Cardiac Biomarkers and Their Role in Identifying Increased Risk of Cardiovascular Complications in COVID-19 Patients
Abstract
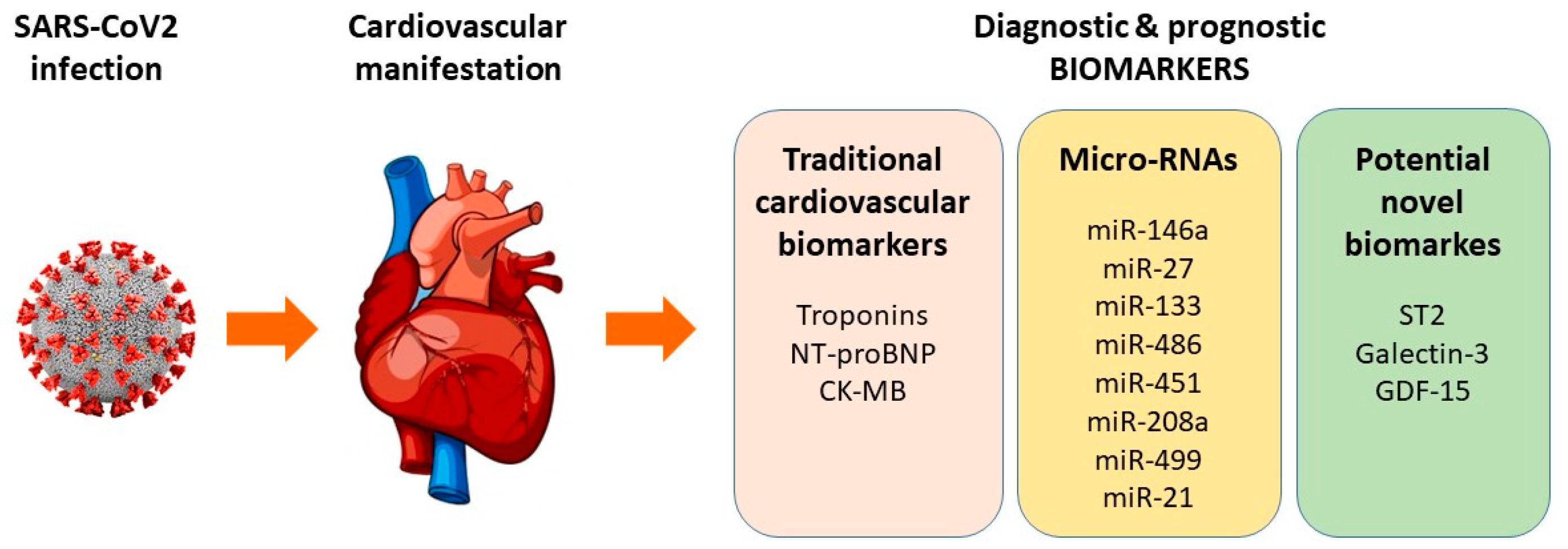
:1. Introduction
2. Traditional Cardiovascular Biomarkers
2.1. Troponin
2.2. B-Type Natriuretic Peptide
2.3. Creatine Kinase-Myocardial Band
3. MicroRNAs (miRNAs) as Potential Biomarkers in COVID-19-Associated Cardiovascular Complications
3.1. MiR-146a
3.2. MiR-27
3.3. MiR-133
3.4. MiR-486
3.5. MiR-451
3.6. Other MiRNAs
3.7. MiRNAs in Relation to Established Cardiac Biomarkers
4. Potential Novel Biomarkers
4.1. ST2
4.2. Galectin-3
4.3. GDF-15
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Rippe, J.M. Lifestyle Strategies for Risk Factor Reduction, Prevention, and Treatment of Cardiovascular Disease. Am. J. Lifestyle Med. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Omran, F.; Kyrou, I.; Osman, F.; Lim, V.G.; Randeva, H.S.; Chatha, K. Cardiovascular Biomarkers: Lessons of the Past and Prospects for the Future. Int. J. Mol. Sci. 2022, 23, 5680. [Google Scholar] [CrossRef]
- Vasan, R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006, 113, 2335–2362. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiology 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januzzi, J.L.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Thygesen, K.; Alpert, J.S.; et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.a.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.a.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef] [Green Version]
- Kapranov, P.; Willingham, A.T.; Gingeras, T.R. Genome-wide transcription and the implications for genomic organization. Nat. Rev. Genet. 2007, 8, 413–423. [Google Scholar] [CrossRef]
- Guterres, A.; de Azeredo Lima, C.H.; Miranda, R.L.; Gadelha, M.R. What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? Infect. Genet. Evol. 2020, 85, 104417. [Google Scholar] [CrossRef]
- Ying, S.-Y.; Chang, D.C.; Lin, S.-L. The MicroRNA (miRNA): Overview of the RNA Genes that Modulate Gene Function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Saçar Demirci, M.D.; Adan, A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ 2020, 8, e9369. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, R.L.; Cullen, B.R. Viruses, microRNAs, and Host Interactions. Annu. Rev. Microbiol. 2010, 64, 123–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. COVID-19 Virulence in Aged Patients Might Be Impacted by the Host Cellular MicroRNAs Abundance/Profile. Aging Dis. 2020, 11, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Arghiani, N.; Nissan, T.; Matin, M.M. Role of microRNAs in COVID-19 with implications for therapeutics. Biomed. Pharmacother. 2021, 144, 112247. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, N.R.; Jethani, N.; Chen, J.; Aphinyanaphongs, Y.; Zhang, R.; Dogra, S.; Alviar, C.L.; Keller, N.; Razzouk, L.; Quinones-Camacho, A.; et al. Myocardial Injury in Adults Hospitalized with COVID-19. Circulation 2020, 142, 2393–2395. [Google Scholar] [CrossRef]
- Sandoval, Y.; Januzzi, J.L.; Jaffe, A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1244–1258. [Google Scholar] [CrossRef]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef]
- Perrone, M.A.; Spolaore, F.; Ammirabile, M.; Romeo, F.; Caciagli, P.; Ceriotti, F.; Bernardini, S. The assessment of high sensitivity cardiac troponin in patients with COVID-19: A multicenter study. Int. J. Cardiology. Heart Vasc. 2021, 32, 100715. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.B.; et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef]
- Pfisterer, M.; Buser, P.; Rickli, H.; Gutmann, M.; Erne, P.; Rickenbacher, P.; Vuillomenet, A.; Jeker, U.; Dubach, P.; Beer, H.; et al. BNP-guided vs. symptom-guided heart failure therapy: The Trial of Intensified vs. Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009, 301, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Troughton, R.W.; Frampton, C.M.; Yandle, T.G.; Espiner, E.A.; Nicholls, M.G.; Richards, A.M. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000, 355, 1126–1130. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. In cGMP: Generators, Effectors and Therapeutic Implications; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 341–366. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, E.; Kass, D.A. Role of Oxidative Stress in Cardiac Hypertrophy and Remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef]
- Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1295. [CrossRef] [Green Version]
- Caro-Codón, J.; Rey, J.R.; Buño, A.; Iniesta, A.M.; Rosillo, S.O.; Castrejon-Castrejon, S.; Rodriguez-Sotelo, L.; Martinez, L.A.; Marco, I.; Merino, C.; et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 456–464. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, D.; Wen, X.-S.; Cheng, X.-C.; Sun, M.; He, B.; You, L.-N.; Lei, P.; Tan, X.-W.; Qin, S.; et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir. Res. 2020, 21, 83. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Lombardi, C.M.; Specchia, C.; Merlo, M.; Nuzzi, V.; Ferraro, I.; Peveri, G.; Oriecuia, C.; Pozzi, A.; Inciardi, R.M.; et al. Combined Role of Troponin and Natriuretic Peptides Measurements in Patients with Covid-19 (from the Cardio-COVID-Italy Multicenter Study). Am. J. Cardiol. 2022, 167, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.S.; Babuin, L.; Apple, F.S. Biomarkers in acute cardiac disease: The present and the future. J. Am. Coll. Cardiol. 2006, 48, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, P.; Zhu, J.; Zhong, Z.; Li, H.; Pang, J.; Li, B.; Zhang, J. Association of elevated inflammatory markers and severe COVID-19: A meta-analysis. Medicine 2020, 99, e23315. [Google Scholar] [CrossRef]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Liu, M.; Sun, M.; Xiao, L.; Chang, Q.; Chen, Y.; Huang, J. Abnormal myocardial enzymes in the prediction of mortality and hypertension in COVID-19 patients: A retrospective study. Aging 2022, 14, 8585–8594. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Kang, J.-S.; Wang, Q.; Kim, T.-E. Cardiac biomarkers and COVID-19: A systematic review and meta-analysis. J. Infect. Public Health 2021, 14, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. JTH 2020, 18, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Zhao, M.; Yang, M.; Zhong, J.; Gu, Y.; Peng, H.; Che, Y.; Huang, C. Identification of a circulating microRNA signature for colorectal cancer detection. PLoS ONE 2014, 9, e87451. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, J.; Wang, J.; Zhang, Z.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.K.; Zhu, J.Q.; Zhang, J.T.; Li, Q.; Li, Y.; He, J.; Qin, Y.W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, R.; Li, Y.; Pu, J.; Lu, Y.; Jiao, J.; Li, K.; Yu, B.; Li, Z.; Wang, R.; et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010, 391, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sakata, Y.; Nakatani, D.; Suna, S.; Mizuno, H.; Shimizu, M.; Usami, M.; Sasaki, T.; Sato, H.; Kawahara, Y.; et al. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2012, 427, 280–284. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. JTH 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatinelli, J.; Giuliani, A.; Matacchione, G.; Latini, S.; Laprovitera, N.; Pomponio, G.; Ferrarini, A.; Svegliati Baroni, S.; Pavani, M.; Moretti, M.; et al. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2021, 193, 111413. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Fatima, R.; Assaly, R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020, 92, 2283–2285. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Wang, X.; Mann, M.; Adamus, T.P.; Wang, D.; Moreira, D.F.; Zhang, Z.; Ouyang, C.; He, X.; Zhang, B.; et al. Myeloid cell-targeted miR-146a mimic inhibits NF-κB-driven inflammation and leukemia progression in vivo. Blood 2020, 135, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Roganović, J. Downregulation of microRNA-146a in diabetes, obesity and hypertension may contribute to severe COVID-19. Med. Hypotheses 2021, 146, 110448. [Google Scholar] [CrossRef]
- Vasuri, F.; Ciavarella, C.; Collura, S.; Mascoli, C.; Valente, S.; Degiovanni, A.; Gargiulo, M.; Capri, M.; Pasquinelli, G. Adventitial Microcirculation Is a Major Target of SARS-CoV-2-Mediated Vascular Inflammation. Biomolecules 2021, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, X.; Zhang, X.; Ha, T.; Ma, H.; Liu, L.; Kalbfleisch, J.H.; Gao, X.; Kao, R.L.; Williams, D.L.; et al. Attenuation of cardiac dysfunction in polymicrobial sepsis by microRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. J. Immunol. 2015, 195, 672–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.G.; Watanabe, S.; Lee, A.; Gorski, P.A.; Lee, P.; Jeong, D.; Liang, L.; Liang, Y.; Baccarini, A.; Sahoo, S.; et al. miR-146a Suppresses SUMO1 Expression and Induces Cardiac Dysfunction in Maladaptive Hypertrophy. Circ. Res. 2018, 123, 673–685. [Google Scholar] [CrossRef]
- Huang, P.; He, X.; Xu, M. The Role of miRNA-146a and Proinflammatory Cytokines in Carotid Atherosclerosis. BioMed Res. Int. 2020, 2020, 6657734. [Google Scholar] [CrossRef]
- Palomer, X.; Capdevila-Busquets, E.; Botteri, G.; Davidson, M.M.; Rodríguez, C.; Martínez-González, J.; Vidal, F.; Barroso, E.; Chan, T.O.; Feldman, A.M.; et al. miR-146a targets Fos expression in human cardiac cells. Dis. Models Mech. 2015, 8, 1081–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyakova, E.A.; Zaraiskii, M.I.; Mikhaylov, E.N.; Baranova, E.I.; Galagudza, M.M.; Shlyakhto, E.V. Association of myocardial and serum miRNA expression patterns with the presence and extent of coronary artery disease: A cross-sectional study. Int. J. Cardiol. 2021, 322, 9–15. [Google Scholar] [CrossRef]
- Barsanti, C.; Trivella, M.G.; D’Aurizio, R.; El Baroudi, M.; Baumgart, M.; Groth, M.; Caruso, R.; Verde, A.; Botta, L.; Cozzi, L.; et al. Differential Regulation of MicroRNAs in End-Stage Failing Hearts Is Associated with Left Ventricular Assist Device Unloading. BioMed Res. Int. 2015, 2015, 592512. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhou, M.; Wang, J.; Zhao, Y.; Li, S.; Zhou, B.; Su, Z.; Xu, C.; Xia, Y.; Qian, H.; et al. Role of microRNA-27a in down-regulation of angiogenic factor AGGF1 under hypoxia associated with high-grade bladder urothelial carcinoma. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gonzalo-Calvo, D.; Benítez, I.D.; Pinilla, L.; Carratalá, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Molinero, M.; González, J.; Torres, G.; Bernal, M.; et al. Circulating microRNA profiles predict the severity of COVID-19 in hospitalized patients. Transl. Res. 2021, 236, 147–159. [Google Scholar] [CrossRef]
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef]
- Gutmann, C.; Khamina, K.; Theofilatos, K.; Diendorfer, A.B.; Burnap, S.A.; Nabeebaccus, A.; Fish, M.; McPhail, M.J.W.; O’Gallagher, K.; Schmidt, L.E.; et al. Association of cardiometabolic microRNAs with COVID-19 severity and mortality. Cardiovasc. Res. 2022, 118, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef]
- Callis, T.E.; Pandya, K.; Seok, H.Y.; Tang, R.-H.; Tatsuguchi, M.; Huang, Z.-P.; Chen, J.-F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Zhou, H.; Tang, Q. miR-133: A Suppressor of Cardiac Remodeling? Front. Pharmacol. 2018, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Barwari, T.; Joshi, A.; Theofilatos, K.; Zampetaki, A.; Barallobre-Barreiro, J.; Singh, B.; Sörensen, N.A.; Neumann, J.T.; Zeller, T.; et al. Comparative Analysis of Circulating Noncoding RNAs Versus Protein Biomarkers in the Detection of Myocardial Injury. Circ. Res. 2019, 125, 328–340. [Google Scholar] [CrossRef]
- Donaldson, A.; Natanek, S.A.; Lewis, A.; Man, W.D.-C.; Hopkinson, N.S.; Polkey, M.I.; Kemp, P.R. Increased skeletal muscle-specific microRNA in the blood of patients with COPD. Thorax 2013, 68, 1140–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.-J.; Schröder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase attracts neutrophils by physical forces. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef] [Green Version]
- Slezak, S.; Jin, P.; Caruccio, L.; Ren, J.; Bennett, M.; Zia, N.; Adams, S.; Wang, E.; Ascensao, J.; Schechter, G.; et al. Gene and microRNA analysis of neutrophils from patients with polycythemia vera and essential thrombocytosis: Down-regulation of micro RNA-1 and -133a. J. Transl. Med. 2009, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Beg, F.; Wang, R.; Saeed, Z.; Devaraj, S.; Masoor, K.; Nakshatri, H. Inflammation-associated microRNA changes in circulating exosomes of heart failure patients. BMC Res. Notes 2017, 10, 751. [Google Scholar] [CrossRef]
- Wang, J.; Tian, X.; Han, R.; Zhang, X.; Wang, X.; Shen, H.; Xue, L.; Liu, Y.; Yan, X.; Shen, J.; et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 2014, 33, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Artiga, E.; Kalyanasundaram, A.; Hansen, B.J.; Webb, A.; Pietrzak, M.; Biesiadecki, B.; Whitson, B.; Mokadam, N.A.; Janssen, P.M.L.; et al. Altered microRNA and mRNA profiles during heart failure in the human sinoatrial node. Sci. Rep. 2021, 11, 19328. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Lu, D.; Bär, C.; Chatterjee, S.; Costa, A.; Riedel, I.; Mooren, F.C.; Zhu, Y.; Huang, Z.; Wei, M.; et al. miR-486 attenuates cardiac ischemia/reperfusion injury and mediates the beneficial effect of exercise for myocardial protection. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 1675–1691. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wu, S. miR-451: A Novel Biomarker and Potential Therapeutic Target for Cancer. OncoTargets Ther. 2019, 12, 11069–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slagsvold, K.H.; Johnsen, A.B.; Rognmo, Ø.; Høydal, M.; Wisløff, U.; Wahba, A. Comparison of left versus right atrial myocardium in patients with sinus rhythm or atrial fibrillation—An assessment of mitochondrial function and microRNA expression. Physiol. Rep. 2014, 2, e12124. [Google Scholar] [CrossRef] [PubMed]
- Van der Weg, K.; Prinzen, F.W.; Gorgels, A.P. Editor’s Choice—Reperfusion cardiac arrhythmias and their relation to reperfusion-induced cell death. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhao, Y.; Li, J.; Liu, C.; Zhu, L.; Zhang, J.; Yu, Y.; Wang, W.-J.; Lei, G.; Yan, J.; et al. Downregulated miR-451a as a feature of the plasma cfRNA landscape reveals regulatory networks of IL-6/IL-6R-associated cytokine storms in COVID-19 patients. Cell. Mol. Immunol. 2021, 18, 1064–1066. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Bautista-Becerril, B.; Pérez-Dimas, G.; Sommerhalder-Nava, P.C.; Hanono, A.; Martínez-Cisneros, J.A.; Zarate-Maldonado, B.; Muñoz-Soria, E.; Aquino-Gálvez, A.; Castillejos-López, M.; Juárez-Cisneros, A.; et al. miRNAs, from Evolutionary Junk to Possible Prognostic Markers and Therapeutic Targets in COVID-19. Viruses 2021, 14, 41. [Google Scholar] [CrossRef]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- Gidlöf, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Liebetrau, C.; Möllmann, H.; Dörr, O.; Szardien, S.; Troidl, C.; Willmer, M.; Voss, S.; Gaede, L.; Rixe, J.; Rolf, A.; et al. Release Kinetics of Circulating Muscle-Enriched MicroRNAs in Patients Undergoing Transcoronary Ablation of Septal Hypertrophy. J. Am. Coll. Cardiol. 2013, 62, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, Y.; Mueller, M.; Haaf, P.; Goretti, E.; Twerenbold, R.; Zangrando, J.; Vausort, M.; Reichlin, T.; Wildi, K.; Moehring, B.; et al. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J. Intern. Med. 2015, 277, 260–271. [Google Scholar] [CrossRef]
- Aoki, S.; Hayakawa, M.; Ozaki, H.; Takezako, N.; Obata, H.; Ibaraki, N.; Tsuru, T.; Tominaga, S.-I.; Yanagisawa, K. ST2 gene expression is proliferation-dependent and its ligand, IL-33, induces inflammatory reaction in endothelial cells. Mol. Cell. Biochem. 2010, 335, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lian, J.; Yue, Y.; Zhang, Y. IL-33/ST2 as a potential target for tumor immunotherapy. Eur. J. Immunol. 2021, 51, 1943–1955. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Takagi, T.; Tsukamoto, T.; Tetsuka, T.; Tominaga, S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993, 318, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, S.; Kuroiwa, K.; Tago, K.; Iwahana, H.; Yanagisawa, K.; Komatsu, N. Presence and expression of a novel variant form of ST2 gene product in human leukemic cell line UT-7/GM. Biochem. Biophys. Res. Commun. 1999, 264, 14–18. [Google Scholar] [CrossRef]
- Demyanets, S.; Konya, V.; Kastl, S.P.; Kaun, C.; Rauscher, S.; Niessner, A.; Pentz, R.; Pfaffenberger, S.; Rychli, K.; Lemberger, C.E.; et al. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2080–2089. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, R.; Hei, H.; Dobner, S.; Lee, R.T. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J. Biol. Chem. 2012, 287, 6941–6948. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Liew, F.Y. The IL-33/ST2 pathway—A new therapeutic target in cardiovascular disease. Pharmacol. Ther. 2011, 131, 179–186. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.J.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [Green Version]
- Seki, K.; Sanada, S.; Kudinova, A.Y.; Steinhauser, M.L.; Handa, V.; Gannon, J.; Lee, R.T. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circulation. Heart Fail. 2009, 2, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Reviews. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Xu, C.; Zhao, R.; Cao, Z. Diagnostic Value of sST2 in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 697837. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Concistrè, A.; Suppa, M.; Galardo, G.; Rosa, A.; Bertazzoni, G.; Pugliese, F.; Letizia, C.; Petramala, L. Prognostic Role of sST2 in Acute Heart Failure and COVID-19 Infection—A Narrative Review on Pathophysiology and Clinical Prospective. Int. J. Mol. Sci. 2022, 23, 8230. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Hong, X.-Y.; Li, Y.; Chen, W.; Ye, G.; Li, Y.; Luo, Y. Serum-soluble ST2 as a novel biomarker reflecting inflammatory status and illness severity in patients with COVID-19. Biomark. Med. 2020, 14, 1619–1629. [Google Scholar] [CrossRef]
- Sánchez-Marteles, M.; Rubio-Gracia, J.; Peña-Fresneda, N.; Garcés-Horna, V.; Gracia-Tello, B.; Martínez-Lostao, L.; Crespo-Aznárez, S.; Pérez-Calvo, J.I.; Giménez-López, I. Early Measurement of Blood sST2 Is a Good Predictor of Death and Poor Outcomes in Patients Admitted for COVID-19 Infection. J. Clin. Med. 2021, 10, 3534. [Google Scholar] [CrossRef]
- Homsak, E.; Gruson, D. Soluble ST2: A complex and diverse role in several diseases. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 507, 75–87. [Google Scholar] [CrossRef]
- Ragusa, R.; Basta, G.; Del Turco, S.; Caselli, C. A possible role for ST2 as prognostic biomarker for COVID-19. Vasc. Pharmacol. 2021, 138, 106857. [Google Scholar] [CrossRef]
- Cao, Q.; Lei, H.; Yang, M.; Wei, L.; Dong, Y.; Xu, J.; Nasser, M.; Liu, M.; Zhu, P.; Xu, L.; et al. Impact of Cardiovascular Diseases on COVID-19: A Systematic Review. Med. Sci. Monit. Med. J. Exp. Clin. Res. 2021, 27, e930032-1–e930032-12. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Balan, V.; Raz, A. Chimeric galectin-3 and collagens: Biomarkers and potential therapeutic targets in fibroproliferative diseases. J. Biol. Chem. 2022, 298, 102622. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of Transforming Growth Factor-β1–driven Lung Fibrosis by Galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Hrynchyshyn, N.; Jourdain, P.; Desnos, M.; Diebold, B.; Funck, F. Galectin-3: A new biomarker for the diagnosis, analysis and prognosis of acute and chronic heart failure. Arch. Cardiovasc. Dis. 2013, 106, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Keng, B.M.H.; Gao, F.; Ewe, S.H.; Tan, R.S.; Teo, L.L.Y.; Xie, B.Q.; Koh, W.; Koh, A.S. Galectin-3 as a candidate upstream biomarker for quantifying risks of myocardial ageing. ESC Heart Fail. 2019, 6, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Fu, W.; Zheng, Y.; Yang, J.; Liu, Y.; Qi, Z.; Wu, M.; Fan, Z.; Yin, K.; Chen, Y.; et al. Galectin 3 enhances platelet aggregation and thrombosis via Dectin-1 activation: A translational study. Eur. Heart J. 2022, 43, 3556–3574. [Google Scholar] [CrossRef]
- Jakobs, K.; Rauch, U. Galectin-3 inhibitors as novel antithrombotic drugs with almost no bleeding risk: Wishful thinking or a realistic vision? Eur. Heart J. 2022, 43, 3575–3577. [Google Scholar] [CrossRef]
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Di Serio, F.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556. [Google Scholar] [CrossRef]
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yücel, Ç.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and Prostaglandin E2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, 74, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gajovic, N.; Markovic, S.S.; Jurisevic, M.; Jovanovic, M.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, M.; Jovanovic, I. Galectin-3 as an important prognostic marker for COVID-19 severity. Sci. Rep. 2023, 13, 1460. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Reviews. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Puccini, M.; Jakobs, K.; Reinshagen, L.; Friebel, J.; Schencke, P.-A.; Ghanbari, E.; Landmesser, U.; Haghikia, A.; Kränkel, N.; Rauch, U. Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19. Int. J. Mol. Sci. 2023, 24, 7683. [Google Scholar] [CrossRef] [PubMed]
- Di Candia, A.M.; de Avila, D.X.; Moreira, G.R.; Villacorta, H.; Maisel, A.S. Growth differentiation factor-15, a novel systemic biomarker of oxidative stress, inflammation, and cellular aging: Potential role in cardiovascular diseases. Am. Heart J. Plus Cardiol. Res. Pract. 2021, 9, 100046. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. GDF15 and Cardiac Cells: Current Concepts and New Insights. Int. J. Mol. Sci. 2021, 22, 8889. [Google Scholar] [CrossRef]
- Eddy, A.C.; Trask, A.J. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021, 57, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Kimenai, D.M.; Marioni, R.E.; Hayward, C.; Campbell, A.; Porteous, D.; Mills, N.L.; O’Rahilly, S.; Sattar, N. Reference ranges for GDF-15, and risk factors associated with GDF-15, in a large general population cohort. Clin. Chem. Lab. Med. 2022, 60, 1820–1829. [Google Scholar] [CrossRef]
- Myhre, P.L.; Prebensen, C.; Strand, H.; Røysland, R.; Jonassen, C.M.; Rangberg, A.; Sørensen, V.; Søvik, S.; Røsjø, H.; Svensson, M.; et al. Growth Differentiation Factor 15 Provides Prognostic Information Superior to Established Cardiovascular and Inflammatory Biomarkers in Unselected Patients Hospitalized with COVID-19. Circulation 2020, 142, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Brenière, C.; Méloux, A.; Pédard, M.; Marie, C.; Thouant, P.; Vergely, C.; Béjot, Y. Growth Differentiation Factor-15 (GDF-15) Is Associated with Mortality in Ischemic Stroke Patients Treated with Acute Revascularization Therapy. Front. Neurol. 2019, 10, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Guadiana Romualdo, L.G.; Mulero, M.D.R.; Olivo, M.H.; Rojas, C.R.; Arenas, V.R.; Morales, M.G.; Abellán, A.B.; Conesa-Zamora, P.; García-García, J.; Hernández, A.C.; et al. Circulating levels of GDF-15 and calprotectin for prediction of in-hospital mortality in COVID-19 patients: A case series. J. Infect. 2021, 82, e40–e42. [Google Scholar] [CrossRef] [PubMed]
- Notz, Q.; Schmalzing, M.; Wedekink, F.; Schlesinger, T.; Gernert, M.; Herrmann, J.; Sorger, L.; Weismann, D.; Schmid, B.; Sitter, M.; et al. Pro- and Anti-Inflammatory Responses in Severe COVID-19-Induced Acute Respiratory Distress Syndrome—An Observational Pilot Study. Front. Immunol. 2020, 11, 581338. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Zhang, J.; Shi, Y.; Liu, Y.; Yang, Y.; He, J.; Luo, S.; Huang, Y.; Liu, Y.; Liu, D.; et al. Comprehensive Profiling of Inflammatory Factors Revealed That Growth Differentiation Factor-15 Is an Indicator of Disease Severity in COVID-19 Patients. Front. Immunol. 2021, 12, 662465. [Google Scholar] [CrossRef]
- Alserawan, L.; Peñacoba, P.; Orozco Echevarría, S.E.; Castillo, D.; Ortiz, E.; Martínez-Martínez, L.; Moga Naranjo, E.; Domingo, P.; Castellví, I.; Juarez, C.; et al. Growth Differentiation Factor 15 (GDF-15): A Novel Biomarker Associated with Poorer Respiratory Function in COVID-19. Diagnostics 2021, 11, 1998. [Google Scholar] [CrossRef]
- Parchwani, D.; Dholariya, S.; Katoch, C.; Singh, R. Growth differentiation factor 15 as an emerging novel biomarker in SARS-CoV-2 infection. World J. Methodol. 2022, 12, 438–447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaluri, N.; Stančáková Yaluri, A.; Žeňuch, P.; Žeňuchová, Z.; Tóth, Š.; Kalanin, P. Cardiac Biomarkers and Their Role in Identifying Increased Risk of Cardiovascular Complications in COVID-19 Patients. Diagnostics 2023, 13, 2508. https://doi.org/10.3390/diagnostics13152508
Yaluri N, Stančáková Yaluri A, Žeňuch P, Žeňuchová Z, Tóth Š, Kalanin P. Cardiac Biomarkers and Their Role in Identifying Increased Risk of Cardiovascular Complications in COVID-19 Patients. Diagnostics. 2023; 13(15):2508. https://doi.org/10.3390/diagnostics13152508
Chicago/Turabian StyleYaluri, Nagendra, Alena Stančáková Yaluri, Pavol Žeňuch, Zuzana Žeňuchová, Štefan Tóth, and Peter Kalanin. 2023. "Cardiac Biomarkers and Their Role in Identifying Increased Risk of Cardiovascular Complications in COVID-19 Patients" Diagnostics 13, no. 15: 2508. https://doi.org/10.3390/diagnostics13152508
APA StyleYaluri, N., Stančáková Yaluri, A., Žeňuch, P., Žeňuchová, Z., Tóth, Š., & Kalanin, P. (2023). Cardiac Biomarkers and Their Role in Identifying Increased Risk of Cardiovascular Complications in COVID-19 Patients. Diagnostics, 13(15), 2508. https://doi.org/10.3390/diagnostics13152508





