Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Vasculitides: A Literature Review
Abstract
:1. Introduction
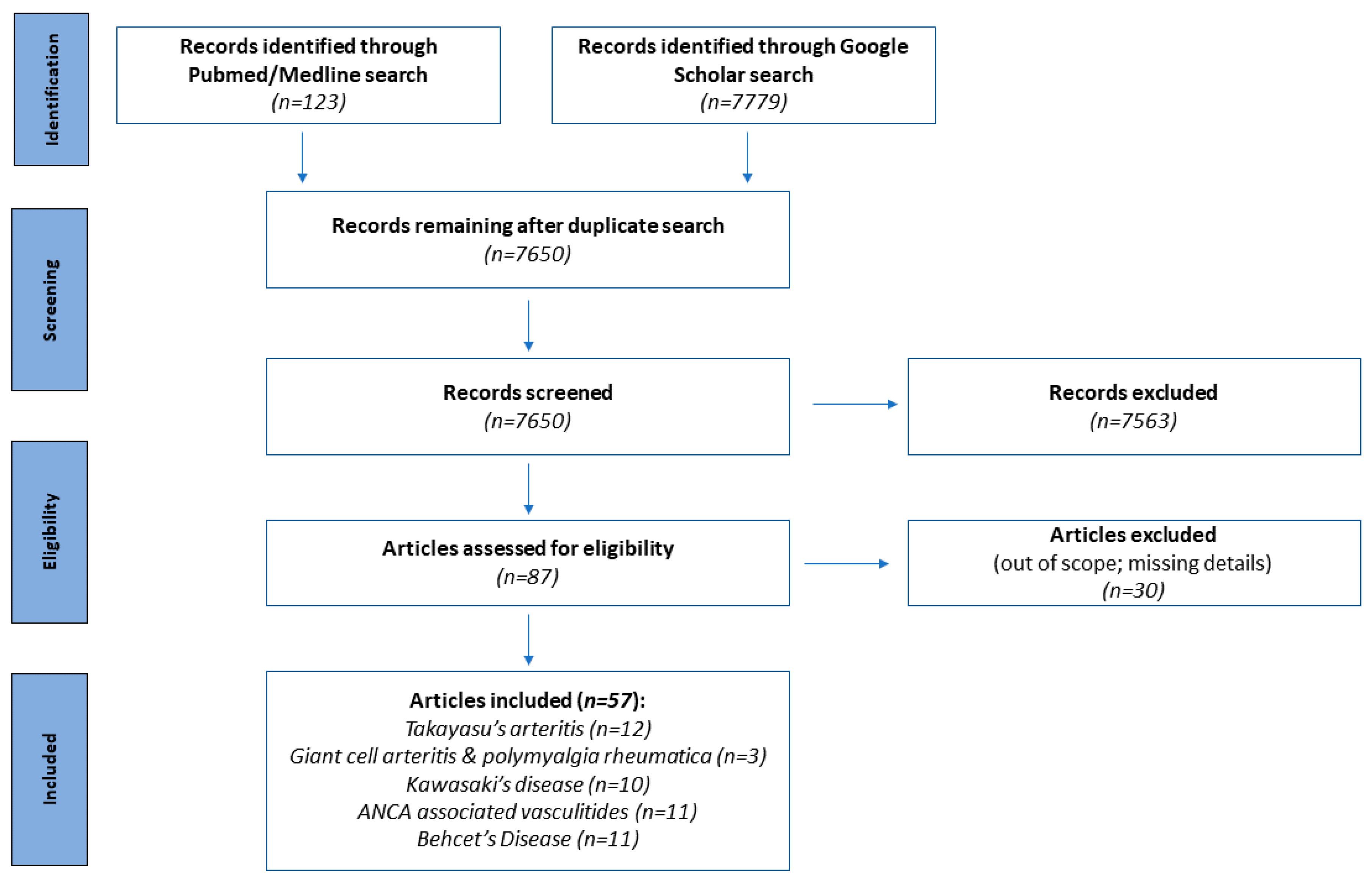
2. Methods
3. Basic Principles of Arterial Stiffness Measurements
4. Aortic/Arterial Stiffness as a Surrogate CV Marker in Patients with Systemic Vasculitis
4.1. Large Vessel Vasculitides
4.1.1. Takayasu’s Arteritis
4.1.2. Giant Cell Arteritis/Polymyalgia Rheumatica
4.2. Medium Vessel Vasculitides
Kawasaki’s Disease
4.3. Small Vessel Vasculitides
ANCA Associated
4.4. Varying Size Vasculitides
Behcet’s Disease
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watts, R.A.; Robson, J. Introduction, epidemiology and classification of vasculitis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin. Exp. Nephrol. 2013, 17, 603–606. [Google Scholar] [CrossRef]
- Demir, S.; Sonmez, H.E.; Ozen, S. Vasculitis: Decade in Review. Curr. Rheumatol. Rev. 2019, 15, 14–22. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Zhang, M.; Ko, S.; Chen, F. An Update on Cardiovascular Risk Factors After Kawasaki Disease. Front. Cardiovasc. Med. 2021, 8, 671198. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.H.; Cohen Tervaert, J.W. Cardiovascular events and the role of accelerated atherosclerosis in systemic vasculitis. Atherosclerosis 2021, 325, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lo Gullo, A.; Giuffrida, C.; Morace, C.; Squadrito, G.; Magnano San Lio, P.; Ricciardi, L.; Salvarani, C.; Mandraffino, G. Arterial Stiffness and Adult Onset Vasculitis: A Systematic Review. Front. Med. 2022, 9, 824630. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract. Res. Clin. Rheumatol. 2013, 27, 33–44. [Google Scholar] [CrossRef]
- Kronbichler, A.; Leierer, J.; Gauckler, P.; Shin, J.I. Comorbidities in ANCA-associated vasculitis. Rheumatology 2020, 59 (Suppl. 3), iii79–iii83. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Reamy, B.V.; Williams, P.M.; Kuckel, D.P. Prevention of Cardiovascular Disease. Prim. Care 2018, 45, 25–44. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyorala, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Cullen, P.; Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation 2002, 105, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Vegas-Revenga, N.; Atienza-Mateo, B.; Corrales-Selaya, C.; Prieto-Pena, D.; Rueda-Gotor, J.; Portilla, V.; Blanco, R.; Castaneda, S.; Ferraz-Amaro, I.; et al. Combined use of QRISK3 and SCORE as predictors of carotid plaques in patients with rheumatoid arthritis. Rheumatology 2021, 60, 2801–2807. [Google Scholar] [CrossRef]
- Zhu, L.; Singh, M.; Lele, S.; Sahakian, L.; Grossman, J.; Hahn, B.; McMahon, M. Assessing the validity of QRISK3 in predicting cardiovascular events in systemic lupus erythematosus. Lupus Sci. Med. 2022, 9, e000564. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial stiffness: A new surrogate end point for cardiovascular disease? J. Nephrol. 2007, 20 (Suppl. S12), S45–S50. [Google Scholar] [PubMed]
- Argyropoulou, O.D.; Protogerou, A.D.; Sfikakis, P.P. Accelerated atheromatosis and arteriosclerosis in primary systemic vasculitides: Current evidence and future perspectives. Curr. Opin. Rheumatol. 2018, 30, 36–43. [Google Scholar] [CrossRef]
- Morioka, T.; Mori, K.; Emoto, M. Is Stiffness Parameter beta Useful for the Evaluation of Atherosclerosis?~ Its Clinical Implications, Limitations, and Future Perspectives ~. J. Atheroscler. Thromb. 2021, 28, 435–453. [Google Scholar] [CrossRef]
- Triantafyllias, K.; Thiele, L.E.; Cavagna, L.; Baraliakos, X.; Bertsias, G.; Schwarting, A. Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Arthritides and Connective Tissue Diseases: A Literature Review. Diagnostics 2023, 13, 1870. [Google Scholar] [CrossRef]
- Nichols, W.W.; Singh, B.M. Augmentation index as a measure of peripheral vascular disease state. Curr. Opin. Cardiol. 2002, 17, 543–551. [Google Scholar] [CrossRef]
- Namba, T.; Masaki, N.; Takase, B.; Adachi, T. Arterial Stiffness Assessed by Cardio-Ankle Vascular Index. Int. J. Mol. Sci. 2019, 20, 3664. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, P.; Krajcoviechova, A.; Seidlerova, J.; Mayer, O.; Bruthans, J.; Filipovsky, J.; Laurent, S.; Cifkova, R. Arterial stiffness parameters: How do they differ? Atherosclerosis 2013, 231, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Sequi-Dominguez, I.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Nunez de Arenas-Arroyo, S.; Martinez-Vizcaino, V. Accuracy of Pulse Wave Velocity Predicting Cardiovascular and All-Cause Mortality. A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Mitchell, G.F. Arterial stiffness: Insights from Framingham and Iceland. Curr. Opin. Nephrol. Hypertens. 2015, 24, 1–7. [Google Scholar] [CrossRef]
- Triantafyllias, K.; De Blasi, M.; Hoffmann, I.; Thomaidis, T.; Drees, P.; Schwarting, A. The count of tender rather than swollen joints correlates with aortic stiffness in patients with rheumatoid arthritis. Springerplus 2016, 5, 428. [Google Scholar] [CrossRef]
- Triantafyllias, K.; de Blasi, M.; Lutgendorf, F.; Cavagna, L.; Stortz, M.; Weinmann-Menke, J.; Konstantinides, S.; Galle, P.R.; Schwarting, A. High cardiovascular risk in mixed connective tissue disease: Evaluation of macrovascular involvement and its predictors by aortic pulse wave velocity. Clin. Exp. Rheumatol. 2019, 37, 994–1002. [Google Scholar]
- Stortz, M.; Triantafyllias, K.; Schwarting, A.; Weinmann-Menke, J. Vascular stiffness: Influencing factors on carotid-femoral pulse wave velocity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2020, 38, 74–81. [Google Scholar]
- Triantafyllias, K.; Cavagna, L.; Klonowski, A.; Drott, U.; Fiehn, C.; Wendel, S.; Bergner, R.; de Blasi, M.; Voll, R.E.; Baulmann, J.; et al. Possible misclassification of cardiovascular risk by SCORE in antisynthetase syndrome: Results of the pilot multicenter study RI. CAR.D.A. Rheumatology 2021, 60, 1300–1312. [Google Scholar] [CrossRef]
- Colaci, M.; Zanoli, L.; Lo Gullo, A.; Sambataro, D.; Sambataro, G.; Aprile, M.L.; Castellino, P.; Malatino, L. The Impaired Elasticity of Large Arteries in Systemic Sclerosis Patients. J. Clin. Med. 2022, 11, 3256. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllias, K.; Stortz, M.; de Blasi, M.; Leistner, C.; Weinmann-Menke, J.; Schwarting, A. Increased aortic stiffness in patients with fibromyalgia: Results of a prospective study on carotid-femoral pulse wave velocity. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S116), 114–115. [Google Scholar] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef]
- Agueda, A.F.; Monti, S.; Luqmani, R.A.; Buttgereit, F.; Cid, M.; Dasgupta, B.; Dejaco, C.; Mahr, A.; Ponte, C.; Salvarani, C.; et al. Management of Takayasu arteritis: A systematic literature review informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis. RMD Open 2019, 5, e001020. [Google Scholar] [CrossRef]
- Keser, G.; Aksu, K.; Direskeneli, H. Takayasu arteritis: An update. Turk. J. Med. Sci. 2018, 48, 681–697. [Google Scholar] [CrossRef]
- Salles Rosa Neto, N.; Levy-Neto, M.; Tolezani, E.C.; Bonfa, E.; Bortolotto, L.A.; Pereira, R.M. Determinants of arterial stiffness in female patients with Takayasu arteritis. J. Rheumatol. 2014, 41, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Yuan, L.J.; Cao, T.S.; Liu, J.; Ren, R.; Duan, Y.Y. Aortic stiffness evaluated by echocardiography in female patients with Takayasu’s arteritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S103), 134–138. [Google Scholar]
- Raninen, R.O.; Kupari, M.M.; Hekali, P.E. Carotid and femoral artery stiffness in Takayasu’s arteritis. An ultrasound study. Scand. J. Rheumatol. 2002, 31, 85–88. [Google Scholar] [CrossRef]
- Liu, Q.; Dang, A.M.; Chen, B.W.; Lv, N.Q.; Wang, X.; Zheng, D.Y. N-terminal Pro-B-type Natriuretic Peptide is Associated with Arterial Stiffness as Measured According to the Brachial-ankle Pulse Wave Velocity in Patients with Takayasu Arteritis. J. Atheroscler. Thromb. 2015, 22, 628–636. [Google Scholar] [CrossRef]
- Tombetti, E.; Hysa, E.; Mason, J.C.; Cimmino, M.A.; Camellino, D. Blood Biomarkers for Monitoring and Prognosis of Large Vessel Vasculitides. Curr. Rheumatol. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- Grotenhuis, H.B.; Aeschlimann, F.A.; Hui, W.; Slorach, C.; Yeung, R.S.M.; Benseler, S.M.; Bradley, T.J.; Grosse-Wortmann, L. Increased Arterial Stiffness Adversely Affects Left Ventricular Mechanics in Patients With Pediatric Takayasu Arteritis From a Toronto Cohort. J. Clin. Rheumatol. 2019, 25, 171–175. [Google Scholar] [CrossRef]
- Ng, W.F.; Fantin, F.; Ng, C.; Dockery, F.; Schiff, R.; Davies, K.A.; Rajkumar, C.; Mason, J.C. Takayasu’s arteritis: A cause of prolonged arterial stiffness. Rheumatology 2006, 45, 741–745. [Google Scholar] [CrossRef]
- Wang, Z.; Dang, A.; Lv, N. Brachial-Ankle Pulse Wave Velocity is Increased and Associated with Disease Activity in Patients with Takayasu Arteritis. J. Atheroscler. Thromb. 2020, 27, 172–182. [Google Scholar] [CrossRef]
- Wang, X.; Dang, A. Prognostic Value of Brachial-Ankle Pulse Wave Velocity in Patients With Takayasu Arteritis with Drug-Eluting Stent Implantation. Arthritis Care Res. 2015, 67, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cheng, N.; Dang, A.; Lv, N. Association between increased arterial stiffness measured by brachial-ankle pulse wave velocity and cardiovascular events in patients with Takayasu’s arteritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S117), 65–71. [Google Scholar] [PubMed]
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef]
- Nesher, G.; Breuer, G.S. Giant Cell Arteritis and Polymyalgia Rheumatica: 2016 Update. Rambam Maimonides Med. J. 2016, 7, e0035. [Google Scholar] [CrossRef] [PubMed]
- Dzhus, M.; Mostbauer, H. Cardiovascular lesions in giant cell arteritis. Reumatologia 2022, 60, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ciofalo, A.; Gulotta, G.; Iannella, G.; Pasquariello, B.; Manno, A.; Angeletti, D.; Pace, A.; Greco, A.; Altissimi, G.; de Vincentiis, M.; et al. Giant Cell Arteritis (GCA): Pathogenesis, Clinical Aspects and Treatment Approaches. Curr. Rheumatol. Rev. 2019, 15, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Cimmino, M.A.; Maradit-Kremers, H.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. 2012 provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2012, 71, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Bartoloni, E.; Pucci, G.; Pirro, M.; Settimi, L.; Alunno, A.; Gerli, R.; Mannarino, E. Aortic stiffness is increased in polymyalgia rheumatica and improves after steroid treatment. Ann. Rheum. Dis. 2012, 71, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Pieringer, H.; Stuby, U.; Hargassner, S.; Biesenbach, G. Treatment with corticosteroids reduces arterial stiffness in patients with polymyalgia rheumatica as measured with pulse wave analysis. Ann. Rheum. Dis. 2008, 67, 279. [Google Scholar] [CrossRef] [PubMed]
- Emamifar, A.; Ellingsen, T.; Hermann, A.P.; Hess, S.; Gerke, O.; Ahangarani Farahani, Z.; Syrak Hansen, P.; Jensen Hansen, I.M.; Thye-Ronn, P. Prognostic impacts of glucocorticoid treatment in patients with polymyalgia rheumatica and giant cell arteritis. Sci. Rep. 2021, 11, 6220. [Google Scholar] [CrossRef]
- Ooyanagi, R.; Fuse, S.; Tomita, H.; Takamuro, M.; Horita, N.; Mori, M.; Tsutsumi, H. Pulse wave velocity and ankle brachial index in patients with Kawasaki disease. Pediatr. Int. 2004, 46, 398–402. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Wong, S.J.; Ho, M.H. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch. Dis. Child. 2007, 92, 43–47. [Google Scholar] [CrossRef]
- Cheung, Y.F.; O, K.; Woo, C.W.; Armstrong, S.; Siow, Y.L.; Chow, P.C.; Cheung, E.W. Oxidative stress in children late after Kawasaki disease: Relationship with carotid atherosclerosis and stiffness. BMC Pediatr. 2008, 8, 20. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Nakagawa, R.; Kuwata, S.; Kurishima, C.; Saiki, H.; Iwamoto, Y.; Sugimoto, M.; Ishido, H.; Masutani, S.; Senzaki, H. Arterial stiffness in patients after Kawasaki disease without coronary artery involvement: Assessment by performing brachial ankle pulse wave velocity and cardio-ankle vascular index. J. Cardiol. 2015, 66, 130–134. [Google Scholar] [CrossRef]
- AlHuzaimi, A.; Al Mashham, Y.; Potts, J.E.; De Souza, A.M.; Sandor, G.G. Echo-Doppler assessment of arterial stiffness in pediatric patients with Kawasaki disease. J. Am. Soc. Echocardiogr. 2013, 26, 1084–1089. [Google Scholar] [CrossRef]
- Oyamada, J.; Toyono, M.; Shimada, S.; Aoki-Okazaki, M.; Takahashi, T. Altered central aortic elastic properties in Kawasaki disease are related to changes in left ventricular geometry and coronary artery aneurysm formation. J. Am. Soc. Echocardiogr. 2012, 25, 690–696. [Google Scholar] [CrossRef]
- Chen, K.Y.; Zannino, D.; Curtis, N.; Cheung, M.; Burgner, D. Increased aortic intima-media thickness following Kawasaki disease. Atherosclerosis 2017, 260, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.K.; Banday, A.Z.; Das, R.R.; Manohari, S.; Jindal, A.K.; Singh, S. Long-term vascular dysfunction in Kawasaki disease: Systematic review and meta-analyses. Cardiol. Young 2022, 33, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Dietz, S.M.; Tacke, C.E.; Gort, J.; Kuipers, I.M.; de Groot, E.; Wiegman, A.; Hutten, B.A.; Kuijpers, T.W. Carotid Intima-Media Thickness in Patients With a History of Kawasaki Disease. Circ. J. 2015, 79, 2682–2687. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Lee, K.H.; Denicolo, S.; Choi, D.; Lee, H.; Ahn, D.; Kim, K.H.; Lee, J.H.; Kim, H.; Hwang, M.; et al. Immunopathogenesis of ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2020, 21, 7319. [Google Scholar] [CrossRef] [PubMed]
- Geetha, D.; Jefferson, J.A. ANCA-Associated Vasculitis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Comarmond, C.; Cacoub, P. Granulomatosis with polyangiitis (Wegener): Clinical aspects and treatment. Autoimmun. Rev. 2014, 13, 1121–1125. [Google Scholar] [CrossRef]
- Furuta, S.; Iwamoto, T.; Nakajima, H. Update on eosinophilic granulomatosis with polyangiitis. Allergol. Int. 2019, 68, 430–436. [Google Scholar] [CrossRef]
- Houben, E.; Penne, E.L.; Voskuyl, A.E.; van der Heijden, J.W.; Otten, R.H.J.; Boers, M.; Hoekstra, T. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: A meta-analysis of observational studies. Rheumatology 2018, 57, 555–562. [Google Scholar] [CrossRef]
- Yildiz, M.; Soy, M.; Kurum, T.; Yildiz, B.S. Arterial distensibility in Wegener’s granulomatosis: A carotid-femoral pulse wave velocity study. Anadolu Kardiyol. Derg. 2007, 7, 281–285. [Google Scholar]
- Slot, M.C.; Kroon, A.A.; Damoiseaux, J.; Theunissen, R.; Houben, A.; de Leeuw, P.W.; Tervaert, J.W.C. CD4+CD28null T Cells are related to previous cytomegalovirus infection but not to accelerated atherosclerosis in ANCA-associated vasculitis. Rheumatol. Int. 2017, 37, 791–798. [Google Scholar] [CrossRef]
- Wilde, B.; Slot, M.; van Paassen, P.; Theunissen, R.; Kemna, M.; Witzke, O.; Cohen Tervaert, J.W. Phosphorylcholine antibodies are diminished in ANCA-associated vasculitis. Eur. J. Clin. Investig. 2015, 45, 686–691. [Google Scholar] [CrossRef]
- Farrah, T.E.; Melville, V.; Czopek, A.; Fok, H.; Bruce, L.; Mills, N.L.; Bailey, M.A.; Webb, D.J.; Dear, J.W.; Dhaun, N. Arterial stiffness, endothelial dysfunction and impaired fibrinolysis are pathogenic mechanisms contributing to cardiovascular risk in ANCA-associated vasculitis. Kidney Int. 2022, 102, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Pacholczak, R.; Bazan-Socha, S.; Iwaniec, T.; Zareba, L.; Kielczewski, S.; Walocha, J.A.; Musial, J.; Dropinski, J. Endothelial dysfunction in patients with granulomatosis with polyangiitis: A case-control study. Rheumatol. Int. 2018, 38, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Rios-Blanco, J.J.; Arpa, J. Accelerated atherosclerosis in ANCA-associated vasculitis. Acta Neurol. Scand. 2017, 136, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Hajj-Ali, R.A.; Major, J.; Langford, C.; Hoffman, G.S.; Clark, T.; Zhang, L.; Sun, Z.; Silverstein, R.L. The interface of inflammation and subclinical atherosclerosis in granulomatosis with polyangiitis (Wegener’s): A preliminary study. Transl. Res. 2015, 166, 366–374. [Google Scholar] [CrossRef]
- Louka, A.M.; Sagris, D.; Ntaios, G. Immunity, Vascular Aging and Stroke. Curr. Med. Chem. 2022, 29, 5510–5521. [Google Scholar] [CrossRef]
- Chanouzas, D.; Dyall, L.; Dale, J.; Moss, P.; Morgan, M.; Harper, L. CD4+CD28- T-cell expansions in ANCA-associated vasculitis and association with arterial stiffness: Baseline data from a randomised controlled trial. Lancet 2015, 385 (Suppl. S1), S30. [Google Scholar] [CrossRef]
- Isozaki, T.; Homma, T.; Sagara, H.; Kasama, T. Role of Cytokines in EGPA and the Possibility of Treatment with an Anti-IL-5 Antibody. J. Clin. Med. 2020, 9, 3890. [Google Scholar] [CrossRef]
- Hatemi, G.; Seyahi, E.; Fresko, I.; Talarico, R.; Hamuryudan, V. One year in review 2020: Behcet’s syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S127), 3–10. [Google Scholar]
- Tong, B.; Liu, X.; Xiao, J.; Su, G. Immunopathogenesis of Behcet’s Disease. Front. Immunol. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F. Behcet’s disease. Int. J. Rheum. Dis. 2018, 21, 2057–2058. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Yong, W.C.; Sanguankeo, A. Increased Arterial Stiffness in Behcet’s Disease: A Systematic Review and Meta-Analysis. Korean Circ. J. 2017, 47, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Caldas, C.A.; Borba, E.F.; Bortolotto, L.A.; Medeiros, D.M.; Bonfa, E.; Goncalves, C.R. Increased arterial stiffness assessed by pulse wave velocity in Behcet’s disease and its association with the lipid profile. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Karakas, M.S.; Kilinc, A.Y.; Altekin, R.E.; Yalcinkaya, A.S. Evaluation of arterial stiffness and subclinical atherosclerosis in patients with Behcet’s disease without cardiovascular involvement. Turk Kardiyol. Dern. Ars. 2016, 44, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Zencirkiran Agus, H.; Yildiz, B.S.; Kahraman, S.; Kalkan, K.; Aladag, N.B.; Yildiz, M. Increased Arterial Stiffness Measured By Carotid Femoral (Aortic) Pulse Wave Velocity In Patients with Inactive Behcet’s Disease. Kardiologiia 2020, 60, 869. [Google Scholar] [CrossRef]
- Protogerou, A.D.; Lekakis, J.; Ikonomidis, I.; Stamatelopoulos, K.; Aznaouridis, K.; Karatzis, E.N.; Papamichael, C.; Markomihelakis, N.; Kaklamanis, P.; Mavrikakis, M. Pressure wave reflections, central blood pressure, and aortic stiffness in patients with Adamantiades-Behcet’s disease: A cross-sectional case-control study underlining the role of chronic corticosteroid treatment. Am. J. Hypertens. 2006, 19, 660–666; discussion 7–8. [Google Scholar] [CrossRef]
- Yilmaz, S.; Celik, G.; Esmen, S.E. Assessment of arterial stiffness in patients with inactive and active Behcet’s disease. Scand. J. Rheumatol. 2014, 43, 63–69. [Google Scholar] [CrossRef]
- Alis, D.; Durmaz, E.S.M.; Civcik, C.; Tutuncu, M.; Saip, S.; Kocer, N.; Islak, C.; Kizilkilic, O. Assessment of the common carotid artery wall stiffness by Shear Wave Elastography in Behcet’s disease. Med. Ultrason. 2018, 20, 446–452. [Google Scholar] [CrossRef]
- Rhee, M.Y.; Chang, H.K.; Kim, S.K. Intima-media thickness and arterial stiffness of carotid artery in Korean patients with Behcet’s disease. J. Korean Med. Sci. 2007, 22, 387–392. [Google Scholar] [CrossRef]
- Celik, G.; Yilmaz, S.; Ergulu Esmen, S. Non-dipping blood pressure patterns and arterial stiffness parameters in patients with Behcet’s disease. Hypertens. Res. 2015, 38, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Kayikcioglu, M.; Aksu, K.; Hasdemir, C.; Keser, G.; Turgan, N.; Kultursay, H.; Doganavsargil, E. Endothelial functions in Behcet’s disease. Rheumatol. Int. 2006, 26, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Khera, R.; Corrales-Medina, V.F.; Townsend, R.R.; Chirinos, J.A. Inflammation and arterial stiffness in humans. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cifkova, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Aortic stiffness for cardiovascular risk prediction: Just measure it, just do it! J. Am. Coll. Cardiol. 2014, 63, 647–649. [Google Scholar] [CrossRef]




| Assessment Methods of Arterial Stiffness | |||
|---|---|---|---|
| Marker | Methodology | Clinical Association | Calculation |
| Pulse wave velocity (PWV) | Oscillometric marker | Reflects the speed of pulse waves traveling between two sites of the arterial tree | Calculated by dividing the distance between these sites by the pressure wave transit time (∆s/∆t) |
| Augmentation index (AIx) | Marker based on applanation tonometry | Represents the supplementary systolic blood pressure increase, which is mainly caused by wave reflections | Calculated as the ratio between (1) the difference between peak systolic pressure and the shoulder of the ascending part of the blood pressure curve, and (2) the pulse pressure |
| Aortic stiffness index (AoSI) | Doppler echocardiography marker | Measures aortic stiffness 3 cm above the aortic valve | Calculated by the following formula: AoSI = ln(SBP/DBP)/(AoS − AoD)/AoD (where SBP = systolic blood pressure and DBP = diastolic blood pressure) |
| β-stiffness index | Elastic parameter usually assessed by carotid ultrasound, coupled with applanation tonometry data | Indirectly measures the change in the internal luminal diameter of the carotid artery in the radial direction | Calculated by the formula: β-stiffness index = ln(SBP/DBP)/[(Ds − Dd)/Dd] |
| Aortic distensibility | Echocardiographic measure | Reflects aortic stiffness | Calculated by the formula: aortic distensibility = (2×)/(AoS − AoD)/AoD/(SBP − DBP) |
| Cardio-ankle vascular index (CAVI) | A marker that is associated with PWV, being however less dependent on arterial pressure | Reflects stiffness of the cardio-ankle part of the arterial tree | Calculated by the formula CAVI = a[(2ρ/(SBP − DBP)) × ln(SBP/DBP) × PWV2 ] + b (a and b are constants; ρ = blood density, PWV: brachial–ankle PWV) |
| Definition of Arterial Stiffness | Loss of Arterial Compliance. Association with CVD Due to Systolic Hypertension and Heightened Pulse Pressure |
|---|---|
| Basic Pathogenetic Mechanisms | |
| Arterial wall (extracellular matrix) composition changes | Fragmentation of elastin fibers, deposition of collagen (stiff wall material), and cross-linking of collagen molecules. |
| Activation of renin–angiotensin system | Changes in the arterial wall by the proliferation of vascular smooth cells, inflammatory activity, and collagen increase. |
| Effects of chronic arterial hypertension | Stretching of the arterial wall by pulse pressure, stiffer vessel ‘‘appearance‘‘ during examination. |
| Functional arterial stiffening | Endothelial dysfunction: reduced NO synthesis, activity, and content. |
| Further pathophysiological mechanisms | Acceleration of atherosclerosis, effects of autoantibodies, metabolic components, and further traditional CV risk factors. |
| Reference | Vasculitis | Marker(s) of Arterial Stiffness | Study Population | Statistical Analysis |
|---|---|---|---|---|
| Salles Rosa Neto et al. [38] | TA | cfPWV and AIx | N = 27 | cfPWV higher in TA than in HC (9.77 ± 3.49 vs. 7.83 ± 1.06 m/s; p = 0.009) |
| Yang Y et al. [39] | TA | cfPWV via echo | N = 25 | cfPWV higher in TA than in HC (8.37 ± 2.23 vs. 6.46 ± 1.15 m/s; p < 0.001) |
| Raninen RO et all. [40] | TA | Carotid and femoral ultrasound | n = 29 | Ep and YM were significantly higher than HC: carotid: p = 0.019 and 0.013/femoral: p = 0.005 and 0.039. Carotid stiffness index is also higher than in HC (p = 0.004). |
| Liu Q et al. [41] | TA | baPWV | n = 72 | CV risk markers significantly higher in the high baPWV group than in the low baPWV group |
| Grotenhuis HB et al. [43] | TA | crPWV, cfPWV | n = 7 | crPWV and cfPWV higher than HC: 8.1 ± 1.8 vs. 6.4 ± 0.6 m/s, p = 0.03 und 8.3 ± 1.9 vs. 5.1 ± 0.8 m/s, p < 0.01, respectively |
| Ng WF et al. [44] | TA | PWV and AIx | n = 10 | cfPWV higher in TA than in HC (p = 0.03) |
| Wang Z et al. [45] | TA | baPWV | n = 67 | baPWV higher than in HC (p < 0.001) |
| Wang X et al. [46] | TA | baPWV | n = 48 | baPWV higher than in HC (17.0 ± 3.8 vs. 13.8 ± 3.0 m/s; p = 0.002) |
| He Y et al. [47] | TA | baPWV | n = 74 | baPWV associated with CV events (OR: 1.132, 95%CI: 1.063–1.204, p < 0.001) |
| Schillaci G et al. [53] | PMR | Aortic PWV | n = 39 | Aortic PWV higher than in HC (12.4 ± 4 vs. 10.2 ± 2 m/s, p < 0.01) |
| Pieringer H et al. [54] | PMR | AIx | n = 13 | Non-significant Aix difference: patients vs. HC (28.5 (9.1%) vs. 24.7 (6.4%); p = 0.19) |
| Emamifar A et al. [55] | PMR and GCA | Aortic PWV and AIx | n = 77 | No PWV differences between patients with different grades of CV risk (all; p > 0.05) |
| Ooyanagi R et al. [56] | KD | PWV and ABI | n = 90 | When a cut-off was set as % of normal predicted PWV (%N PWV) ≥ 120%, and ABI ≤ 0.9, KD- history patients had higher PWV than HC |
| Cheung YF et al. [57] | KD | Carotid artery stiffness index and brPWV | n = 72 | cIMT correlated positively with carotid artery stiffness index (r = 0.40, p = 0.001) and brPWV (r = 0.28, p = 0.016) |
| Cheung YF et al. [58] | KD | Carotid stiffness index | n = 51 | Biomarker levels correlated positively with carotid IMT (p < 0.001 and p = 0.034, respectively), and stiffness index (p = 0.001 and p = 0.021) |
| Nakagawa R et al. [60] | KD | baPWV, CAVI | n = 201 | baPWV significantly higher than in HC (913 ± 121 cm/s vs. 886 ± 135 cm/s, p = 0.04) CAVI not significantly different between the two groups (p = 0.9) |
| AlHuzaimi A et al. [61] | KD | Aortic PWV | n = 42 | PWV higher than in HC (495 vs. 370 cm/s, p = 0.0008) |
| Oyamada J et al. [62] | KD | Echo | n = 75 | Coronary aneurysms and left ventricular mass index were independently relevant to aortic stiffness index and aortic distensibility |
| Chen KY et al. [63] | KD | PWV, carotid distensibility, and diameter compliance | n = 60 | Patients with coronary artery abnormalities had reduced carotid distensibility compared to controls (15.16% (95% CI 13.67–16.65) vs. 17.50 (95% CI 16.43–18.58), p = 0.02) |
| Yildiz M et al. [71] | GPA | Aortic PWV | N = 10 | cfPWV higher in GPA than in HC (p = 0.04) |
| Slot MC et al. [72] | AAV | Aortic PWV | N = 78 | cfPWV higher in AAV than in HC (9.80 ± 2.50 m/s vs. 8.72 ± 1.68; p = 0.04) |
| Wilde B et al. [73] | AAV | PWV, A. femoralis | N = 83 | PWV in AAV vs. HC: 9.8 ± 2.8 vs. 9.0 ± 2.2, p = 0.4 |
| Farrah TE et al. [74] | AVV | PMV and AIx | N = 64 | PMV and AIx higher in AVV than HC. (PWV: 7.3 ± 1.3 vs. 6.4 ± 1.0 m/s (p = 0.016); AIx: 26% ± 11% vs. 20% ± 10% (p = 0.031)) |
| Caldas CA et al. [85] | BD | PWV | N = 46 | PWV higher than HC (8.48 ± 1.14 vs. 7.53 ± 1.40 m/s, p = 0.017). |
| Yıldırım A et al. [86] | BD | PWV | N = 30 | PWV higher than HC (6.35 ± 1.05 vs. 5.75 ± 0.83; p = 0.017). |
| Zencirkiran Agus H et al. [87] | BD | cfPWV | N = 90 | PWV higher than HC (9.57 ± 1.88 vs. 8.47 ± 1.13 m/s; p = 0.003) |
| Protogerou AD et al. [88] | BD | AIx and AoSI | N = 98 | AIx, but not AoSI, was lower in steroid taking patients than in steroid-free patients and similar to controls (21 ± 14% vs. 12 ± 14%, p < 0.05) |
| Yilmaz S et al. [89] | BD | PWV | N = 96 | PWV values were higher in patients with active BD than in patients with inactive BD (p < 0.05) |
| Alis D et al. [90] | BD | cIMT and SWE | N = 62 | Mean right (3.72 ± 0.94 m/s) and left (3.57 ± 0.72 m/s) SWE higher than mean right (2.42 ± 0.49 m/s) and left (2.56 ± 0.49 m/s) in HC (p < 0.001 for both) |
| Rhee MY et al. [91] | BD | DC, β and Einc | N = 94 | DC (23.10 ± 9.5 vs. 27.90 ± 10.14, p = 0.021), β (3.26 ± 0.45 vs. 3.04 ± 0.32, p = 0.007), and Einc (0.64 ± 0.33 vs. 0.49 ± 0.16, p = 0.008) higher than in HC |
| Celik G et al. [92] | BD | AIx and PWV | N = 156 | BD patients with high AIx had higher PWV (5.91 ± 1.26 vs. 5.20 ± 0.60; p = 0.013) |
| Kayikçioğlu M et al. [93] | BD | EDVD of brachial artery | N = 95 | EDVD significantly impaired in patients with BD compared with HC (11.4 +/− 6.3 vs. 20.4 +/− 9.1%, p = 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllias, K.; Thiele, L.-E.; Mandel, A.; Cavagna, L.; Baraliakos, X.; Bertsias, G.; Hasseli, R.; Minnich, P.; Schwarting, A. Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Vasculitides: A Literature Review. Diagnostics 2023, 13, 3603. https://doi.org/10.3390/diagnostics13243603
Triantafyllias K, Thiele L-E, Mandel A, Cavagna L, Baraliakos X, Bertsias G, Hasseli R, Minnich P, Schwarting A. Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Vasculitides: A Literature Review. Diagnostics. 2023; 13(24):3603. https://doi.org/10.3390/diagnostics13243603
Chicago/Turabian StyleTriantafyllias, Konstantinos, Leif-Erik Thiele, Anna Mandel, Lorenzo Cavagna, Xenofon Baraliakos, George Bertsias, Rebecca Hasseli, Pascal Minnich, and Andreas Schwarting. 2023. "Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Vasculitides: A Literature Review" Diagnostics 13, no. 24: 3603. https://doi.org/10.3390/diagnostics13243603
APA StyleTriantafyllias, K., Thiele, L.-E., Mandel, A., Cavagna, L., Baraliakos, X., Bertsias, G., Hasseli, R., Minnich, P., & Schwarting, A. (2023). Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Vasculitides: A Literature Review. Diagnostics, 13(24), 3603. https://doi.org/10.3390/diagnostics13243603






