating deep and radiomics features based on gadobenate dimeglumine-enhanced MRI for postoperative early recurrence of hepatocellular carcinoma. Radiol. Med. 2022, 127, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, H.; Tanaka, S.; Kabata, D.; Takemura, S.; Amano, R.; Kimura, K.; Kinoshita, M.; Kubo, S. The Prognostic Impact of Tumor Differentiation on Recurrence and Survival after Resection of Hepatocellular Carcinoma Is Dependent on Tumor Size. Liver Cancer 2021, 10, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Lee, Y.J.; Lee, K.B.; Han, J.K. Added Value of sequentially performed gadoxetic acid-enhanced liver MRI for the diagnosis of small (10–19 mm) or atypical hepatic observations at contrast-enhanced CT: A prospective comparison: Value of EOB-MR in Small or Atypical Nodules. J. Magn. Reson. Imaging 2019, 49, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.H.; Mohammed, Z.; Qayyum, T.; Horgan, P.G.; McMillan, D.C. The prognostic value of histological tumor necrosis in solid organ malignant disease: A systematic review. Futur. Oncol. 2011, 7, 1223–1235. [Google Scholar] [CrossRef]
- Bijelic, L.; Rubio, E.R. Tumor Necrosis in Hepatocellular Carcinoma—Unfairly Overlooked? Ann. Surg. Oncol. 2021, 28, 600–601. [Google Scholar] [CrossRef]
- Li, X.; Yao, Q.; Liu, C.; Wang, J.; Zhang, H.; Li, S.; Cai, P. Macrotrabecular-Massive Hepatocellular Carcinoma: What Should We Know? J. Hepatocell. Carcinoma 2022, 9, 379–387. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, Y.; Lin, F.; Luo, X.; Lin, Z.; Zhang, Y.; Li, L.; Li, Z.-P.; Feng, S.-T.; Cai, H.; et al. Prediction of Early Treatment Response to Initial Conventional Transarterial Chemoembolization Therapy for Hepatocellular Carcinoma by Machine-Learning Model Based on Computed Tomography. J. Hepatocell. Carcinoma 2021, 8, 1473–1484. [Google Scholar] [CrossRef]
- Tan, C.H.; Thng, C.H.; Low, A.S.C.; Tan, V.K.M.; Hartono, S.; Koh, T.S.; Goh, B.K.P.; Cheow, P.C.; Tan, Y.M.; Chung, A.Y.F.; et al. Wash-out of hepatocellular carcinoma: Quantitative region of interest analysis on CT. Ann. Acad. Med. Singap. 2011, 40, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, M.D.; Lythgoe, M.F.; Mookerjee, R.P.; Taylor, S. Vascular assessment of liver disease—Towards a new frontier in MRI. Br. J. Radiol. 2016, 89, 20150675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lai, S.-L.; Chen, J.; Xie, D.; Wu, F.-X.; Jin, G.-Q.; Su, D.-K. Validated preoperative computed tomography risk estimation for postoperative hepatocellular carcinoma recurrence. World J. Gastroenterol. 2017, 23, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Jin, X.-L.; Suh, S.; Hong, S.Y.; Hong, K.; Han, E.S.; Lee, J.-M.; Choi, Y.; Yi, N.-J.; Lee, K.-W.; et al. Different Risk Factors for Early and Late Recurrence After Curative Resection of Hepatocellular Carcinoma. World J. Surg. 2022, 46, 197–206. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.-L.; Liu, Q.-P.; Sun, S.-W.; Zhang, J.; Zhu, F.-P.; Yang, G.; Yan, X.; Zhang, Y.-D.; Liu, X.-S. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J. Hepatol. 2019, 70, 1133–1144. [Google Scholar] [CrossRef]
- Liu, L.; Shui, Y.; Yu, Q.; Guo, Y.; Zhang, L.; Zhou, X.; Yu, R.; Lou, J.; Wei, S.; Wei, Q. Narrow-Margin Hepatectomy Resulted in Higher Recurrence and Lower Overall Survival for R0 Resection Hepatocellular Carcinoma. Front. Oncol. 2021, 10, 610636. [Google Scholar] [CrossRef]
- Huo, Y.R.; Chan, M.V.; Chan, C. Resection Plus Post-operative Adjuvant Transcatheter Arterial Chemoembolization (TACE) Compared with Resection Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2020, 43, 572–586. [Google Scholar] [CrossRef]
- Liu, F.; Liu, D.; Wang, K.; Xie, X.; Su, L.; Kuang, M.; Huang, G.; Peng, B.; Wang, Y.; Lin, M.; et al. Deep Learning Radiomics Based on Contrast-Enhanced Ultrasound Might Optimize Curative Treatments for Very-Early or Early-Stage Hepatocellular Carcinoma Patients. Liver Cancer 2020, 9, 397–413. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Huang, G.; Zhang, J. Predicting the Initial Treatment Response to Transarterial Chemoembolization in Intermediate-Stage Hepatocellular Carcinoma by the Integration of Radiomics and Deep Learning. Front. Oncol. 2021, 11, 730282. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, L.; Zhong, B.; Zhou, H.; Zhu, H.; Zhou, H.; Song, J.; Guo, J.; Zhu, X.; Ji, J.; et al. Machine-learning analysis of contrast-enhanced computed tomography radiomics predicts patients with hepatocellular carcinoma who are unsuitable for initial transarterial chemoembolization monotherapy: A multicenter study. Transl. Oncol. 2021, 14, 101034. [Google Scholar] [CrossRef]
- Mokrane, F.-Z.; Lu, L.; Vavasseur, A.; Otal, P.; Peron, J.-M.; Luk, L.; Yang, H.; Ammari, S.; Saenger, Y.; Rousseau, H.; et al. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur. Radiol. 2020, 30, 558–570. [Google Scholar] [CrossRef] [PubMed]




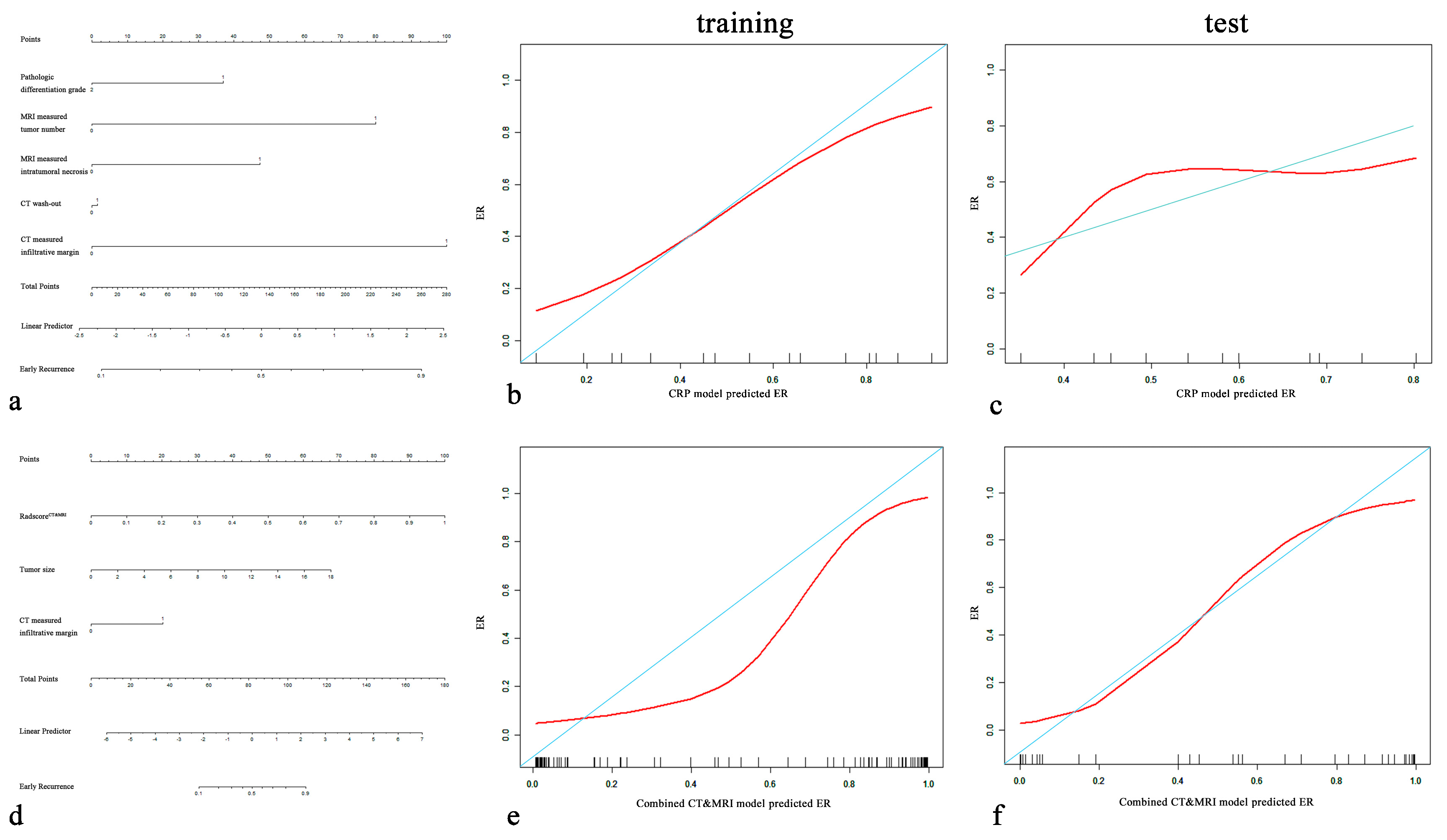

| Variables | Non-ER (n = 59) | ER (n = 60) | p-Value | Training Group (n = 83) | Test Group (n = 36) | p-Value |
|---|---|---|---|---|---|---|
| Age(years), mean ± SD | 62.68 ± 9.00 | 60.23 ± 12.18 | 0.431 | 61.88 ± 10.39 | 60.44 ± 11.62 | 0.506 |
| Sex, n (%) | 0.668 | 0.936 | ||||
| Male | 49 (83.1) | 48 (80.0) | 67 (80.7) | 30 (83.3) | ||
| female | 10 (16.9) | 12 (20.0) | 16 (19.3) | 6 (16.7) | ||
| AFP, n (%) | 0.777 | 0.261 | ||||
| ≤400 ng/mL | 48 (81.4) | 50 (83.3) | 71 (85.5) | 27 (75) | ||
| >400 ng/mL | 11 (18.6) | 10 (16.6) | 12 (14.5) | 9 (25) | ||
| Pathogenesis, n (%) | 0.422 | 0.845 | ||||
| HBV | 46 (78.0) | 43 (71.7) | 63 (75.9) | 26 (72.2) | ||
| HCV | 2 (3.4) | 6 (10.0) | 5 (6.0) | 3 (8.3) | ||
| Other | 11 (18.6) | 11 (18.3) | 15 (18.1) | 7 (8.4) | ||
| CNLC, n (%) | 0.779 | 0.476 | ||||
| Ia | 25 (42.4) | 28 (46.7) | 34 (41) | 19 (52.8) | ||
| Ib | 18 (30.5) | 19 (31.7) | 27 (32.5) | 10 (27.8) | ||
| IIa | 16 (27.1) | 13 (21.7) | 22 (26.5) | 7 (19.4) | ||
| BCLC, n (%) | 0.973 | 0.476 | ||||
| 0 + A | 52 (88.1) | 53 (88.3) | 72 (86.7) | 33 (91.7) | ||
| B | 7 (11.9) | 7 (11.7) | 11 (13.3) | 3 (8.3) | ||
| Pathologic MVI, n (%) | 0.013 | 0.649 | ||||
| Absent | 34 (57.6) | 21 (35.0) | 40 (48.2) | 15 (41.7) | ||
| Present | 25 (42.4) | 39 (65.0) | 43 (51.8) | 21 (58.3) | ||
| Satellite nodules, n (%) | 0.615 | 0.381 | ||||
| Absent | 46 (78.0) | 49 (81.7) | 64 (77.1) | 31 (86.1) | ||
| Present | 13 (22.0) | 11 (18.3) | 19 (22.9) | 5 (13.9) | ||
| Pathologic differentiation grade, n (%) | 0.044 | 0.312 | ||||
| Low | 8 (13.6) | 18 (30.0) | 15 (18.1) | 11 (30.6) | ||
| Median | 37 (62.7) | 35 (58.3) | 53 (63.9) | 19 (52.8) | ||
| High | 14 (23.7) | 7 (11.7) | 15 (18.1) | 6 (16.7) | ||
| Tumor size, cm | 4.28 ± 2.52 | 5.71 ± 2.62 | <0.001 | 5.09 ± 2.82 | 4.79 ± 2.27 | 0.571 |
| Radiologic evidence of cirrhosis, n (%) | 0.523 | 1.000 | ||||
| Absent | 24 (40.7) | 21 (35.0) | 31 (37.3) | 14 (38.9) | ||
| Present | 35 (59.32%) | 39 (65.00%) | 52 (62.7) | 22 (61.1) | ||
| CT wash-in, n (%) | 0.714 | 0.600 | ||||
| Absent | 3 (5.1) | 4 (6.7) | 6 (7.2) | 1 (2.8) | ||
| Present | 56 (94.9) | 56 (93.3) | 77 (92.8) | 35 (97.2) | ||
| CT wash-out, n (%) | 0.045 | 0.408 | ||||
| Absent | 7 (11.9) | 5 (8.3) | 8 (9.6) | 4 (11.1) | ||
| Present | 52 (88.1) | 55 (91.7) | 75 (90.4) | 32 (88.9) | ||
| CT measured infiltrative margin, n (%) | <0.001 | 1.000 | ||||
| Absent | 41 (69.5) | 20 (33.3) | 43 (51.8) | 18 (50) | ||
| Present | 18 (30.5) | 40 (66.7) | 40 (48.2) | 18 (50) | ||
| CT measured tumor number, n (%) | 0.099 | 0.838 | ||||
| single | 42 (71.2) | 34 (56.7) | 54 (65.1) | 22 (61.1) | ||
| multiple | 17 (28.8) | 26 (43.3) | 29 (34.9) | 14 (38.9) | ||
| CT measured intratumoral necrosis, n (%) | 0.637 | 0.851 | ||||
| Absent | 34 (57.6) | 32 (53.3) | 47 (56.6) | 19 (52.8) | ||
| Present | 25 (42.4) | 28 (46.7) | 36 (43.4) | 17 (47.2) | ||
| CT measured pseudocapsule, n (%) | 0.397 | 0.708 | ||||
| Absent | 36 (61.0) | 32 (53.3) | 46 (55.4) | 22 (61.1) | ||
| Present | 23 (39.0) | 28 (46.7) | 37 (44.6) | 14 (38.9) | ||
| MRI wash-in, n (%) | 0.978 | 1.000 | ||||
| Absent | 5 (8.5) | 5 (8.3) | 7 (8.4) | 3 (8.3) | ||
| Present | 54 (91.5) | 55 (91.7) | 76 (91.6) | 33 (91.7) | ||
| MRI wash-out, n (%) | 0.978 | 1.000 | ||||
| Absent | 5 (8.5) | 5 (8.3) | 5 (6.0) | 5 (13.9) | ||
| Present | 54 (91.5) | 55 (91.7) | 78 (94) | 31 (86.1) | ||
| MRI measured infiltrative margin, n (%) | 0.388 | 0.769 | ||||
| Absent | 38 (64.4) | 34 (56.7) | 49 (59) | 23 (63.9) | ||
| Present | 21 (35.6) | 26 (43.3) | 34 (41) | 13 (36.1) | ||
| MRI measured tumor number, n (%) | 0.015 | 0.424 | ||||
| single | 45 (76.3) | 33 (55.0) | 52 (62.7) | 26 (72.2) | ||
| multiple | 14 (23.7) | 27 (45.0) | 31 (37.3) | 10 (27.8) | ||
| MRI measured intratumoral necrosis, n (%) | <0.001 | 1.000 | ||||
| Absent | 45 (76.3) | 27 (45.0) | 50 (60.2) | 22 (61.1) | ||
| Present | 14 (23.7) | 33 (55.0) | 33 (39.8) | 14 (38.9) | ||
| MRI measured pseudocapsule, n (%) | 0.035 | 0.224 | ||||
| Absent | 36 (61.0) | 25 (41.7) | 39 (47) | 22 (61.1) | ||
| Present | 23 (39.0) | 35 (58.3) | 44 (53) | 14 (38.9) | ||
| LI_RADS, n (%) | 0.402 | 0.444 | ||||
| 3 | 4 (6.8) | 4 (6.7) | 4 (4.8) | 4 (11.1) | ||
| 4 | 5 (8.5) | 10 (16.7) | 11 (13.3) | 4 (11.1) | ||
| 5 | 50 (84.7) | 46 (76.6) | 68 (81.9) | 28 (77.8) | ||
| RadscoreCT | 0.38 ± 0.21 | 0.65 ± 0.25 | <0.001 | 0.50 ± 0.27 | 0.55 ± 0.28 | 0.340 |
| RadscoreMRI | 0.38 ± 0.24 | 0.68 ± 0.22 | <0.001 | 0.51 ± 0.29 | 0.58 ± 0.24 | 0.201 |
| RadscoreCT&MRI | 0.26 ± 0.23 | 0.76 ± 0.23 | <0.001 | 0.49 ± 0.35 | 0.56 ± 0.31 | 0.343 |
| CRP Model | Combined CT | Combined MRI | Combined CT and MRI | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%) | p | |
| Tumor size | 0.53 (0.26–1.10) | 0.555 | 1.34 (1.09–1.64) | 0.002 * | 1.18 (0.96–1.45) | 0.11 | 1.37 (1.04–1.80) | 0.021 * |
| Pathologic differentiation grade | 0.46 (0.23–0.92) | 0.029 * | 0.65 (0.30–1.42) | 0.279 | 0.57 (0.27–1.20) | 0.14 | 0.54 (0.20, 1.45) | 0.221 |
| MRI measured tumor number | 3.91 (1.52–10.07) | 0.005 * | NA | NA | 2.24 (0.82–6.15) | 0.12 | 3.20 (0.70, 14.52) | 0.132 |
| MRI measured intratumoral necrosis | 2.74 (1.10–6.82) | 0.031 * | NA | NA | 3.13 (1.12–8.81) | 0.03 * | 4.60 (0.83, 25.54) | 0.080 |
| CT measured infiltrative margin | 4.74 (1.91–11.77) | 0.001 * | 4.77 (1.74–13.09) | 0.003 * | NA | NA | 5.87 (1.25–27.44) | <0.024 * |
| CT wash-out | 3.80 (1.07–13.51) | 0.039 * | 2.43 (0.57–10.33) | 0.231 | NA | NA | 2.45 (0.36, 16.96) | 0.363 |
| RadscoreCT | NA | NA | 16.32 (9.19–138.84) | <0.001 * | NA | NA | NA | NA |
| RadscoreMRI | NA | NA | NA | NA | 23.27 (2.38–227.60) | <0.01 * | NA | NA |
| RadscoreCT&MRI | NA | NA | NA | NA | NA | NA | 30.98 (17.88–536.4) | <0.001 * |
| Training | Test | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95%CI) | ACC | Sen | Spec | AUC (95%CI) | ACC | Sen | Spec | Training Set | Test Set | ||
| (1) RadiomicsCT | 0.820 (0.731, 0.909) | 0.759 | 0.643 | 0.878 | 0.742 (0.552, 0.887) | 0.778 | 0.722 | 0.833 | 1 vs. 3 | 0.001 * | 0.032 * |
| (2) RadiomicsMRI | 0.833 (0.749, 0.916) | 0.747 | 0.524 | 0.976 | 0.753 (0.559, 0.868) | 0.750 | 0.833 | 0.667 | 2 vs. 3 | 0.001 * | 0.039 * |
| (3) RadiomicsCT&MRI | 0.931 (0.879, 0.983) | 0.867 | 0.809 | 0.927 | 0.909 (0.765, 0.957) | 0.833 | 0.999 | 0.667 | 1 vs. 2 | 0.850 | 0.911 |
| (a) Combined CT | 0.894 (0.804, 0.948) | 0.867 | 0.857 | 0.878 | 0.784 (0.585, 0.884) | 0.750 | 0.778 | 0.722 | a vs. c | 0.044 * | 0.010 * |
| (b) Combined MRI | 0.856 (0.756, 0.922) | 0.783 | 0.667 | 0.902 | 0.787 (0.612, 0.900) | 0.750 | 0.833 | 0.667 | b vs. c | 0.001 * | 0.024* |
| (c) Combined CT and MRI | 0.955 (0.890, 0.981) | 0.927 | 0.905 | 0.951 | 0.951 (0.792, 0.961) | 0.916 | 0.990 | 0.833 | a vs. b | 0.464 | 0.983 |
| 3 vs. c | 0.083 | 0.434 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Sheng, Y.; Jiang, Z.; Liu, H.; Lu, H.; Xing, W. What Imaging Modality Is More Effective in Predicting Early Recurrence of Hepatocellular Carcinoma after Hepatectomy Using Radiomics Analysis: CT or MRI or Both? Diagnostics 2023, 13, 2012. https://doi.org/10.3390/diagnostics13122012
Wang Q, Sheng Y, Jiang Z, Liu H, Lu H, Xing W. What Imaging Modality Is More Effective in Predicting Early Recurrence of Hepatocellular Carcinoma after Hepatectomy Using Radiomics Analysis: CT or MRI or Both? Diagnostics. 2023; 13(12):2012. https://doi.org/10.3390/diagnostics13122012
Chicago/Turabian StyleWang, Qing, Ye Sheng, Zhenxing Jiang, Haifeng Liu, Haitao Lu, and Wei Xing. 2023. "What Imaging Modality Is More Effective in Predicting Early Recurrence of Hepatocellular Carcinoma after Hepatectomy Using Radiomics Analysis: CT or MRI or Both?" Diagnostics 13, no. 12: 2012. https://doi.org/10.3390/diagnostics13122012
APA StyleWang, Q., Sheng, Y., Jiang, Z., Liu, H., Lu, H., & Xing, W. (2023). What Imaging Modality Is More Effective in Predicting Early Recurrence of Hepatocellular Carcinoma after Hepatectomy Using Radiomics Analysis: CT or MRI or Both? Diagnostics, 13(12), 2012. https://doi.org/10.3390/diagnostics13122012






