Surgical Anatomy of the Liver—Significance in Ovarian Cancer Surgery
Abstract
:1. Introduction
2. Anatomical Lobes of the Liver
3. Morphological Variations of the Liver
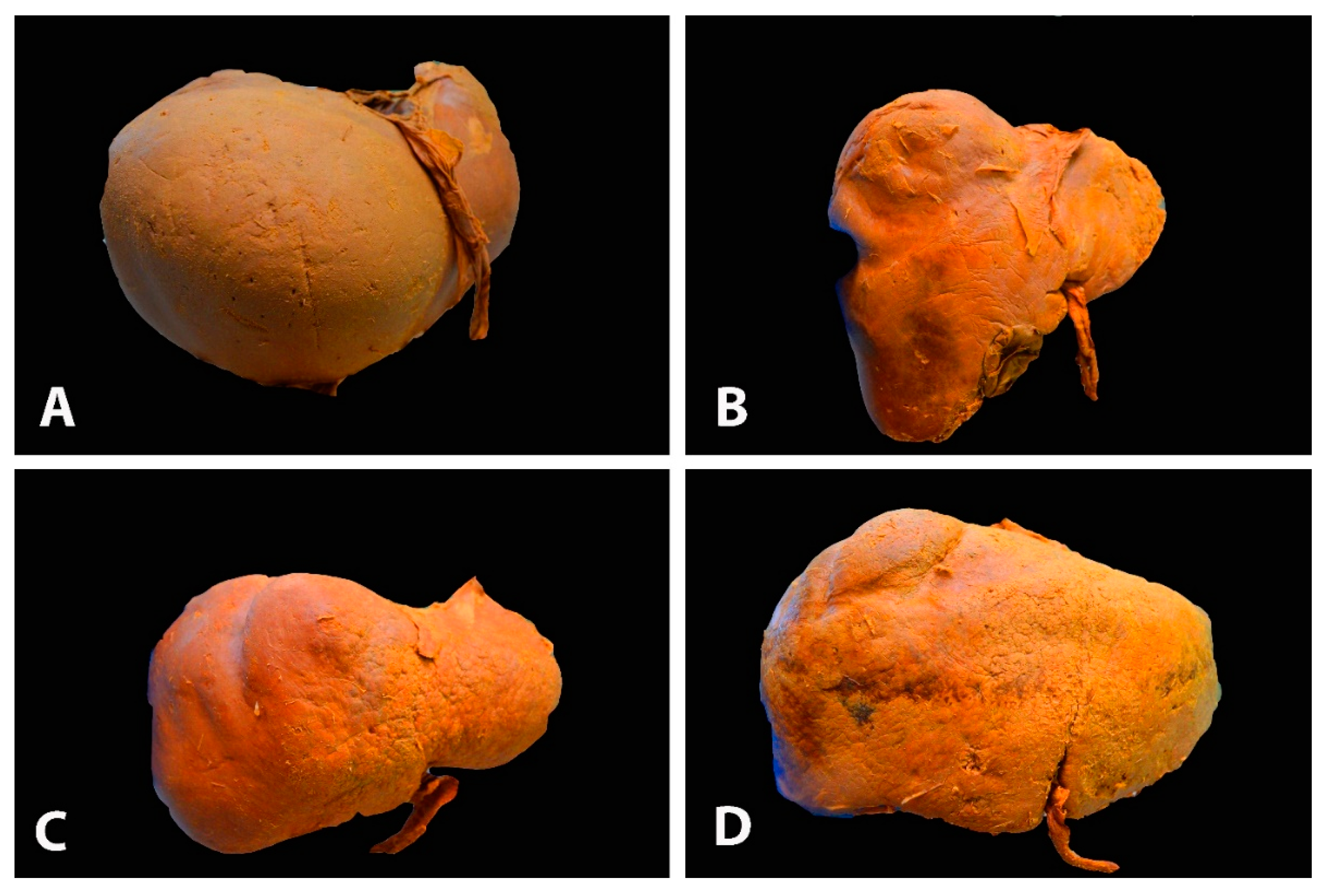
3.1. Liver Shape Variations
Significance of Liver Shape Variations in Ovarian Cancer Surgery
3.2. Congenital and Acquired Variations of the Liver
3.2.1. Accessory Liver Fissures
3.2.2. Deep Diaphragmatic Grooves
3.2.3. Significance of Liver Fissures and Grooves in Ovarian Cancer Surgery
3.3. Riedel’s Lobe
Significance of Riedel’s Lobe in Ovarian Cancer Surgery
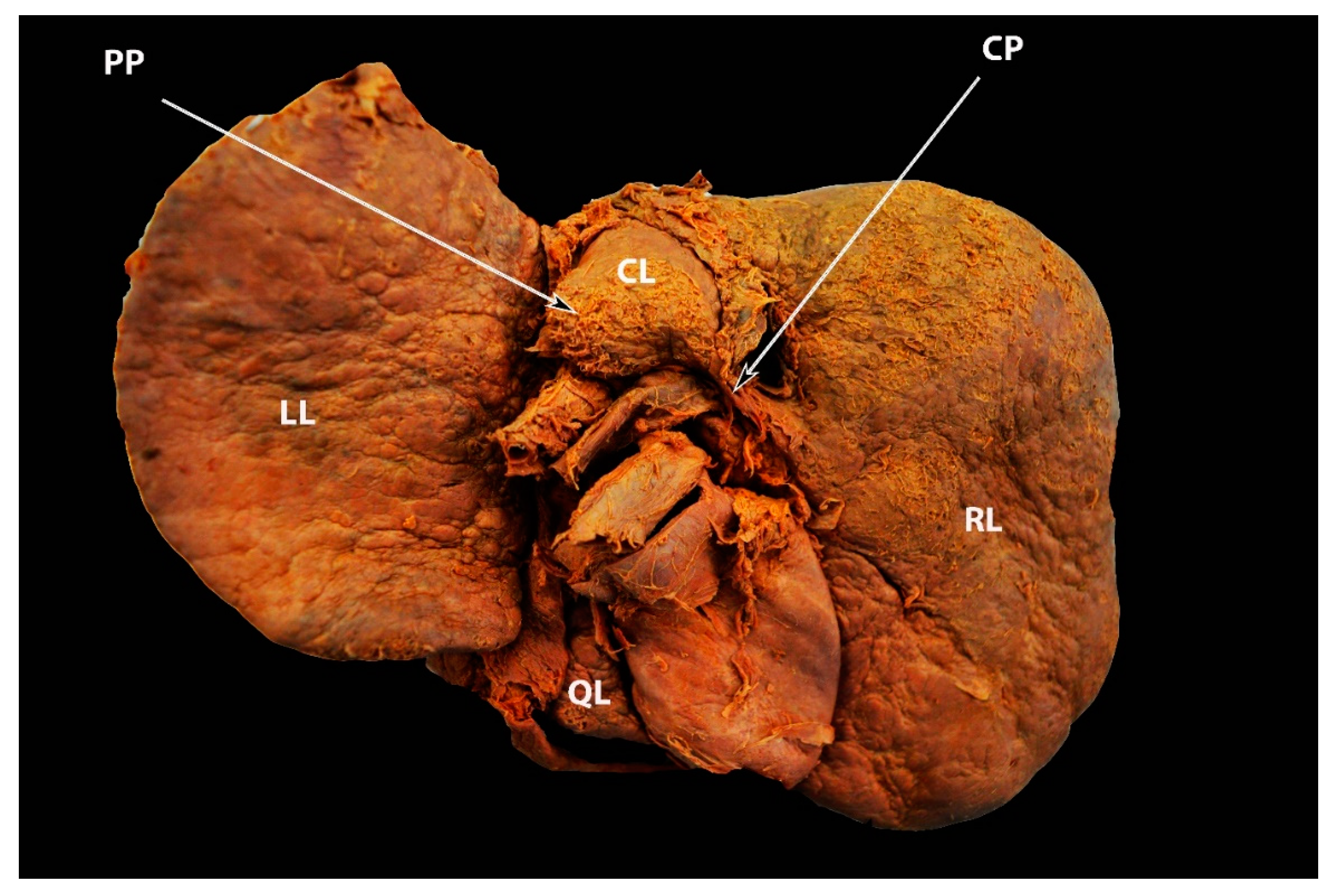
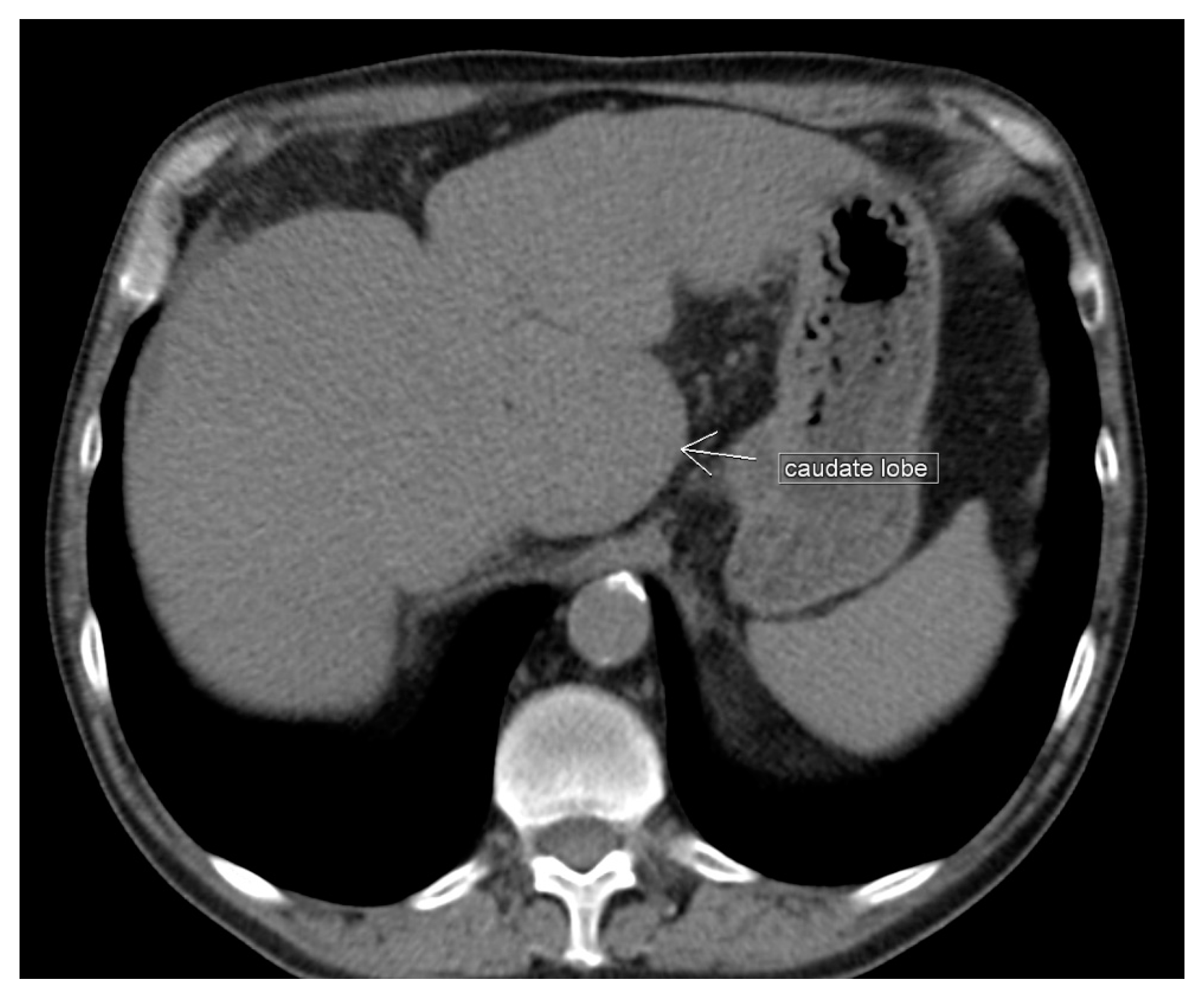
3.4. Papillary Process (Spiegel’s Lobe) and Caudate Process of the Caudate Lobe
Significance of the Papillary Process (Spiegel’s Lobe) and Caudate Process of the Caudate Lobe of the Liver in Ovarian-Cancer Surgery
3.5. Pons Hepatis
Significance of Pons Hepatis in Ovarian Cancer Surgery
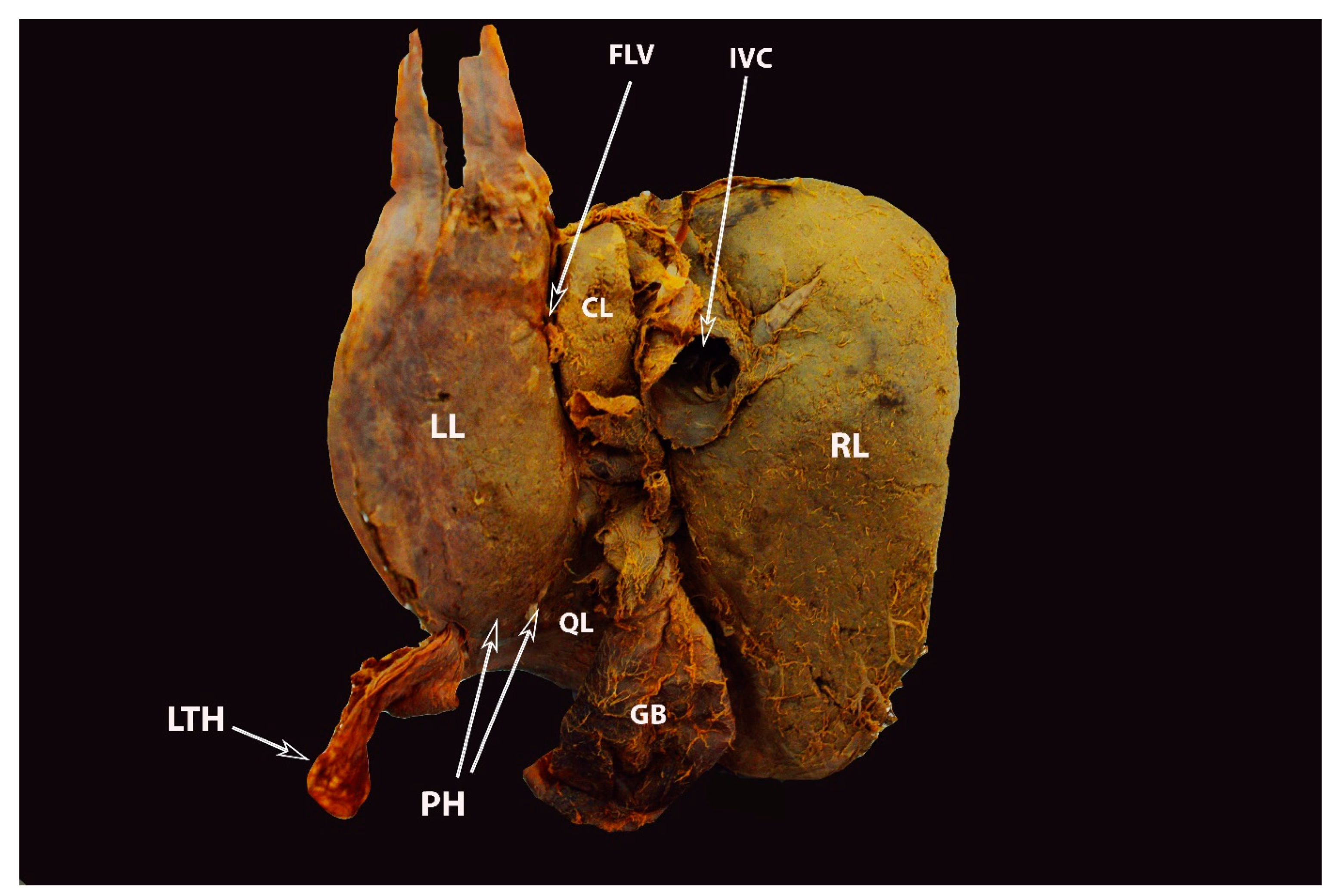
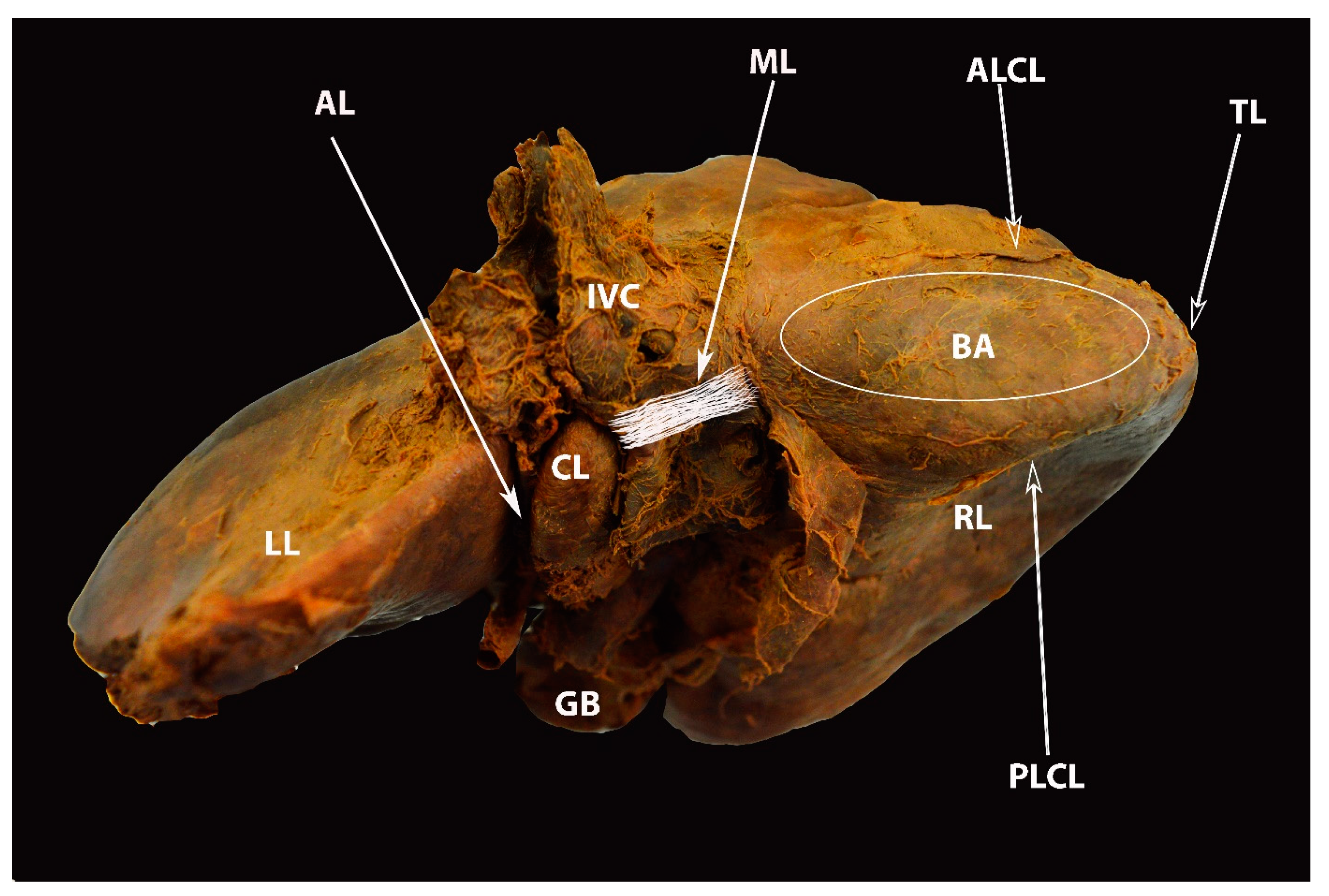
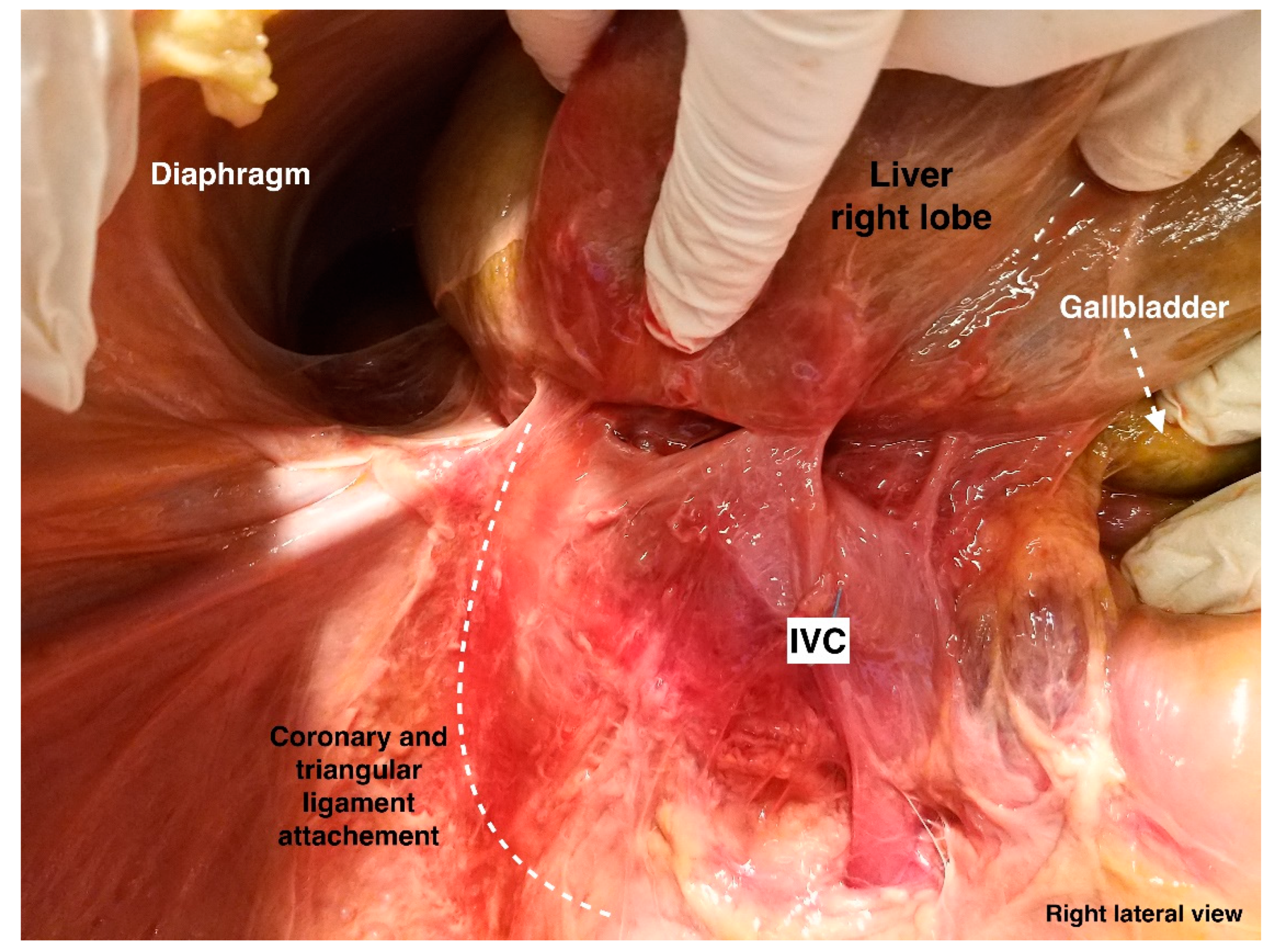
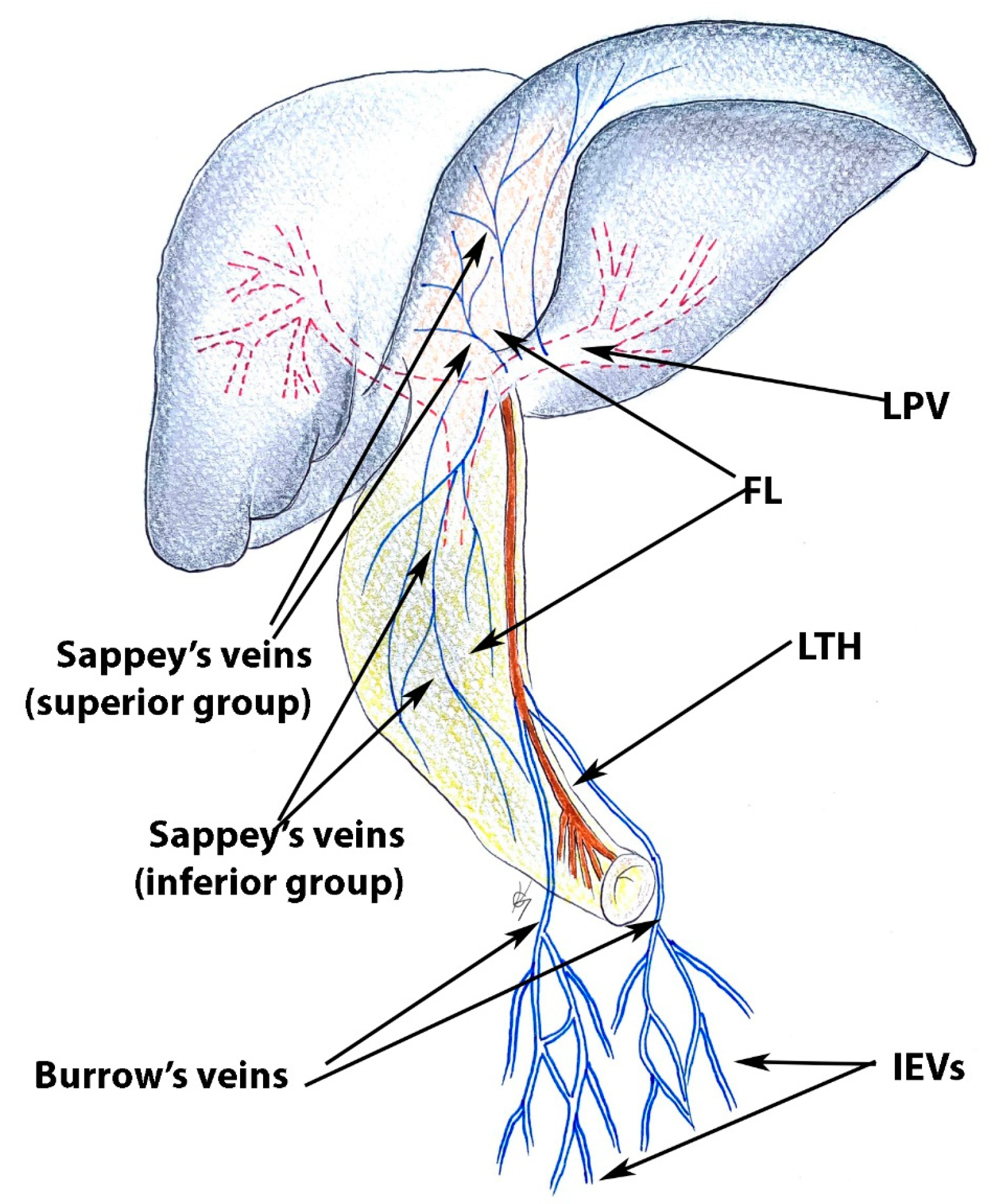
4. Ligamentous Attachments of the Liver
5. Liver Segments
6. Vascular Anatomy of the Liver
6.1. Arterial Supply of the Liver
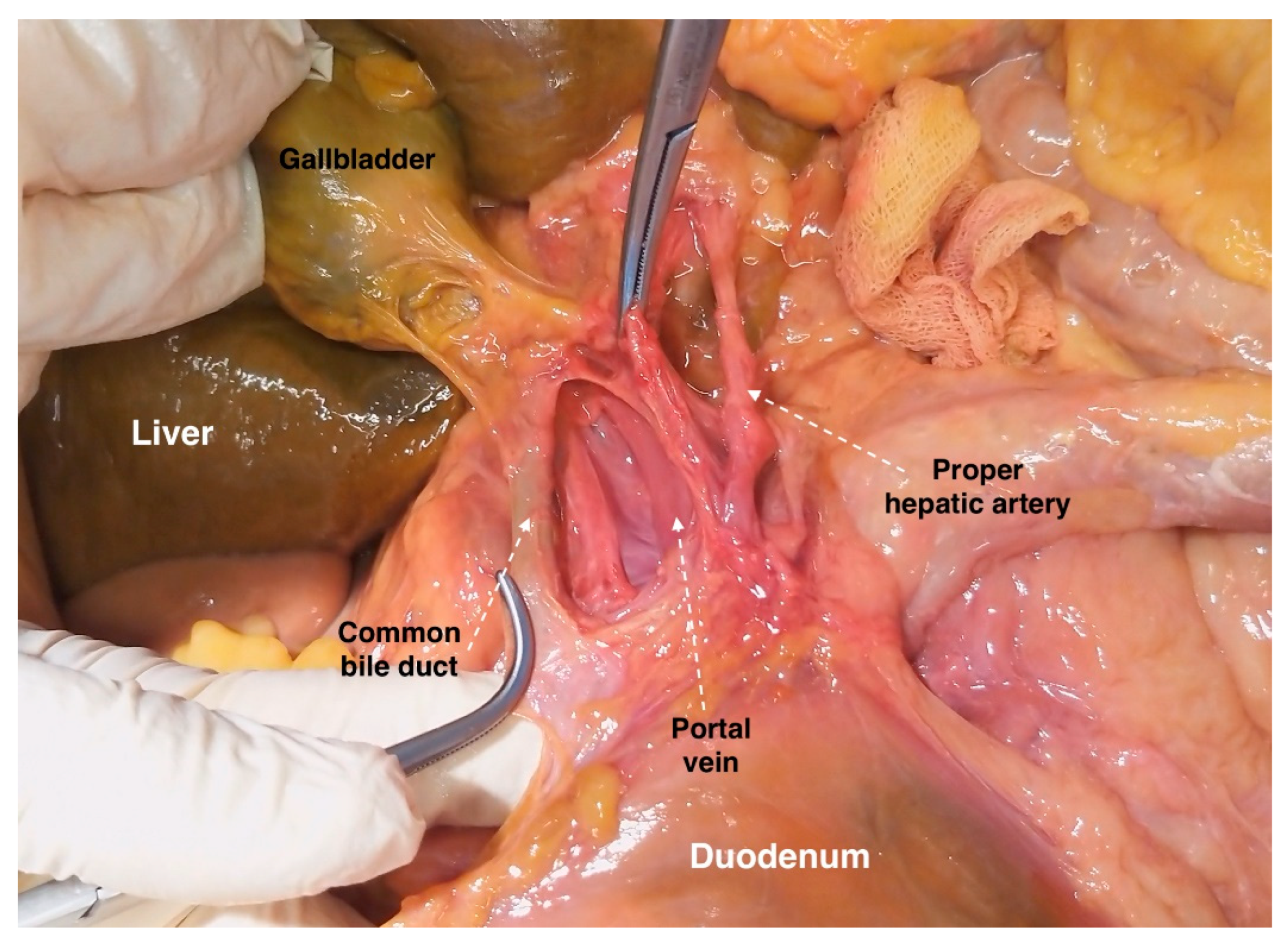
6.2. The Portal Vein
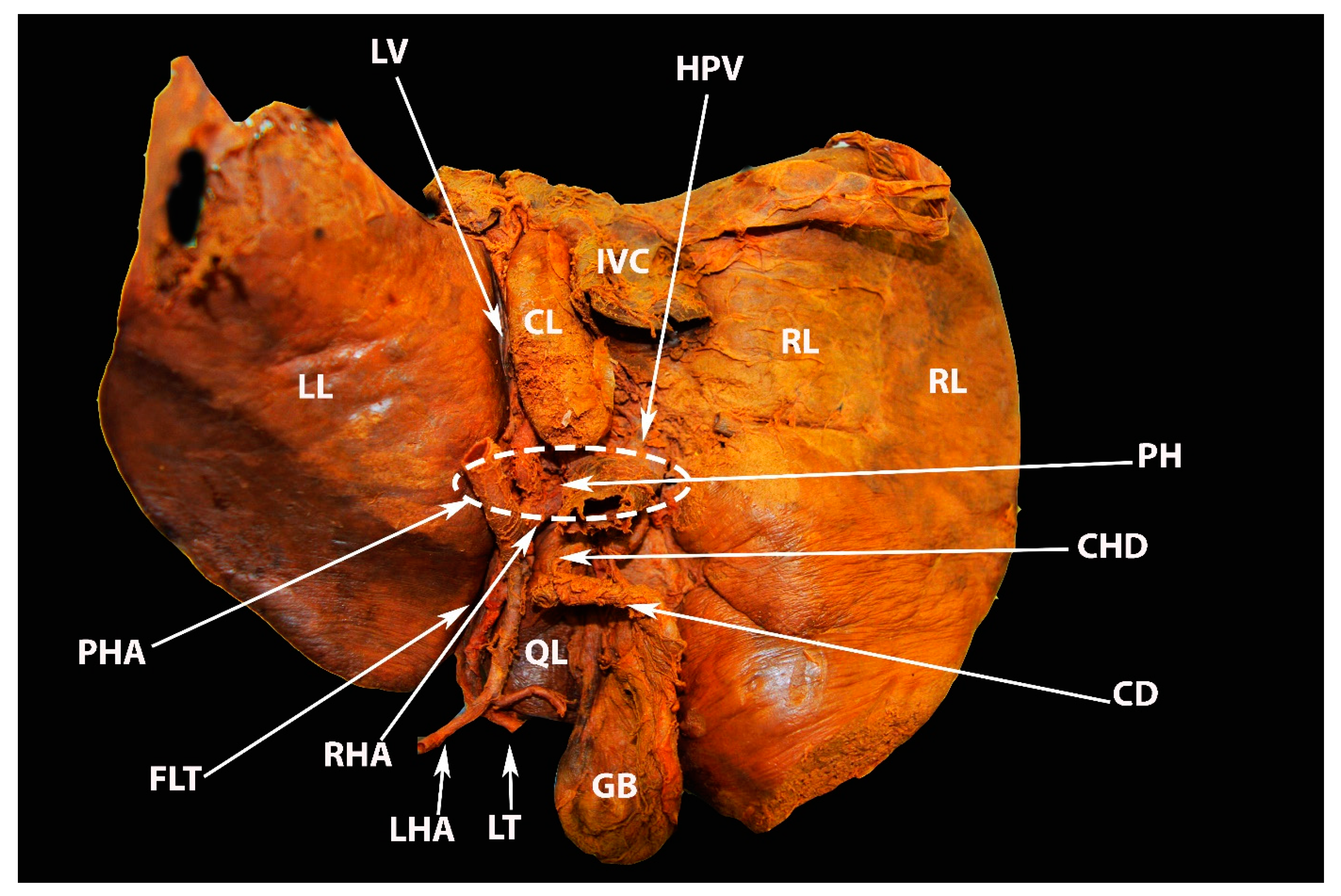
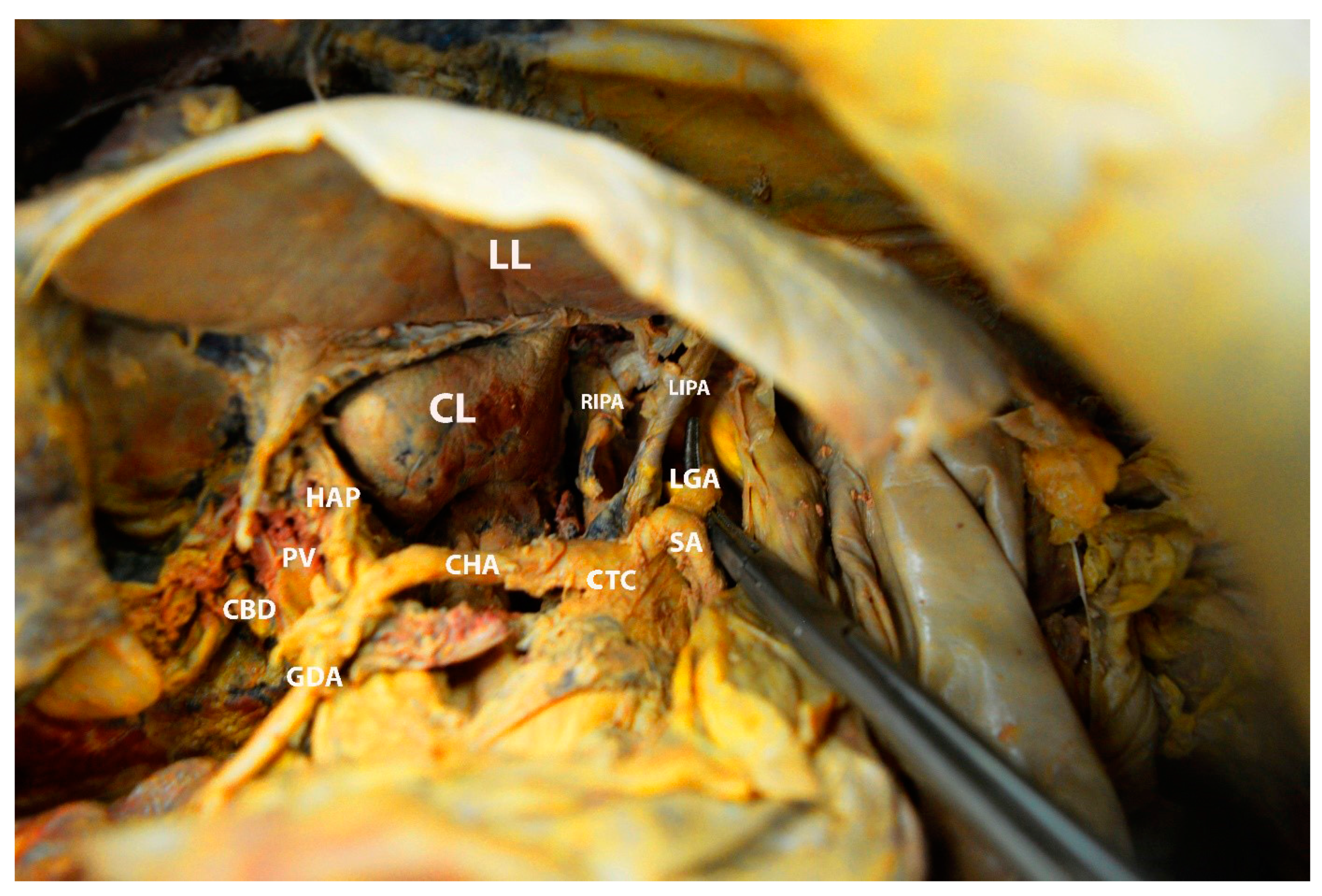
6.3. Porta Hepatis
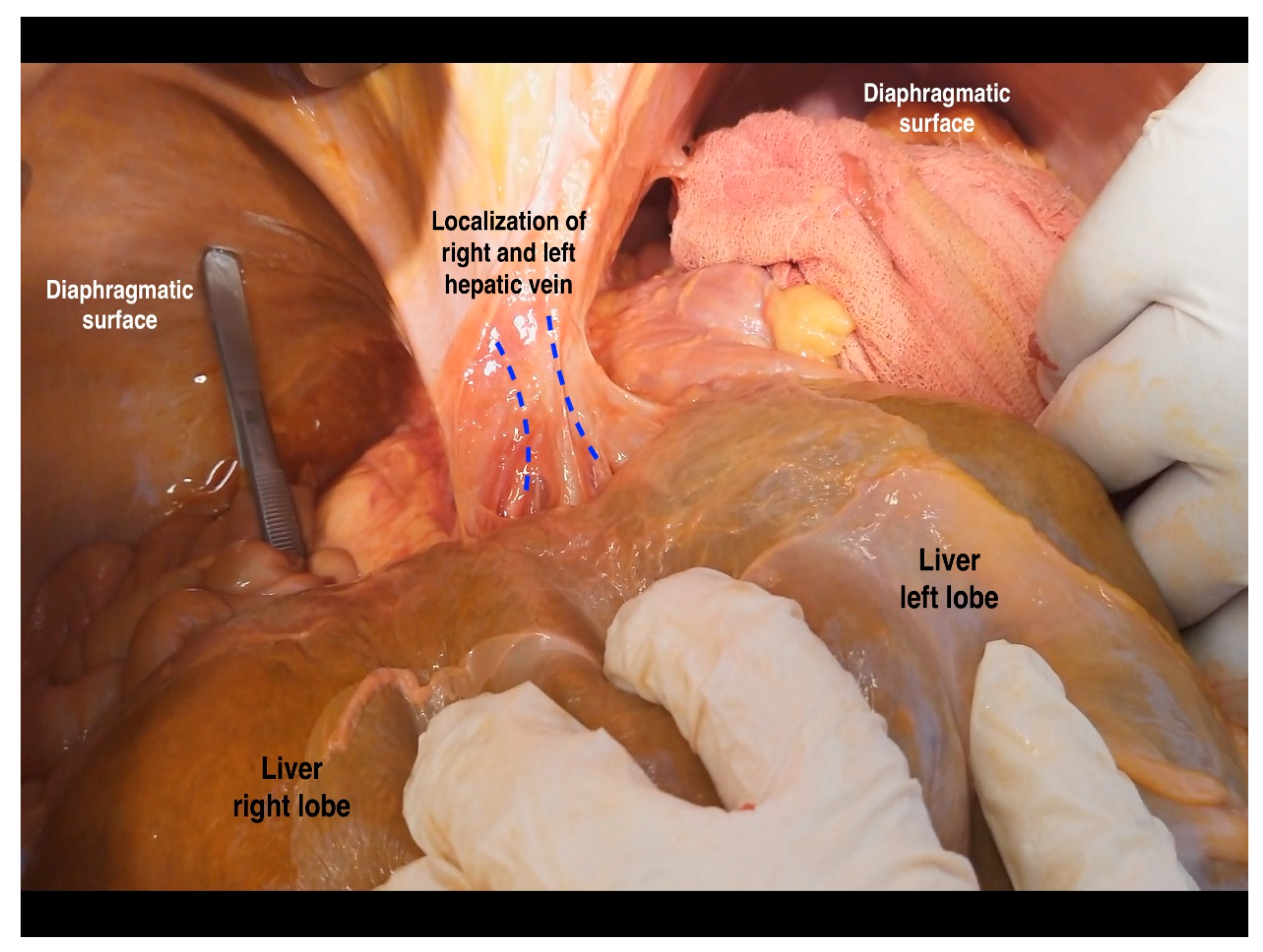
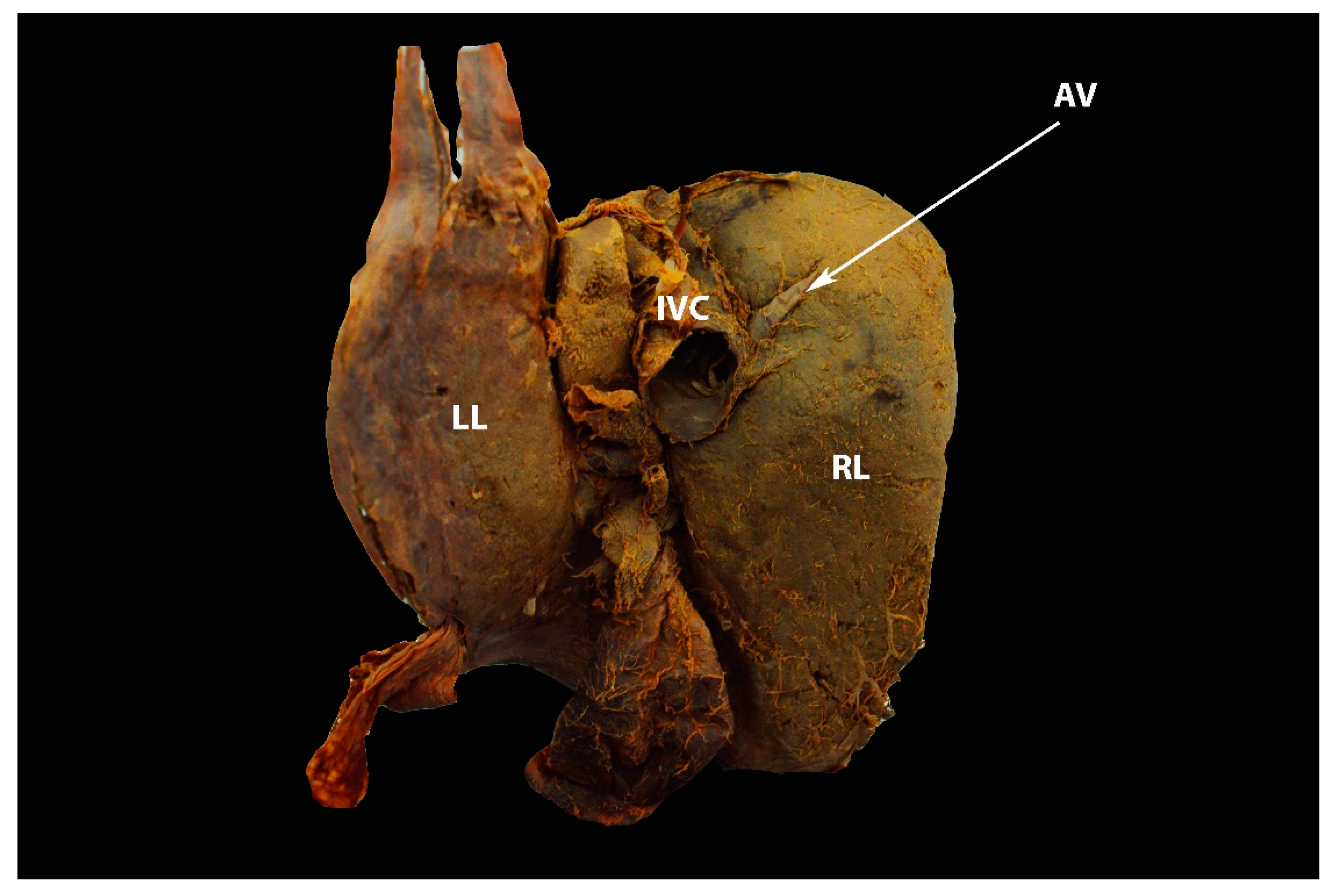
6.4. Hepatic Veins and IVC
7. Macroscopic and Microscopic Anatomy of the Liver—One Entity
8. Significance of Liver Anatomy in Ovarian Cancer Surgery
8.1. Liver Mobilization
- Step 1: dissection and transection of the falciform ligament containing ligamentum teres in a cranial direction up to the level of its bifurcation into the coronary ligament (transection of the falciform ligament with an electrosurgical device is preferable);
- Step 2: dissection and division of the left triangular ligament;
- Step 3: dissection of the anterior and posterior layers of the coronary ligament on the left liver lobe;
- Step 4: dissection and division of the hepatogastric ligament;
- Step 5: dividing (from medial to lateral) the anterior layer of the coronary ligament in the right liver lobe;
- Step 6: dividing the right triangular ligament;
- Step 7: dissection and transection (from lateral to medial) of the posterior layer of the coronary ligament.
- Step 8: dissection between the liver surface and the ventral aspect of the IVC.
8.1.1. Step 1. Tips, Tricks, and Attention during Dissection
8.1.2. Step 2 and 3. Tips, Tricks, and Attention during Dissection
8.1.3. Step 4. Tips, Tricks and Attention during Dissection
8.1.4. Steps 5 and 6. Tips, Tricks, and Attention during Dissection
8.1.5. Steps 7 and 8. Tips, Tricks, and Attention during Dissection
8.2. Porta Hepatis and Hepatoduodenal Ligament Dissection
8.3. Gall Bladder Fossa Dissection
9. Preventing Iatrogenic Injury during Liver Mobilization
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taskiran, C.; Vatansever, D.; Giray, B.; Eraslan, A.; Tanju, S.; Arvas, M.; Balik, E. Upper abdominal debulking surgery for ovarian cancer total colectomy, total peritonectomy, and extended upper abdominal debulking surgery. Int. J. Gynecol. Cancer 2020, 30, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obs. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Karlan, B.Y.; Chi, D.S. Surgery for Ovarian Cancer, 3rd ed.; Taylor & Francis Group, LLC.: Abingdon, UK, 2016. [Google Scholar]
- Pannu, H.K.; Oliphant, M. The subperitoneal space and peritoneal cavity: Basic concepts. Abdom. Imaging 2015, 40, 2710–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanyan, A.; Malakyan, Z.; Alaverdyan, A.; Davtyan, H.; Hovhannisyan, T. Right upper quadrant peritonectomy. Answering frequently asked questions. Int. J. Gynecol. Cancer 2021, 31, 1305–1306. [Google Scholar] [CrossRef]
- Lago, V.; Domingo, S.; Matute, L.; Padilla-Iserte, P.; Gurrea, M. Radical en bloc peritonectomy in advanced ovarian cancer. Ecancermedicalscience 2018, 12, 808. [Google Scholar] [CrossRef] [Green Version]
- Rosati, A.; De Rose, A.M.; Sala, E.; Giuliante, F.; Scambia, G.; Fagotti, A. Ovarian cancer metastases in the liver area: Proposal of a standardized anatomo-surgical classification. Int. J. Gynecol. Cancer 2022, 32, 955–956. [Google Scholar] [CrossRef]
- Juza, R.M.; Pauli, E.M. Clinical and surgical anatomy of the liver: A review for clinicians. Clin. Anat. 2014, 27, 764–769. [Google Scholar] [CrossRef]
- Abdel-Misih, S.R.; Bloomston, M. Liver anatomy. Surg. Clin. N. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Bismuth, H.; Balzarotti, R.; Majno, P. Liver Surgical Anatomy. In Extreme Hepatic Surgery and Other Strategies; Springer: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar] [CrossRef]
- Chamberlain, R.S. Essential Functional Hepatic and Biliary Anatomy for the Surgeon. In Hepatic Surgery; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Gray, H.; Standring, S.; Hrold Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175. [Google Scholar]
- Phad, V.V.; Syed, S.A.; Joshi, R.A. Morphological variations of liver. Int. J. Health Sci. Res. 2014, 4, 119–124. [Google Scholar]
- Chaudhari, H.J.; Ravat, M.K.; Vaniya, V.H.; Bhedi, A.N. Morphological Study of Human Liver and Its Surgical Importance. J. Clin. Diagn. Res. 2017, 11, AC09–AC12. [Google Scholar] [CrossRef]
- Singh, H.R.; Rabi, S. Study of morphological variations of liver in human. Transl. Res. Anat. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Srimani, P.; Saha, A. Liver morphology: Anatomical study about the outer aspects. Surg. Radiol. Anat. 2020, 42, 1425–1434. [Google Scholar] [CrossRef]
- Vinnakota, S.; Jayasree, N. A new insight into the morphology of the human liver: A cadaveric study. ISRN Anat. 2013, 2013, 689564. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.Y. Different Approaches to Liver Resection; Springer: Singapore, 2021; pp. 155–169. [Google Scholar]
- Netter, F.H. Atlas of Human Anatomy, 2nd ed.; 18 Guilford Press: New York, NY, USA, 2000. [Google Scholar]
- Macchi, V.; Feltrin, G.; Parenti, A.; De Caro, R. Diaphragmatic sulci and portal fissures. J. Anat. 2003, 202 Pt 3, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Auh, Y.H.; Lim, J.H.; Kim, K.W.; Lee, D.H.; Lee, M.G.; Cho, K.S. Loculated fluid collections in hepatic fissures and recesses: CT appearance and potential pitfalls. Radiographics 1994, 14, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Cawich, S.O.; Spence, R.; Mohammed, F.; Gardner, M.T.; Sinanan, A.; Naraynsingh, V. The liver and Chilaiditi’s syndrome: Significance of hepatic surface grooves. SAGE Open Med. Case Rep. 2017, 5, 2050313X17744979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuz, A.; Batur, A.; Bulut, M.D.; Bora, A.; Göya, C.; Andic, C.; Beyazal, M.; Olmez, S. Anterior hepatic grooves accompanied by Chilaiditi sign: A retrospective radiological analysis of a neglected anatomical fact. Surg. Radiol. Anat. 2015, 37, 483–492. [Google Scholar] [CrossRef]
- Chilaiditi, D. Zur Frage der Hepatoptose und Ptose im allegemeinen im Anschluss an drei Fallevon temporarer, partieller Leberverlagerung. Fortschr. Geb Rontgenstr. Nukl. 1910, 16, 173–208. [Google Scholar]
- Gulati, M.S.; Wafula, J.; Aggarwal, S. Chilaiditi’s sign possibly associated with malposition of chest tube placement. J. Postgrad. Med. 2008, 54, 138–139. [Google Scholar] [CrossRef]
- Moaven, O.; Hodin, R.A. Chilaiditi syndrome: A rare entity with important differential diagnoses. Gastroenterol. Hepatol. 2012, 8, 276–278. [Google Scholar]
- Sohal, R.J.; Adams, S.H.; Phogat, V.; Durer, C.; Harish, A. Chilaiditi’s Sign: A Case Report. Cureus 2019, 11, e6230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savopoulos, C.; Kakaletsis, N.; Kaiafa, G.; Iliadis, F.; Kalogera-Fountzila, A.; Hatzitolios, A.I. Riedel’s lobe of the liver: A case report. Medicine 2015, 94, e430. [Google Scholar] [CrossRef]
- Riedel, I. Ueber den zungenfrmigen Fortsatz des rechten Leberlappens und seine pathognostische Bedeutung für die Erkrankung der Gallenblase nebst Bemerkungen über Gallensteinoperationen. Berl. Klin. Wochenschr. 1888, 25, 577–602. [Google Scholar]
- Sham, R.; Sain, A.; Silver, L. Hypertrophic Riedel’s lobe of the liver. Clin. Nucl. Med. 1978, 3, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.H.; Patel, M.C.; Abrahams, P.H.; Dixon, A.K. Riedel’s lobe of the liver: Fact or fiction? Clin. Anat. 1998, 11, 47–49. [Google Scholar] [CrossRef]
- Soo, M.S.; Adatepe, M.H. Metastatic lesions arising in a Riedel’s lobe. Findings from a sulfur colloid liver-spleen scan. Clin. Nucl. Med. 1990, 15, 814–815. [Google Scholar] [CrossRef]
- Zamfir, R.; Braşoveanu, V.; Boroş, M.; Herlea, V.; Popescu, I. Hepatocellular carcinoma in Riedel’s lobe. Chirurgia 2008, 103, 121–123. [Google Scholar]
- Al-Handola, R.; Chinnappan, J.; Bakeer, M.; Ayad, S. Incidental Finding of Riedel’s Lobe of the Liver and Intrahepatic Cholangiocarcinoma. Cureus 2023, 15, e40683. [Google Scholar] [CrossRef]
- Kudo, M. Riedel’s lobe of the liver and its clinical implication. Intern Med. 2000, 39, 87–88. [Google Scholar] [CrossRef] [Green Version]
- Lane, D. Masses in the right hypochondrium and Riedel’s lobe. Med. J. Aust. 1966, 1, 896–899. [Google Scholar] [CrossRef]
- De Simoni, O.; Barina, A.; Gruppo, M.; Scapinello, A.; Mourmouras, V.; Pilati, P.; Franzato, B. A Potentially Misleading Hepatocellular Carcinoma. Medicina 2021, 57, 850. [Google Scholar] [CrossRef] [PubMed]
- Auh, Y.H.; Rosen, A.; Rubenstein, W.A.; Engel, I.A.; Whalen, J.P.; Kazam, E. CT of the papillary process of the caudate lobe of the liver. AJR Am. J. Roentgenol. 1984, 142, 535–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.D.; Joshi, S.S.; Athavale, S.A. Some interesting observations on the surface features of the liver and their clinical implications. Singapore Med. J. 2009, 50, 715–719. [Google Scholar] [PubMed]
- Mittal, A.; Goyal, G.L.; Kamath, V.G. Variations in Hepatic Segmentation on the Surface of Liver—A Cadaveric Study. JK Sci. 2021, 23, 43–46. Available online: https://journal.jkscience.org/index.php/JK-Science/article/view/43 (accessed on 12 January 2023).
- Donmez, B.O.; Sarikcioglu, L.; Gokhan, G.; Elpek, G.O.; Ucar, Y. Pons hepatis: Report of two cases. Acta Gastroenterol. Belg. 2009, 72, 279–280. [Google Scholar] [PubMed]
- Couinaud, C. (Ed.) Surgical Anatomy of the Liver Revisited; C. Couinaud 15 rue Spontini F 75116 Paris: Paris, France, 1989. [Google Scholar]
- Onitsuka, A.; Katagiri, Y.; Miyauchi, T.; Shimamoto, T.; Mimoto, H.; Ozeki, Y. Metastatic hepatoma originating from the pons hepatis presenting extrahepatic growth--classification of different patterns covering REX’s recessus. Hepatogastroenterology 2003, 50, 235–237. [Google Scholar]
- Ramirez, P.T.; Frumovitz, M.; Abu-Rustum, N.R. (Eds.) Principles of Gynecologic Oncology Surgery; Elsevier: Amsterdam, The Netherlands, 2018; p. 5. ISBN 978032342878. [Google Scholar]
- Ibukuro, K.; Fukuda, H.; Tobe, K.; Akita, K.; Takeguchi, T. The vascular anatomy of the ligaments of the liver: Gross anatomy, imaging and clinical applications. Br. J. Radiol. 2016, 89, 20150925. [Google Scholar] [CrossRef] [Green Version]
- Dahmane, R.; Morjane, A.; Ravnik, D.; Hribernik, M. Anatomy of the ligamentum venosum arantii and its contribution to the left hepatic vein and common trunk control. A study on cadaveric livers. Cells Tissues Organs. 2009, 190, 297–300. [Google Scholar] [CrossRef]
- Joshi, S.; Gorin, M.A.; Ciancio, G. Release of the inferior vena cava ligament during caval thrombectomy causing tumor thrombus embolization. Urol Int. 2013, 90, 490–492. [Google Scholar] [CrossRef]
- Makuuchi, M.; Yamamoto, J.; Takayama, T.; Kosuge, T.; Gunvén, P.; Yamazaki, S.; Hasegawa, H. Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology 1991, 38, 176–179. [Google Scholar]
- Kogure, K.; Ishizaki, M.; Nemoto, M.; Kuwano, H.; Yorifuji, H.; Ishikawa, H.; Takata, K.; Makuuchi, M. Close relation between the inferior vena cava ligament and the caudate lobe in the human liver. J. Hepatobiliary Pancreat. Surg. 2007, 14, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Morjane, A.; Dahmane, R.; Ravnik, D.; Hribernik, M. Anatomy and Surgical Relevance of the Hepatocaval Ligament. Cells Tissues Organs 2008, 187, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.kenhub.com/en/library/anatomy/liver-ligaments (accessed on 3 February 2023).
- Cantlie, J. On a new arrangement of the right and left lobes of the liver. Proc.–Anat. Soc. Great Br. Irel. 1897, 32, 4–9. [Google Scholar]
- Couinaud, C. Anatomic principles of left and right regulated hepatectomy: Technics. J. Chir. 1954, 70, 933–966. [Google Scholar]
- Lowe, M.C.; D’Angelica, M.I. Anatomy of Hepatic Resectional Surgery. Surg. Clin. N. Am. 2016, 96, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldırım, Y.; Gultekin, E. Cytoreductive Surgery in Gynecologic Oncology: A Multidisciplinary Approach. In Surgical Anatomy of Upper Abdominal Solid Organs for Gynecologic Oncologists; Yildirim, Y., Ed.; Transworld Research Network: Kerala, India, 2010; pp. 13–34. [Google Scholar]
- Carneiro, C.; Brito, J.; Bilreiro, C.; Barros, M.; Bahia, C.; Santiago, I.; Caseiro-Alves, F. All about portal vein: A pictorial display to anatomy, variants and physiopathology. Insights Imaging 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okudaira, M. Anatomy of the Portal Vein System and Hepatic Vasculature. In Portal Hypertension; Okuda, K., Benhamou, J.P., Eds.; Springer: Tokyo, Japan, 1991; pp. 3–12. [Google Scholar] [CrossRef]
- Neginhal, D.D.; Kulkarni, U.K. Normal anatomy of porta hepatis—A cadaveric study. Natl. J. Clin. Anat. 2019, 8, 22–26. [Google Scholar]
- Krishna, M. Microscopic anatomy of the liver. Clin. Liver Dis. 2013, 2 (Suppl. S1), S4–S7. [Google Scholar] [CrossRef]
- Kehoe, S.M.; Eisenhauer, E.L.; Chi, D.S. Upper abdominal surgical procedures: Liver mobilization and diaphragm peritonectomy/resection, splenectomy, and distal pancreatectomy. Gynecol. Oncol. 2008, 111 (Suppl. S2), S51–S55. [Google Scholar] [CrossRef]
- Shin, W.; Mun, J.; Park, S.Y.; Lim, M.C. Narrative review of liver mobilization, diaphragm peritonectomy, full-thickness diaphragm resection, and reconstruction. Gland. Surg. 2021, 10, 1212–1217. [Google Scholar] [CrossRef]
- Casullo, J.; Zeng, H.; Belley, G.; Artho, G. CT of the paraumbilical and ensiform veins in patients with superior vena cava or left brachiocephalic vein obstruction. PLoS ONE 2018, 13, e0196093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bills, D.; Moore, S. The falciform ligament and the ligamentum teres: Friend or foe. ANZ J. Surg. 2009, 79, 678–680. [Google Scholar] [CrossRef]
- Martin, B.F.; Tudor, R.G. The umbilical and paraumbilical veins of man. J. Anat. 1980, 130 Pt 2, 305–322. [Google Scholar] [PubMed]
- Yoshimitsu, K.; Honda, H.; Kuroiwa, T.; Irie, H.; Aibe, H.; Shinozaki, K.; Masuda, K. Unusual hemodynamics and pseudolesions of the noncirrhotic liver at CT. Radiographics 2001, 21, S81–S96. [Google Scholar] [CrossRef]
- Sappey, C. Recherches sur quelques veines portes accessoires. C R Seances Soc. Biol. Fil. 1859, 11, 3–13. [Google Scholar]
- Burow, K.A. Beitrag zur Gefässlehre des Fötus. Arch. Anat. Physiol. 1838, 44–45. [Google Scholar]
- Selçuk, İ.; Öztürk Başarır, Z.; Ohri, N.; Akar, B.; Çalışkan, E.; Güngör, T. Comparative surgical resection of the ligamentum teres hepatis in a cadaveric model and a patient with ovarian cancer. J. Turk. Ger. Gynecol Assoc. 2019, 20, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. The hepatic bridge. Eur. J. Surg. Oncol. 2018, 44, 1083–1086. [Google Scholar] [CrossRef]
- Veerapong, J.; Solomon, H.; Helm, C.W. Division of the pont hepatique of the liver in cytoreductive surgery for peritoneal malignancy. Gynecol. Oncol. 2013, 128, 133. [Google Scholar] [CrossRef]
- Gulmez, S.; Polat, E.; Duman, U.; Senger, A.S.; Uzun, O.; Ozduman, O.; Oz, A.; Subasi, I.E.; Duman, M. Hepatic bridge and round ligament of the liver during cytoreductive surgery: A retrospective cohort. Langenbecks Arch Surg. 2022, 407, 1201–1207. [Google Scholar] [CrossRef]
- Togami, S.; Kato, T.; Oi, T.; Ishikawa, M.; Onda, T.; Ikeda, S.; Kasamatsu, T. A rare case of recurrent ovarian cancer presenting as a round ligament metastasis. World J. Surg. Oncol. 2011, 9, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://eacademy.esgo.org/esgo/2018/surgical-videos/298843/faculty.presenters.right.diaphragmatic.peritonectomy.in.10.steps.html?f=menu%3D17%2Alisting%3D0%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D10%2Atopic%3D19424%2Afeatured%3D16241 (accessed on 10 September 2022).
- Sureka, B.; Sharma, N.; Khera, P.S.; Garg, P.K.; Yadav, T. Hepatic vein variations in 500 patients: Surgical and radiological significance. Br. J. Radiol. 2019, 92, 20190487. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Huang, T.L.; Chen, C.L.; Chen, T.Y.; Huang, C.C.; Ko, S.F.; Lee, T.Y. Variations of the left and middle hepatic veins: Application in living related hepatic transplantation. J. Clin. Ultrasound. 1996, 24, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Orguc, S.; Tercan, M.; Bozoklar, A.; Akyildiz, M.; Gurgan, U.; Celebi, A.; Nart, D.; Karasu, Z.; Icoz, G.; Zeytunlu, M.; et al. Variations of hepatic veins: Helical computerized tomography experience in 100 consecutive living liver donors with emphasis on right lobe. Transpl. Proc. 2004, 36, 2727–2732. [Google Scholar] [CrossRef]
- de Oliveira, C.V.; Horvat, N.; de Azambuja, R.L. A rare case of left hepatic vein anomalous drainage to the coronary sinus. Radiol. Case Rep. 2020, 15, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Karolczak, M.A.; Mądry, W.; Zacharska-Kokot, E. Anomalous connection of the left hepatic vein to coronary sinus in a child with PAPVD. Surgical significance and diagnostic difficulties. Kardiochir Torakochirurgia Pol. 2016, 13, 49–51. [Google Scholar] [CrossRef]
- Lee, C.; Saremi, F. Anomalous left hepatic vein draining into coronary sinus imaged with multidetector computed tomography. Clin. Anat. 2013, 26, 987–989. [Google Scholar] [CrossRef]
- Yee, K.F. Anomalous termination of a hepatic vein in the left atrium. Arch. Pathol. 1968, 85, 219–223. [Google Scholar]
- Kloppenburg, G.T.; Post, M.C.; Mager, H.J.; Schepens, M.A. Rerouting anomalous hepatic venous connection to the left atrium. Ann. Thorac. Surg. 2010, 90, 638–640. [Google Scholar] [CrossRef]
- Frantzides, C.T.; Carlson, M.A.; Shostrom, V.K.; Roberts, J.; Stavropoulos, G.; Ayiomamitis, G.; Frantzides, A. A survey of dumping symptomatology after gastric bypass with or without lesser omental transection. Obes. Surg. 2011, 21, 186–193. [Google Scholar] [CrossRef]
- Brenkman, H.J.F.; van der Wielen, N.I.; Ruurda, J.P.; van Leeuwen, M.S.; Scheepers, J.J.G.; van der Peet, D.L.; van Hillegersberg, R.; Bleys, R.L.A.W.; Cuesta, M.A. Surgical anatomy of the omental bursa and the stomach based on a minimally invasive approach: Different approaches and technical steps to resection and lymphadenectomy. J. Thorac. Dis. 2017, 9 (Suppl. S8), S809–S816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, B.; Douard, R.; Chevallier, J.M.; Delmas, V. L’artère hépatique gauche, variations anatomiques et implications cliniques [Left hepatic artery: Anatomical variations and clinical implications]. Morphologie 2008, 92, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Sawai, K.; Taniguchi, H.; Takahashi, T. Aberrant left hepatic artery arising from the left gastric artery and liver function after radical gastrectomy for gastric cancer. World J. Surg. 1993, 17, 70–73, discussion 74. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Nishi, N.; Matsuo, Y.; Watadani, T.; Kimura, F. The common hepatic artery arising from the left gastric artery. Surg. Radiol. Anat. 2010, 32, 703–705. [Google Scholar] [CrossRef]
- Available online: https://eacademy.esgo.org/esgo/2000/dvds/50936/david.cibula.debulking.procedures.-.liver.mobilisation.and.diaphragm.stripping.html?f=menu%3D17%2Alisting%3D0%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D10%2Atopic%3D19424%2Afeatured%3D16241 (accessed on 2 March 2023).
- Available online: https://eacademy.esgo.org/esgo/2018/ovarian-cancer-surgery-course/209145/luis.chiva.liver.mobilization.26.cardio-phrenic.lnd.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D6%2Amedia%3D1%2Ace_id%3D1331%2Aces_id%3D14147 (accessed on 2 March 2023).
- Nakamura, S.; Tsuzuki, T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg. Gynecol. Obstet. 1981, 152, 43–50. [Google Scholar]
- Shilal, P.; Tuli, A. Anatomical variations in the pattern of the right hepatic veins draining the posterior segment of the right lobe of the liver. J. Clin. Diagn Res. 2015, 9, AC08-12. [Google Scholar] [CrossRef]
- Cawich, S.O.; Naraynsingh, V.; Pearce, N.W.; Deshpande, R.R.; Rampersad, R.; Gardner, M.T.; Mohammed, F.; Dindial, R.; Barrow, T.A. Surgical relevance of anatomic variations of the right hepatic vein. World J. Transplant. 2021, 11, 231–243. [Google Scholar] [CrossRef]
- Shan, Y.; Jin, Y.; Pan, L. Hepatic metastases in ovarian cancer. Hepatobiliary Surg. Nutr. 2022, 11, 924–926. [Google Scholar] [CrossRef]
- Steinbrück, K.; Fernandes, R.; Bento, G.; Vasconcelos, R.; Stoduto, G.; Auel, T.; Pacheco-Moreira, L.F. Inferior Right Hepatic Vein: A Useful Anatomic Variation for Isolated Resection of Segment VIII. Case Rep. Surg. 2013, 2013, 371264. [Google Scholar] [CrossRef]
- Saylisoy, S.; Atasoy, C.; Ersöz, S.; Karayalçin, K.; Akyar, S. Multislice CT angiography in the evaluation of hepatic vascular anatomy in potential right lobe donors. Diagn. Interv. Radiol. 2005, 11, 51–59. [Google Scholar]
- Chouillard, E.K.; Gumbs, A.A.; Cherqui, D. Vascular clamping in liver surgery: Physiology, indications and techniques. Ann. Surg. Innov. Res. 2010, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Raspagliesi, F.; Ditto, A.; Martinelli, F.; Haeusler, E.; Lorusso, D. Advanced ovarian cancer: Omental bursa, lesser omentum, celiac, portal and triad nodes spread as cause of inaccurate evaluation of residual tumor. Gynecol. Oncol. 2013, 129, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.; Traill, Z.; Garruto Campanile, R.; Ferrari, F.; Soleymani Majd, H.; Nieuwstad, J.; Hardern, K.; Gubbala, K. Porta hepatis peritonectomy and hepato-celiac lymphadenectomy in patients with stage IIIC-IV ovarian cancer: Diagnostic pathway, surgical technique and outcomes. Gynecol. Oncol. 2016, 143, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Giannini, A.; D’Oria, O.; Schiavi, M.C.; Di Pinto, A.; Fischetti, M.; Lecce, F.; Perniola, G.; Battaglia, F.; Berloco, P.; et al. Hepatobiliary Disease Resection in Patients with Advanced Epithelial Ovarian Cancer: Prognostic Role and Optimal Cytoreduction. Ann. Surg. Oncol. 2021, 28, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Livani, A.; Angelis, S.; Skandalakis, P.N.; Filippou, D. The Story Retold: The Kocher Manoeuvre. Cureus 2022, 14, e29409. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Chang, S.J. Cholecystectomy, porta hepatis stripping, and omental bursectomy. Gland. Surg. 2021, 10, 1230–1234. [Google Scholar] [CrossRef]
- Mownah, O.A.; Aroori, S. The Pringle maneuver in the modern era: A review of techniques for hepatic inflow occlusion in minimally invasive liver resection. Ann. Hepatobiliary Pancreat. Surg. 2023, 27, 131–140. [Google Scholar] [CrossRef]
- Bhandoria, G.; Bhatt, A.; Mehta, S.; Glehen, O. Upper-Abdominal Cytoreduction for Advanced Ovarian Cancer—Therapeutic Rationale, Surgical Anatomy and Techniques of Cytoreduction. Surg. Tech. Dev. 2023, 12, 1–33. [Google Scholar] [CrossRef]
- Coenegrachts, K. Magnetic resonance imaging of the liver: New imaging strategies for evaluating focal liver lesions. World J. Radiol. 2009, 1, 72–85. [Google Scholar] [CrossRef]
- Donadon, M.; Torzilli, G. Intraoperative ultrasound in patients with hepatocellular carcinoma: From daily practice to future trends. Liver Cancer 2013, 2, 16–24. [Google Scholar] [CrossRef]
- Barat, M.; Cottereau, A.-S.; Kedra, A.; Dermine, S.; Palmieri, L.-J.; Coriat, R.; Dautry, R.; Tselikas, L.; Soyer, P.; Dohan, A. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J. Clin. Med. 2020, 9, 2302. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Naguib, N.N.; Lehnert, T.; Nour-Eldin, N.-E.A.; Eichler, K.; Zangos, S.; Gruber-Rouh, T. Initial experience with repetitive transarterial chemoembolization (TACE) as a third line treatment of ovarian cancer metastasis to the liver: Indications, outcomes and role in patient’s management. Gynecol. Oncol. 2012, 124, 225–229. [Google Scholar] [CrossRef] [PubMed]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Selçuk, I.; Watrowski, R.; Dineva, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Yordanov, A. Surgical Anatomy of the Liver—Significance in Ovarian Cancer Surgery. Diagnostics 2023, 13, 2371. https://doi.org/10.3390/diagnostics13142371
Kostov S, Selçuk I, Watrowski R, Dineva S, Kornovski Y, Slavchev S, Ivanova Y, Dzhenkov D, Yordanov A. Surgical Anatomy of the Liver—Significance in Ovarian Cancer Surgery. Diagnostics. 2023; 13(14):2371. https://doi.org/10.3390/diagnostics13142371
Chicago/Turabian StyleKostov, Stoyan, Ilker Selçuk, Rafał Watrowski, Svetla Dineva, Yavor Kornovski, Stanislav Slavchev, Yonka Ivanova, Deyan Dzhenkov, and Angel Yordanov. 2023. "Surgical Anatomy of the Liver—Significance in Ovarian Cancer Surgery" Diagnostics 13, no. 14: 2371. https://doi.org/10.3390/diagnostics13142371
APA StyleKostov, S., Selçuk, I., Watrowski, R., Dineva, S., Kornovski, Y., Slavchev, S., Ivanova, Y., Dzhenkov, D., & Yordanov, A. (2023). Surgical Anatomy of the Liver—Significance in Ovarian Cancer Surgery. Diagnostics, 13(14), 2371. https://doi.org/10.3390/diagnostics13142371









