An Overview of Clinical Examinations in the Evaluation and Assessment of Arterial and Venous Insufficiency Wounds
Abstract
:1. Introduction
2. Arterial Ulcers and Diabetic Foot Ulcers
2.1. Overview
2.2. Pulsation and Hand-Held Doppler
2.3. Ankle Brachial Index (ABI)
2.4. Transcutaneous Oxygen Measurement (TcPO2)
2.5. Absolute Toe Systolic Pressure
2.6. Arterial Doppler Studies
2.7. Arteriography
2.8. Other Emerging Methods
3. Venous Ulcer
3.1. Overview
3.2. Scoring System of Clinical Venous Disorders and Venous Ulcers
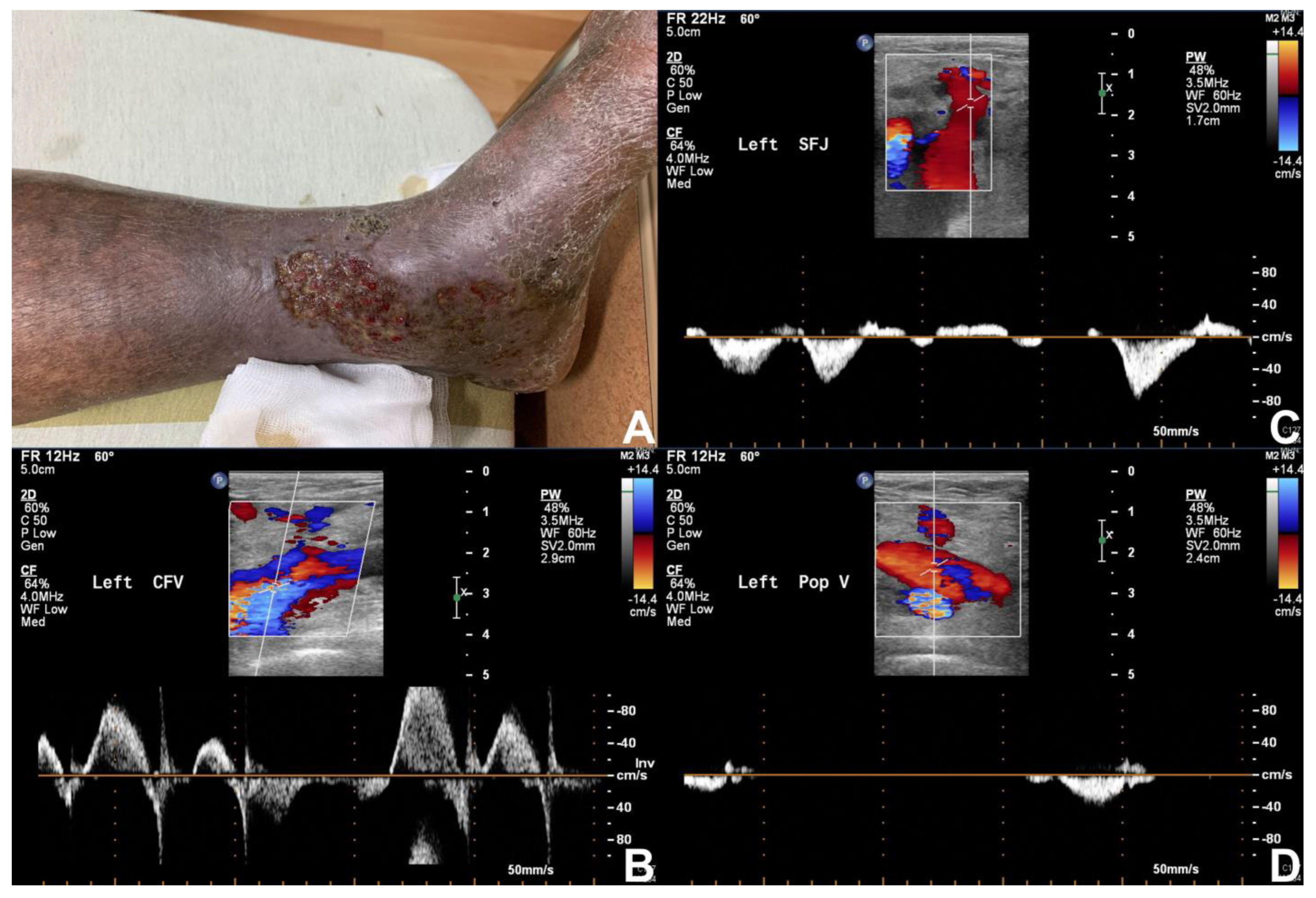
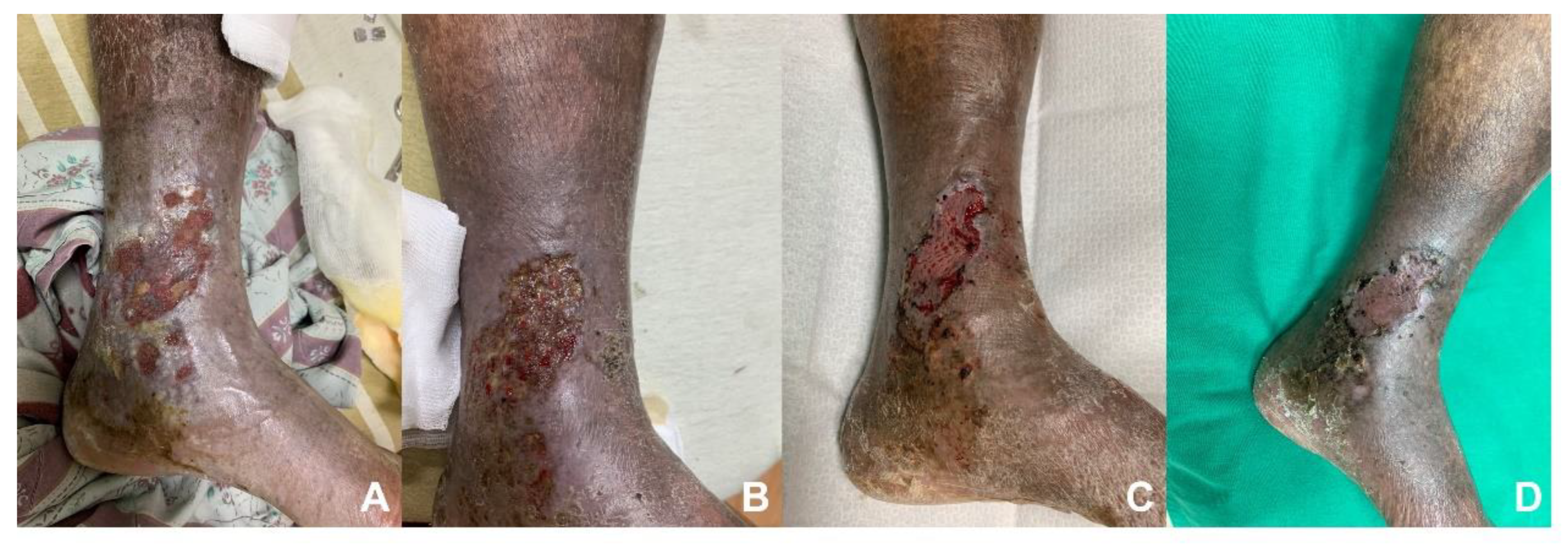
3.3. Duplex Ultrasonography
3.4. Venography (Venogram)
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Grey, J.E.; Harding, K.G.; Enoch, S. Venous and arterial leg ulcers. BMJ 2006, 332, 347–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawall, H.; Huppert, P.; Espinola-Klein, C.; Rümenapf, G. The Diagnosis and Treatment of Peripheral Arterial Vascular Disease. Dtsch. Ärzteblatt Int. 2016, 113, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Dormandy, J.A.; Rutherford, R.B. Management of peripheral arterial disease (PAD). J. Vasc. Surg. 2000, 31, S1–S296. [Google Scholar]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.K.; Golzarian, J.; Gornik, H.L.; Halperin, J.L.; Jaff, M.R.; et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 124, 2020–2045. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Konradsen, L.; Wounlund, J.; Holstein, P. Chronic critical leg ischaemia must include leg ulcers. Eur. J. Vasc. Endovasc. Surg. 1996, 11, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Axisa, B.; Fishwick, G.; Bolia, A.; Thompson, M.M.; London, N.J.M.; Bell, P.R.F.; Naylor, A.R. Complications following peripheral angioplasty. Ann. R. Coll. Surg. Engl. 2002, 84, 39–42. [Google Scholar] [PubMed]
- Hunink, M.G.; Donaldson, M.C.; Meyerovitz, M.F.; Polak, J.F.; Whittemore, A.D.; Kandarpa, K.; Grassi, C.J.; Aruny, J.; Harrington, D.P.; Mannick, A.J. Risks and benefits of femoropopliteal percutaneous balloon angioplasty. J. Vasc. Surg. 1993, 17, 183–192, discussion 192–184. [Google Scholar] [CrossRef] [Green Version]
- Morris-Stiff, G.; Moawad, M.; Appleton, N.; Davies, G.; Hicks, E.; Davies, C.; Lewis, M. Long-term clinical outcome following lower limb arterial angioplasty. Ann. R. Coll. Surg. Engl. 2011, 93, 250–254. [Google Scholar] [CrossRef]
- Aoki, J.; Tanabe, K. Mechanisms of drug-eluting stent restenosis. Cardiovasc. Interv. Ther. 2021, 36, 23–29. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Van Belle, E.; Bauters, C.; Hubert, E.; Bodart, J.-C.; Abolmaali, K.; Meurice, T.; McFadden, E.P.; Lablanche, J.-M.; Bertrand, M.E. Restenosis rates in diabetic patients: A comparison of coronary stenting and balloon angioplasty in native coronary vessels. Circulation 1997, 96, 1454–1460. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, A.; Bakri, F.; Khader, Y.; Lazzarini, P.; Ajlouni, K. Prevalence and Associates of Foot Deformities among Patients with Diabetes in Jordan. Curr. Diabetes Rev. 2020, 16, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Nash, F.; Baker, N.; Fowler, D.; Rayman, G. Reduction in diabetic amputations over 11 years in a defined UK population: Benefits of multidisciplinary team work and continuous prospective audit. Diabetes Care 2008, 31, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.F.; Weinberg, M.D.; Olin, J.W. Peripheral artery disease. Part 1: Clinical evaluation and noninvasive diagnosis. Nat. Rev. Cardiol. 2011, 8, 405–418. [Google Scholar] [CrossRef]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. (Eds.) Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Mowlavi, A.; Whiteman, J.; Wilhelmi, B.J.; Neumeister, M.W.; McLafferty, R. Dorsalis pedis arterial pulse: Palpation using a bony landmark. Postgrad. Med. J. 2002, 78, 746–747. [Google Scholar] [CrossRef] [Green Version]
- Mohler, E.R., 3rd. Peripheral arterial disease: Identification and implications. Arch. Intern. Med. 2003, 163, 2306–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attinger, C.E.; Evans, K.K.; Bulan, E.; Blume, P.; Cooper, P. Angiosomes of the foot and ankle and clinical implications for limb salvage: Reconstruction, incisions, and revascularization. Plast. Reconstr. Surg. 2006, 117, 261S–293S. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.R.; Doomernik, D.E.; Maltha, K.V.; Kooloos, J.G.M.; Kozicz, T.L.; Reijnen, M. Collateral artery pathways of the femoral and popliteal artery. J. Surg. Res. 2017, 211, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Deakin, C.D.; Low, J.L. Accuracy of the advanced trauma life support guidelines for predicting systolic blood pressure using carotid, femoral, and radial pulses: Observational study. BMJ 2000, 321, 673–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitz, J.I.; Byrne, J.; Clagett, G.P.; Farkouh, M.E.; Porter, J.M.; Sackett, D.L.; Strandness, D.E.; Taylor, L.M. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation 1996, 94, 3026–3049. [Google Scholar] [CrossRef]
- Pan, C.R.; Staessen, J.A.; Li, Y.; Wang, J.G. Comparison of three measures of the ankle-brachial blood pressure index in a general population. Hypertens. Res. 2007, 30, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Ankle Brachial Index Collaboration; Fowkes, F.G.; Murray, G.D. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Dachun, X.; Jue, L.; Liling, Z.; Xu, Y.; Hu, D.; Pagoto, S.L.; Ma, Y. Sensitivity and specificity of the ankle-brachial index to diagnose peripheral artery disease: A structured review. Vasc. Med. 2010, 15, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikstrom, J.; Hansen, T.; Johansson, L.; Lind, L.; Ahlstrom, H. Ankle brachial index <0.9 underestimates the prevalence of peripheral artery occlusive disease assessed with whole-body magnetic resonance angiography in the elderly. Acta Radiol. 2008, 49, 143–149. [Google Scholar]
- Premalatha, G.; Ravikumar, R.; Sanjay, R.; Deepa, R.; Mohan, V. Comparison of colour duplex ultrasound and ankle-brachial pressure index measurements in peripheral vascular disease in type 2 diabetic patients with foot infections. J. Assoc. Physicians India 2002, 50, 1240–1244. [Google Scholar]
- Vega, J.; Romani, S.; Garciperez, F.J.; Vicente, L.; Pacheco, N.; Zamorano, J.; Gómez-Barrado, J.J.; Muñoz-Torrero, J.F.S. Peripheral arterial disease: Efficacy of the oscillometric method. Rev. Esp. Cardiol. 2011, 64, 619–621. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Rosamond, W.D.; Chambless, L.E.; Nieto, F.J.; Barnes, R.W.; Hutchinson, R.G.; Tyroler, H.A.; Heiss, G.; ARIC Investigators. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Prev. Med. 2005, 29, 42–49. [Google Scholar] [PubMed]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and Interpretation of the Ankle-Brachial Index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.H.; Byrne, C.D.; Smith, F.B.; Lee, A.J.; Fowkes, F.G. Low ankle-brachial pressure index predicts increased risk of cardiovascular disease independent of the metabolic syndrome and conventional cardiovascular risk factors in the Edinburgh Artery Study. Diabetes Care 2006, 29, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Criqui, M.H.; McClelland, R.L.; McDermott, M.M.; Allison, M.A.; Blumenthal, R.S.; Aboyans, V.; Ix, J.H.; Burke, G.L.; Liu, K.; Shea, S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010, 56, 1506–1512. [Google Scholar] [CrossRef]
- Aboyans, V.; Ho, E.; Denenberg, J.O.; Ho, L.A.; Natarajan, L.; Criqui, M.H. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J. Vasc. Surg. 2008, 48, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Suominen, V.; Rantanen, T.; Venermo, M.; Saarinen, J.; Salenius, J. Prevalence and risk factors of PAD among patients with elevated ABI. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 709–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruhashi, T.; Matsui, S.; Yusoff, F.M.; Kishimoto, S.; Kajikawa, M.; Higashi, Y. Falsely normalized ankle-brachial index despite the presence of lower-extremity peripheral artery disease: Two case reports. J. Med. Case Rep. 2021, 15, 622. [Google Scholar]
- Zubair, M.; Al Amri, M.; Ahmad, J. A retrospective study of ABI and TBI during the healing of ulcer among diabetic patients. Diabetes Metab. Syndr. 2019, 13, 78–83. [Google Scholar] [CrossRef]
- Kmiec, M.M.; Hou, H.; Kuppusamy, M.L.; Drews, T.M.; Prabhat, A.; Petryakov, S.V.; Demidenko, E.; Schaner, P.E.; Buckey, J.C.; Blank, A.; et al. Transcutaneous oxygen measurement in humans using a paramagnetic skin adhesive film. Magn. Reason. Med. 2019, 81, 781–794. [Google Scholar] [CrossRef]
- Byrne, P.; Provan, J.L.; Ameli, F.M.; Jones, D.P. The use of transcutaneous oxygen tension measurements in the diagnosis of peripheral vascular insufficiency. Ann. Surg. 1984, 200, 159–165. [Google Scholar] [CrossRef]
- Caselli, A.; Latini, V.; Lapenna, A.; Di Carlo, S.; Pirozzi, F.; Benvenuto, A.; Uccioli, L. Transcutaneous oxygen tension monitoring after successful revascularization in diabetic patients with ischaemic foot ulcers. Diabet. Med. 2005, 22, 460–465. [Google Scholar] [CrossRef]
- Ladurner, R.; Kuper, M.; Konigsrainer, I.; Löb, S.; Wichmann, D.; Königsrainer, A.; Coerper, S.; Beckert, S. Predictive value of routine transcutaneous tissue oxygen tension (tcpO2) measurement for the risk of non-healing and amputation in diabetic foot ulcer patients with non-palpable pedal pulses. Med. Sci. Monit. 2010, 16, CR273–CR277. [Google Scholar]
- Zubair, M.; Ahmad, J. Transcutaneous oxygen pressure (TcPO2) and ulcer outcome in diabetic patients: Is there any correlation? Diabetes Metab. Syndr. 2019, 13, 953–958. [Google Scholar] [CrossRef]
- Romanos, M.T.; Raspovic, A.; Perrin, B.M. The reliability of toe systolic pressure and the toe brachial index in patients with diabetes. J. Foot Ankle Res. 2010, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.; Dean, R.; Patel, S.; Wu, B.; Molyneaux, L.; Yue, D.K. TBI or not TBI: That is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients? Diabet. Med. 2001, 18, 528–532. [Google Scholar] [CrossRef]
- Bhamidipaty, V.; Dean, A.; Yap, S.; Firth, J.; Barron, M.; Allard, B.; Chan, S. Second Toe Systolic Pressure Measurements are Valid Substitutes for First Toe Systolic Pressure Measurements in Diabetic Patients: A Prospective Study. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 77–82. [Google Scholar]
- Tay, W.L.; Lo, Z.J.; Hong, Q.; Yong, E.; Chandrasekar, S.; Tan, G.W.L. Toe Pressure in Predicting Diabetic Foot Ulcer Healing: A Systematic Review and Meta-analysis. Ann. Vasc. Surg. 2019, 60, 371–378. [Google Scholar]
- Scanlon, C.; Park, K.; Mapletoft, D.; Begg, L.; Burns, J. Interrater and intrarater reliability of photoplethysmography for measuring toe blood pressure and toe-brachial index in people with diabetes mellitus. J. Foot Ankle Res. 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Holland, C.K.; Brown, J.M.; Scoutt, L.M.; Taylor, K.J. Lower extremity volumetric arterial blood flow in normal subjects. Ultrasound Med. Biol. 1998, 24, 1079–1086. [Google Scholar] [CrossRef]
- Hussain, S.T. Blood flow measurements in lower limb arteries using duplex ultrasound. Ann. R. Coll. Surg. Engl. 1997, 79, 323–330. [Google Scholar]
- Lewis, P.; Psaila, J.V.; Davies, W.T.; McCarty, K.; Woodcock, J.P. Measurement of volume flow in the human common femoral artery using a duplex ultrasound system. Ultrasound Med. Biol. 1986, 12, 777–784. [Google Scholar] [CrossRef]
- Hussain, S.T.; Smith, R.E.; Wood, R.F.; Bland, M. Observer variability in volumetric blood flow measurements in leg arteries using duplex ultrasound. Ultrasound Med. Biol. 1996, 22, 287–291. [Google Scholar] [CrossRef]
- Thorley, P.J.; Sheard, K.L.; Rees, M.R. Does peripheral angioplasty compromise limb blood flow? Br. J. Radiol. 1993, 66, 506–509. [Google Scholar]
- Tehan, P.E.; Chuter, V.H. Use of hand-held Doppler ultrasound examination by podiatrists: A reliability study. J. Foot Ankle Res. 2015, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Barberán, J.; Granizo, J.-J.; Aguilar, L.; Alguacil, R.; Sainz, F.; Menéndez, M.-A.; Giménez, M.-J.; Martínez, D.; Prieto, J. Predictive model of short-term amputation during hospitalization of patients due to acute diabetic foot infections. Enfermedades Infecc. Microbiol. Clin. 2010, 28, 680–684. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Chu, S.-Y.; Wen, Y.-W.; Hsu, L.-A.; Chen, C.-C.; Peng, S.-H.; Huang, C.-H.; Sun, J.-H.; Huang, Y.-Y. The value of Doppler waveform analysis in predicting major lower extremity amputation among dialysis patients treated for diabetic foot ulcers. Diabetes Res. Clin. Pract. 2013, 100, 181–188. [Google Scholar]
- Elgzyri, T.; Ekberg, G.; Peterson, K.; Lundell, A.; Apelqvist, J. Can duplex arterial ultrasonography reduce unnecessary angiography? J. Wound Care. 2008, 17, 497–500. [Google Scholar]
- Sun, Z. Diagnostic accuracy of multislice CT angiography in peripheral arterial disease. J. Vasc. Interv. Radiol. 2006, 17, 1915–1921. [Google Scholar] [CrossRef]
- Pomposelli, F. Arterial imaging in patients with lower extremity ischemia and diabetes mellitus. J. Vasc. Surg. 2010, 52, 81S–91S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samal, A.K.; White, C.J. Percutaneous management of access site complications. Catheter. Cardiovasc. Interv. 2002, 57, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Hackl, G.; Gary, T.; Belaj, K.; Hafner, F.; Eller, P.; Brodmann, M. Risk Factors for Puncture Site Complications after Endovascular Procedures in Patients with Peripheral Arterial Disease. Vasc. Endovasc. Surg. 2015, 49, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Cochran, S.T. Anaphylactoid reactions to radiocontrast media. Curr. Allergy Asthma Rep. 2005, 5, 28–31. [Google Scholar] [CrossRef]
- Parfrey, P.S.; Griffiths, S.M.; Barrett, B.J.; Paul, M.D.; Genge, M.; Withers, J.; Farid, N.; McManamon, P.J. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N. Engl. J. Med. 1989, 320, 143–149. [Google Scholar] [CrossRef]
- Hall, K.; Wong, R.; Hunter, G.; Camazine, B.; Rappaport, W.; Smyth, S.; Bull, D.; McIntyre, K.; Bernhard, V.; Misiorowski, R. Contrast-induced nephrotoxicity: The effects of vasodilator therapy. J. Surg. Res. 1992, 53, 317–320. [Google Scholar] [CrossRef]
- Meaney, J.F. Magnetic resonance angiography of the peripheral arteries: Current status. Eur. Radiol. 2003, 13, 836–852. [Google Scholar] [CrossRef]
- Attenberger, U.I.; Haneder, S.; Morelli, J.N.; Diehl, S.J.; Schoenberg, S.O.; Michaely, H.J. Peripheral arterial occlusive disease: Evaluation of a high spatial and temporal resolution 3-T MR protocol with a low total dose of gadolinium versus conventional angiography. Radiology 2010, 257, 879–887. [Google Scholar]
- Haider, C.R.; Glockner, J.F.; Stanson, A.W.; Riederer, S.J. Peripheral vasculature: High-temporal- and high-spatial-resolution three-dimensional contrast-enhanced MR angiography. Radiology 2009, 253, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, H.A.; Kashanian, F.K.; Blumetti, R.F.; Holyoak, W.L.; Hugo, F.P.; Blumenfield, D.M. Safety assessment of gadopentetate dimeglumine in US clinical trials. Radiology 1990, 174, 17–23. [Google Scholar] [CrossRef]
- Niendorf, H.P.; Haustein, J.; Cornelius, I.; Alhassan, A.; Clauss, W. Safety of gadolinium-DTPA: Extended clinical experience. Magn. Reason. Med. 1991, 22, 222–228, discussion 229–232. [Google Scholar] [CrossRef]
- Sam, A.D., 2nd; Morasch, M.D.; Collins, J.; Song, G.; Chen, R.; Pereles, F.S. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J. Vasc. Surg. 2003, 38, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledneva, E.; Karie, S.; Launay-Vacher, V.; Janus, N.; Deray, G. Renal safety of gadolinium-based contrast media in patients with chronic renal insufficiency. Radiology 2009, 250, 618–628. [Google Scholar] [CrossRef]
- Leyba, K.; Wagner, B. Gadolinium-based contrast agents: Why nephrologists need to be concerned. Curr. Opin. Nephrol. Hypertens. 2019, 28, 154–162. [Google Scholar]
- Met, R.; Bipat, S.; Legemate, D.A.; Reekers, J.A.; Koelemay, M.J. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA 2009, 301, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Korn, A.; Ketelsen, D.; Danz, S.; Tsifikas, I.; Claussen, C.D.; Ernemann, U.; Heuschmid, M. Automatic lumen segmentation in calcified plaques: Dual-energy CT versus standard reconstructions in comparison with digital subtraction angiography. AJR Am. J. Roentgenol. 2010, 194, 1590–1595. [Google Scholar]
- De Santis, D.; De Cecco, C.N.; Schoepf, U.J.; Nance, J.W.; Yamada, R.T.; Thomas, B.A.; Otani, K.; Jacobs, B.E.; Turner, D.A.; Wichmann, J.L.; et al. Modified calcium subtraction in dual-energy CT angiography of the lower extremity runoff: Impact on diagnostic accuracy for stenosis detection. Eur. Radiol. 2019, 29, 4783–4793. [Google Scholar] [PubMed]
- Bajwa, A.; Wesolowski, R.; Patel, A.; Saha, P.; Ludwinski, F.; Smith, A.; Nagel, E.; Modarai, B. Assessment of Tissue Perfusion in the Lower Limb. Circ. Cardiovasc. Imaging 2014, 7, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Wang, X.; Yang, X.; Wu, D.; Cheng, Z.; Wu, L. Diagnostic efficiency of low-dose CT angiography compared with conventional angiography in peripheral arterial occlusions. AJR Am. J. Roentgenol. 2013, 201, W906–W914. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Wood, E.H. Indocyanine green: Physical and physiologic properties. Proc. Staff. Meet. Mayo Clin. 1960, 35, 732–744. [Google Scholar] [PubMed]
- Kang, Y.; Lee, J.; Kwon, K.; Choi, C. Dynamic fluorescence imaging of indocyanine green for reliable and sensitive diagnosis of peripheral vascular insufficiency. Microvasc. Res. 2010, 80, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.M.; Bulsara, S.S.; Banerjee, S.; Sahu, T.; Sheorain, V.K.; Grover, T.; Parakh, R. Indocyanine Green Angiography to Prognosticate Healing of Foot Ulcer in Critical Limb Ischemia: A Novel Technique. Ann. Vasc. Surg. 2018, 51, 86–94. [Google Scholar] [CrossRef]
- Mennes, O.A.; van Netten, J.J.; van Baal, J.G.; Steenbergen, W. Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol. Meas. 2019, 40, 065002. [Google Scholar] [CrossRef]
- Galanakis, N.; Maris, T.G.; Kontopodis, N.; Tsetis, K.; Kehagias, E.; Tsetis, D. Perfusion imaging techniques in lower extremity peripheral arterial disease. Br. J. Radiol. 2022, 95, 20211203. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Huellner, M.W.; Banyai, M.; Mafeld, S.; Heverhagen, J.; Strobel, K.; Sah, B.-R. CT perfusion in peripheral arterial disease-hemodynamic differences before and after revascularisation. Eur. Radiol. 2021, 31, 5507–5513. [Google Scholar] [CrossRef]
- Sah, B.R.; Veit-Haibach, P.; Strobel, K.; Banyai, M.; Huellner, M.W. CT-perfusion in peripheral arterial disease—Correlation with angiographic and hemodynamic parameters. PLoS ONE 2019, 14, e0223066. [Google Scholar] [CrossRef]
- Barfett, J.; Velauthapillai, N.; Kloeters, C.; Mikulis, D.J.; Jaskolka, J.D. An en bloc approach to CT perfusion for the evaluation of limb ischemia. Int. J. Cardiovasc. Imaging 2012, 28, 2073–2083. [Google Scholar] [CrossRef]
- Iezzi, R.; Santoro, M.; Dattesi, R.; La Torre, M.F.; Snider, F.; Bonomo, L. Foot CT perfusion in patients with peripheral arterial occlusive disease (PAOD): A feasibility study. Eur. J. Radiol. 2013, 82, e455–e464. [Google Scholar]
- Gordon, Y.; Partovi, S.; Müller-Eschner, M.; Amarteifio, E.; Baeuerle, T.; Weber, M.-A.; Kauczor, H.-U.; Rengier, F. Dynamic contrast-enhanced magnetic resonance imaging: Fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc. Diagn. Ther. 2014, 4, 147–164. [Google Scholar]
- Thompson, R.B.; Aviles, R.J.; Faranesh, A.Z.; Raman, V.K.; Wright, V.; Balaban, R.S.; McVeigh, E.R.; Lederman, R.J. Measurement of skeletal muscle perfusion during postischemic reactive hyperemia using contrast-enhanced MRI with a step-input function. Magn. Reason. Med. 2005, 54, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Isbell, D.C.; Epstein, F.H.; Zhong, X.; DiMaria, J.M.; Berr, S.S.; Meyer, C.H.; Rogers, W.J.; Harthun, N.L.; Hagspiel, K.D.; Weltman, A.; et al. Calf muscle perfusion at peak exercise in peripheral arterial disease: Measurement by first-pass contrast-enhanced magnetic resonance imaging. J. Magn. Reason. Imaging 2007, 25, 1013–1020. [Google Scholar]
- Wong, E.C. An introduction to ASL labeling techniques. J. Magn. Reason. Imaging 2014, 40, 1–10. [Google Scholar] [CrossRef]
- Wu, W.C.; Mohler, E.; 3rd Ratcliffe, S.J.; Wehrli, F.W.; Detre, J.A.; Floyd, T.F. Skeletal muscle microvascular flow in progressive peripheral artery disease: Assessment with continuous arterial spin-labeling perfusion magnetic resonance imaging. J. Am. Coll. Cardiol. 2009, 53, 2372–2377. [Google Scholar] [PubMed] [Green Version]
- Ledermann, H.P.; Schulte, A.-C.; Heidecker, H.-G.; Aschwanden, M.; Jäger, K.A.; Scheffler, K.; Steinbrich, W.; Bilecen, D. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation 2006, 113, 2929–2935. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Törngren, K.; Eriksson, S.; Arvidsson, J.; Falkenberg, M.; Johnsson, A.; Sjöberg, C.; Lagerstrand, K.; Nordanstig, J. A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls. J. Clin. Med. 2021, 10, 3643. [Google Scholar] [PubMed]
- O’Meara, S.; Al-Kurdi, D.; Ovington, L.G. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst. Rev. 2008, Cd003557. [Google Scholar] [CrossRef]
- Callam, M.J. Epidemiology of varicose veins. Br. J. Surg. 1994, 81, 167–173. [Google Scholar] [CrossRef]
- Abbade, L.P.; Lastória, S. Venous ulcer: Epidemiology, physiopathology, diagnosis and treatment. Int. J. Dermatol. 2005, 44, 449–456. [Google Scholar] [PubMed]
- McAree, B.J.; Berridge, D.C. Investigation of the patient with a venous ulcer. Phlebology 2010, 25, 20–27. [Google Scholar]
- Meissner, M.H.; Eklof, B.; Smith, P.C.; Dalsing, M.C.; DePalma, R.G.; Gloviczki, P.; Moneta, G.; Neglén, P.; Donnell, T.O.; Partsch, H.; et al. Secondary chronic venous disorders. J. Vasc. Surg. 2007, 46, 68s–83s. [Google Scholar] [PubMed] [Green Version]
- Collins, L.; Seraj, S. Diagnosis and treatment of venous ulcers. Am. Fam. Physician 2010, 81, 989–996. [Google Scholar]
- Nicolaides, A.N. Investigation of chronic venous insufficiency: A consensus statement (France, March 5–9, 1997). Circulation 2000, 102, E126–E163. [Google Scholar]
- Browse, N.L.; Burnand, K.G. The cause of venous ulceration. Lancet 1982, 2, 243–245. [Google Scholar]
- Falanga, V.; Eaglstein, W.H. The ‘trap’ hypothesis of venous ulceration. Lancet 1993, 341, 1006–1008. [Google Scholar] [CrossRef]
- Coleridge Smith, P.D.; Thomas, P.; Scurr, J.H.; Dormandy, J.A. Causes of venous ulceration: A new hypothesis. Br. Med. J. 1988, 296, 1726–1727. [Google Scholar]
- Powell, C.C.; Rohrer, M.J.; Barnard, M.R.; Peyton, B.D.; Furman, M.I.; Michelson, A.D. Chronic venous insufficiency is associated with increased platelet and monocyte activation and aggregation. J. Vasc. Surg. 1999, 30, 844–853. [Google Scholar] [PubMed] [Green Version]
- Galvan, L. Assessing venous ulcers and venous insufficiency. Nursing 2005, 35, 70. [Google Scholar] [CrossRef]
- Gillespie, D.L.; Writing Group III of the Pacific Vascular Symposium 6. Venous ulcer diagnosis, treatment, and prevention of recurrences. J. Vasc. Surg. 2010, 52, 8s–14s. [Google Scholar] [CrossRef] [Green Version]
- Kokkosis, A.A.; Labropoulos, N.; Gasparis, A.P. Investigation of venous ulcers. Semin. Vasc. Surg. 2015, 28, 15–20. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Padberg, F.T., Jr.; Comerota, A.J.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L. Venous severity scoring: An adjunct to venous outcome assessment. J. Vasc. Surg. 2000, 31, 1307–1312. [Google Scholar] [CrossRef]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2s–48s. [Google Scholar]
- Hess, C.T. Venous Ulcer Assessment and Management: Using the Updated CEAP Classification System. Adv. Ski. Wound Care 2020, 33, 614–615. [Google Scholar] [CrossRef]
- Mallick, S.; Sarkar, T.; Gayen, T.; Naskar, B.; Datta, A.; Sarkar, S. Correlation of Venous Clinical Severity Score and Venous Disability Score with Dermatology Life Quality Index in Chronic Venous Insufficiency. Indian J. Dermatol. 2020, 65, 489–494. [Google Scholar]
- Necas, M. Duplex ultrasound in the assessment of lower extremity venous insufficiency. Australas. J. Ultrasound Med. 2010, 13, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Srisuwan, T.; Inmutto, N.; Kattipathanapong, T.; Rerkasem, A.; Rerkasem, K. Ultrasound Use in Diagnosis and Management of Venous Leg Ulcer. Int. J. Low. Extrem. Wounds 2020, 19, 305–314. [Google Scholar] [CrossRef]
- Magnusson, M.B.; Nelzén, O.; Risberg, B.; Sivertsson, R. A colour Doppler ultrasound study of venous reflux in patients with chronic leg ulcers. Eur. J. Vasc. Endovasc. Surg. 2001, 21, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Lurie, F.; Comerota, A.; Eklof, B.; Kistner, R.L.; Labropoulos, N.; Lohr, J.; Marston, W.; Meissner, M.; Moneta, G.; Neglén, P.; et al. Multicenter assessment of venous reflux by duplex ultrasound. J. Vasc. Surg. 2012, 55, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zygmunt, J.A. Duplex ultrasound for chronic venous insufficiency. J. Invasive Cardiol. 2014, 26, E149–E155. [Google Scholar]
- Carty, G.A.; Steele, J.L.; Clemens, J. Standing versus Supine Evaluation for Superficial Venous Reflux. J. Vasc. Ultrasound 2013, 37, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Cavezzi, A.; Labropoulos, N.; Partsch, H.; Ricci, S.; Caggiati, A.; Myers, K.; Nicolaides, A.; Smith, P.C. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part II. Anatomy. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Kavros, S.J.; Coronado, R. Diagnostic and Therapeutic Ultrasound on Venous and Arterial Ulcers: A Focused Review. Adv. Skin Wound Care. 2018, 31, 55–65. [Google Scholar] [CrossRef]
- Mustafa, B.O.; Rathbun, S.W.; Whitsett, T.L.; Raskob, G.E. Sensitivity and specificity of ultrasonography in the diagnosis of upper extremity deep vein thrombosis: A systematic review. Arch. Intern. Med. 2002, 162, 401–404. [Google Scholar] [CrossRef]
- Goodacre, S.; Sampson, F.; Thomas, S.; van Beek, E.; Sutton, A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med. Imaging 2005, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Alavi, A.; Sibbald, R.G.; Phillips, T.J.; Miller, O.F.; Margolis, D.J.; Marston, W.; Woo, K.; Romanelli, M.; Kirsner, R.S. What’s new: Management of venous leg ulcers: Approach to venous leg ulcers. J. Am. Acad. Dermatol. 2016, 74, 627–640, quiz 641–622. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Shyu, V.B.-H.; Chiu, W.-K.; Huang, R.-W.; Lai, B.-R.; Tsai, C.-H. An Overview of Clinical Examinations in the Evaluation and Assessment of Arterial and Venous Insufficiency Wounds. Diagnostics 2023, 13, 2494. https://doi.org/10.3390/diagnostics13152494
Wang S-H, Shyu VB-H, Chiu W-K, Huang R-W, Lai B-R, Tsai C-H. An Overview of Clinical Examinations in the Evaluation and Assessment of Arterial and Venous Insufficiency Wounds. Diagnostics. 2023; 13(15):2494. https://doi.org/10.3390/diagnostics13152494
Chicago/Turabian StyleWang, Szu-Han, Victor Bong-Hang Shyu, Wen-Kuan Chiu, Ren-Wen Huang, Bo-Ru Lai, and Chia-Hsuan Tsai. 2023. "An Overview of Clinical Examinations in the Evaluation and Assessment of Arterial and Venous Insufficiency Wounds" Diagnostics 13, no. 15: 2494. https://doi.org/10.3390/diagnostics13152494




