A Novel Paradigm for Non-Invasive Prenatal Genetic Screening: Trophoblast Retrieval and Isolation from the Cervix (TRIC)
Abstract
:1. Introduction
2. Cell-Free Fetal DNA (cffDNA) Analysis Methods
2.1. Cell-Free DNA Fragments
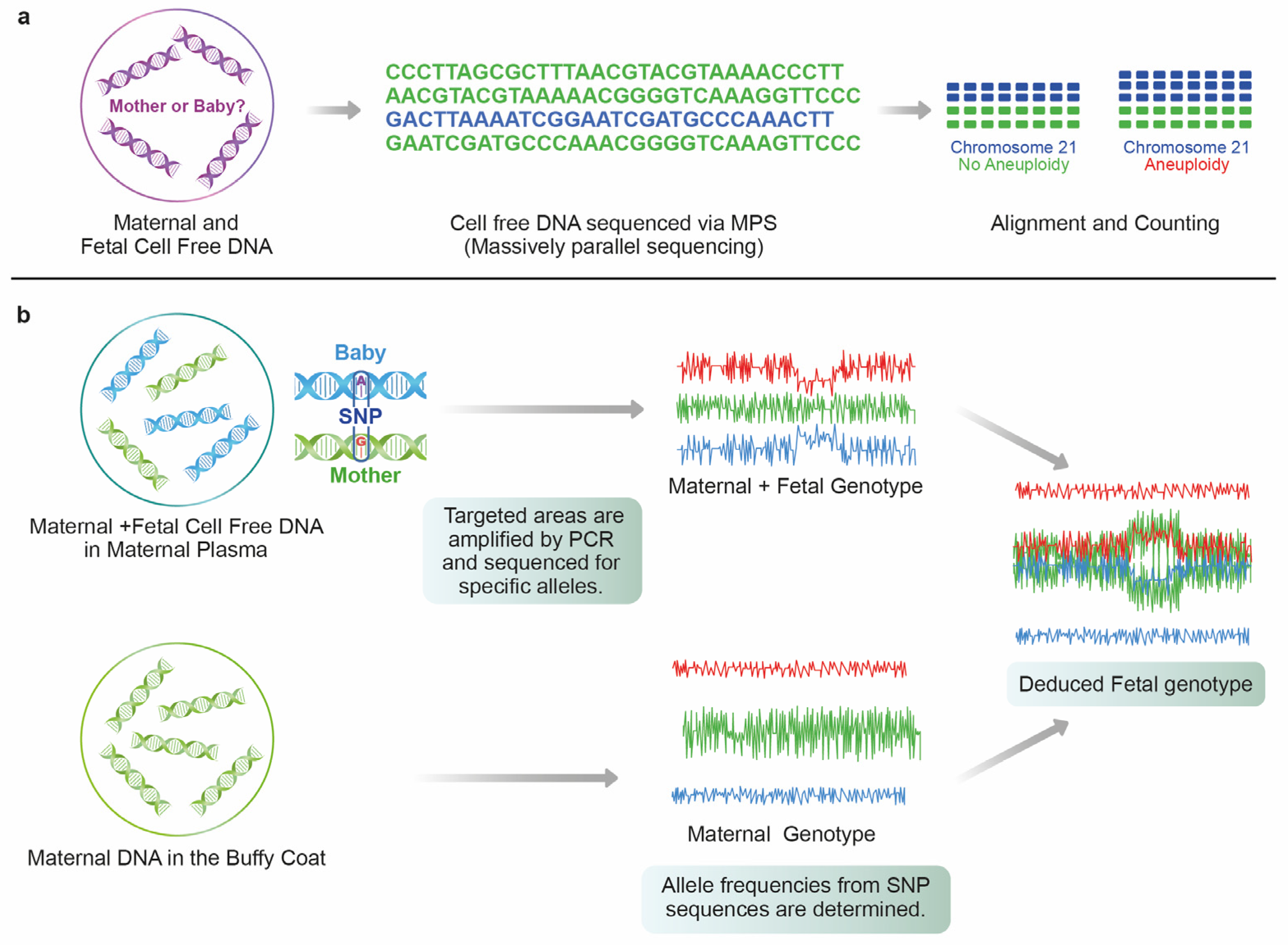
2.2. Massively Parallel Shotgun Sequencing (MPSS)
2.3. Single Nucleotide Polymorphism (SNP)
3. Limitations of NIPT
3.1. Fetal Fraction (FF)
3.2. Chromosomal Mosaicism
3.3. Maternal Malignancies
3.4. Vanishing Twins (VT)
4. Novel Approach for Non-Invasively Retrieving Fetal Cell
5. Trophoblasts Migrating to the Cervix
6. Technical Development of EVT Cell Isolation and Identification
7. TRIC Methods
7.1. Sampling
7.2. Fixation
7.3. Isolation
7.4. Analysis
8. Refining Updates for TRIC Techniques
9. Future Expectations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osterman, M.; Hamilton, B.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020. Natl. Vital. Stat. Rep. 2021, 70, 17. [Google Scholar]
- OECD. OECD Economic Surveys: Korea 2022; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Frick, A.P. Advanced maternal age and adverse pregnancy outcomes. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 92–100. [Google Scholar] [CrossRef]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Womens Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Gantt, A.; Metz, T.D.; Kuller, J.A.; Louis, J.M.; Cahill, A.G.; Turrentine, M.A. Obstetric Care Consensus #11, Pregnancy at age 35 years or older. Am. J. Obstet. Gynecol. 2022, 228, B25–B40. [Google Scholar] [CrossRef]
- Akolekar, R.; Beta, J.; Picciarelli, G.; Ogilvie, C.; D’Antonio, F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review and meta-analysis. Ultrasound Obs. Gynecol 2015, 45, 16–26. [Google Scholar] [CrossRef]
- Ravitsky, V.; Roy, M.-C.; Haidar, H.; Henneman, L.; Marshall, J.; Newson, A.J.; Ngan, O.M.Y.; Nov-Klaiman, T. The Emergence and Global Spread of Noninvasive Prenatal Testing. Annu. Rev. Genom. Hum. Genet. 2021, 22, 309–338. [Google Scholar] [CrossRef]
- Allyse, M.; Minear, M.A.; Berson, E.; Sridhar, S.; Rote, M.; Hung, A.; Chandrasekharan, S. Non-invasive prenatal testing: A review of international implementation and challenges. Int. J. Womens Health 2015, 7, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Quaresima, P.; Visconti, F.; Greco, E.; Venturella, R.; Di Carlo, C. Prenatal tests for chromosomal abnormalities detection (PTCAD): Pregnant women’s knowledge in an Italian Population. Arch Gynecol Obstet. 2021, 303, 1185–1190. [Google Scholar] [CrossRef]
- Kim, K.; Craft, L.K. Non-invasive prenatal testing in mitigating concerns from invasive prenatal diagnostic testing: Retrospective assessment of utility in an academic healthcare system in the US. BMJ Open 2022, 12, e057658. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, P.; Chan, K.C.; Wong, J.; Cheng, Y.K.; Liang, R.H.; Chan, W.K.; Ma, E.S.; Chan, S.L.; Cheng, S.H.; et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Natl. Acad. Sci. USA 2015, 112, E5503–E5512. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Wilkins-Haug, L.; Bianchi, D.W. Review: Cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta 2014, 35, S64–S68. [Google Scholar] [CrossRef] [Green Version]
- Alberry, M.; Maddocks, D.; Jones, M.; Abdel Hadi, M.; Abdel-Fattah, S.; Avent, N.; Soothill, P.W. Free fetal DNA in maternal plasma in anembryonic pregnancies: Confirmation that the origin is the trophoblast. Prenat. Diagn. 2007, 27, 415–418. [Google Scholar] [CrossRef]
- Lo, Y.M.; Tein, M.S.; Lau, T.K.; Haines, C.J.; Leung, T.N.; Poon, P.M.; Wainscoat, J.S.; Johnson, P.J.; Chang, A.M.; Hjelm, N.M. Quantitative analysis of fetal DNA in maternal plasma and serum: Implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet 1998, 62, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Scotchman, E.; Shaw, J.; Paternoster, B.; Chandler, N.; Chitty, L.S. Non-invasive prenatal diagnosis and screening for monogenic disorders. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 320–327. [Google Scholar] [CrossRef]
- Lo, Y.D.; Chan, K.A.; Sun, H.; Chen, E.Z.; Jiang, P.; Lun, F.M.; Zheng, Y.W.; Leung, T.Y.; Lau, T.K.; Cantor, C.R. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci. Transl. Med. 2010, 2, 61ra91. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, P.; Wong, A.I.C.; Cheng, Y.K.Y.; Cheng, S.H.; Zhang, H.; Chan, K.C.A.; Leung, T.Y.; Chiu, R.W.K.; Lo, Y.M.D. Size-tagged preferred ends in maternal plasma DNA shed light on the production mechanism and show utility in noninvasive prenatal testing. Proc. Natl. Acad. Sci. 2018, 115, E5106–E5114. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.C.Y.; Jiang, P.; Peng, W.; Cheng, S.H.; Cheung, Y.T.T.; Tse, O.Y.O.; Shang, H.; Poon, L.C.; Leung, T.Y.; Chan, K.C.A.; et al. Single-molecule sequencing reveals a large population of long cell-free DNA molecules in maternal plasma. Proc. Natl. Acad. Sci. 2021, 118, e2114937118. [Google Scholar] [CrossRef]
- Chiu, R.W.K.; Akolekar, R.; Zheng, Y.W.L.; Leung, T.Y.; Sun, H.; Chan, K.C.A.; Lun, F.M.F.; Go, A.T.J.I.; Lau, E.T.; To, W.W.K.; et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: Large scale validity study. BMJ 2011, 342, c7401. [Google Scholar] [CrossRef] [Green Version]
- Benn, P.; Cuckle, H.; Pergament, E. Non-invasive prenatal testing for aneuploidy: Current status and future prospects. Ultrasound Obstet. Gynecol. 2013, 42, 15–33. [Google Scholar] [CrossRef]
- Chiu, R.W.; Chan, K.C.; Gao, Y.; Lau, V.Y.; Zheng, W.; Leung, T.Y.; Foo, C.H.; Xie, B.; Tsui, N.B.; Lun, F.M.; et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc. Natl. Acad. Sci. USA 2008, 105, 20458–20463. [Google Scholar] [CrossRef]
- Fan, H.C.; Blumenfeld, Y.J.; Chitkara, U.; Hudgins, L.; Quake, S.R. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc. Natl. Acad. Sci. USA 2008, 105, 16266–16271. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; Gil, M.; Atanasova, V.; Markova, D. Validation of targeted sequencing of single-nucleotide polymorphisms for non-invasive prenatal detection of aneuploidy of chromosomes 13, 18, 21, X, and Y. Prenat. Diagn. 2013, 33, 575–579. [Google Scholar] [CrossRef]
- Sparks, A.B.; Struble, C.A.; Wang, E.T.; Song, K.; Oliphant, A. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: Evaluation for trisomy 21 and trisomy 18. Am J Obs. Gynecol 2012, 206, e311–e319. [Google Scholar] [CrossRef]
- Samura, O. Update on noninvasive prenatal testing: A review based on current worldwide research. J Obs. Gynaecol Res 2020, 46, 1246–1254. [Google Scholar] [CrossRef]
- Chan, K.C.; Zhang, J.; Hui, A.B.; Wong, N.; Lau, T.K.; Leung, T.N.; Lo, K.W.; Huang, D.W.; Lo, Y.M. Size distributions of maternal and fetal DNA in maternal plasma. Clin. Chem. 2004, 50, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Alberry, M.S.; Aziz, E.; Ahmed, S.R.; Abdel-Fattah, S. Non invasive prenatal testing (NIPT) for common aneuploidies and beyond. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 424–429. [Google Scholar] [CrossRef]
- Malone, F.D.; Canick, J.A.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Bukowski, R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N. Engl. J. Med. 2005, 353, 2001–2011. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, D.W.; Platt, L.D.; Goldberg, J.D.; Abuhamad, A.Z.; Sehnert, A.J.; Rava, R.P. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obs. Gynecol 2012, 119, 890–901. [Google Scholar] [CrossRef]
- Zimmermann, B.; Hill, M.; Gemelos, G.; Demko, Z.; Banjevic, M.; Baner, J.; Ryan, A.; Sigurjonsson, S.; Chopra, N.; Dodd, M.; et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat. Diagn. 2012, 32, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.C.; Kaimal, A.J.; Dugoff, L.; Norton, M.E.; American College of Obstetricians and Gynecologists. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet. Gynecol. 2020, 136, e48–e69. [Google Scholar] [CrossRef]
- Dungan, J.S.; Klugman, S.; Darilek, S.; Malinowski, J.; Akkari, Y.M.N.; Monaghan, K.G.; Erwin, A.; Best, R.G. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2023, 25, 100336. [Google Scholar] [CrossRef]
- Steele, C.D.; Wapner, R.J.; Smith, J.B.; Haynes, M.K.; Jackson, L.G. Prenatal diagnosis using fetal cells isolated from maternal peripheral blood: A review. Clin Obs. Gynecol 1996, 39, 801–813. [Google Scholar] [CrossRef]
- Wong, F.C.; Lo, Y.M. Prenatal Diagnosis Innovation: Genome Sequencing of Maternal Plasma. Annu. Rev. Med. 2016, 67, 419–432. [Google Scholar] [CrossRef]
- Fiorentino, F.; Bono, S.; Pizzuti, F.; Mariano, M.; Polverari, A.; Duca, S.; Sessa, M.; Baldi, M.; Diano, L.; Spinella, F. The importance of determining the limit of detection of non-invasive prenatal testing methods. Prenat. Diagn. 2016, 36, 304–311. [Google Scholar] [CrossRef]
- Vora, N.L.; Johnson, K.L.; Basu, S.; Catalano, P.M.; Hauguel-De Mouzon, S.; Bianchi, D.W. A multifactorial relationship exists between total circulating cell-free DNA levels and maternal BMI. Prenat. Diagn. 2012, 32, 912–914. [Google Scholar] [CrossRef]
- Juul, L.A.; Hartwig, T.S.; Ambye, L.; Sørensen, S.; Jørgensen, F.S. Noninvasive prenatal testing and maternal obesity: A review. Acta Obstet. Gynecol. Scand. 2020, 99, 744–750. [Google Scholar] [CrossRef]
- Putra, M.; Idler, J.; Patek, K.; Contos, G.; Walker, C.; Olson, D.; Hicks, M.A.; Chaperon, J.; Korzeniewski, S.J.; Patwardhan, S.C.; et al. The association of HBB-related significant hemoglobinopathies and low fetal fraction on noninvasive prenatal screening for fetal aneuploidy. J. Matern. Fetal. Neonatal. Med. 2021, 34, 3657–3661. [Google Scholar] [CrossRef]
- Kuhlmann-Capek, M.; Chiossi, G.; Singh, P.; Monsivais, L.; Lozovyy, V.; Gallagher, L.; Kirsch, N.; Florence, E.; Petruzzi, V.; Chang, J.; et al. Effects of medication intake in early pregnancy on the fetal fraction of cell-free DNA testing. Prenat. Diagn. 2019, 39, 361–368. [Google Scholar] [CrossRef]
- Deng, C.; Liu, S. Factors Affecting the Fetal Fraction in Noninvasive Prenatal Screening: A Review. Front. Pediatr. 2022, 10, 812781. [Google Scholar] [CrossRef]
- Chan, R.W.; Jiang, P.; Peng, X.; Tam, L.S.; Liao, G.J.; Li, E.K.; Wong, P.C.; Sun, H.; Chan, K.C.; Chiu, R.W.; et al. Plasma DNA aberrations in systemic lupus erythematosus revealed by genomic and methylomic sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, E5302–E5311. [Google Scholar] [CrossRef]
- Suzumori, N.; Ebara, T.; Yamada, T.; Samura, O.; Yotsumoto, J.; Nishiyama, M.; Miura, K.; Sawai, H.; Murotsuki, J.; Kitagawa, M.; et al. Fetal cell-free DNA fraction in maternal plasma is affected by fetal trisomy. J. Hum. Genet. 2016, 61, 647–652. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Wilkins-Haug, L. Integration of noninvasive DNA testing for aneuploidy into prenatal care: What has happened since the rubber met the road? Clin. Chem. 2014, 60, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Kinnings, S.L.; Geis, J.A.; Almasri, E.; Wang, H.; Guan, X.; McCullough, R.M.; Bombard, A.T.; Saldivar, J.S.; Oeth, P.; Deciu, C. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat. Diagn. 2015, 35, 816–822. [Google Scholar] [CrossRef]
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Yang, J.; Qi, Y.; Guo, F.; Peng, H.; Wang, D.; Wang, Y.; Luo, X.; Li, Y.; Yin, A. Factors affecting cell-free DNA fetal fraction: Statistical analysis of 13,661 maternal plasmas for non-invasive prenatal screening. Hum Genom. 2019, 13, 62. [Google Scholar] [CrossRef]
- Hartwig, T.S.; Ambye, L.; Sørensen, S.; Jørgensen, F.S. Discordant non-invasive prenatal testing (NIPT)—A systematic review. Prenat. Diagn. 2017, 37, 527–539. [Google Scholar] [CrossRef]
- Malvestiti, F.; Agrati, C.; Grimi, B.; Pompilii, E.; Izzi, C.; Martinoni, L.; Gaetani, E.; Liuti, M.R.; Trotta, A.; Maggi, F.; et al. Interpreting mosaicism in chorionic villi: Results of a monocentric series of 1001 mosaics in chorionic villi with follow-up amniocentesis. Prenat. Diagn. 2015, 35, 1117–1127. [Google Scholar] [CrossRef]
- Kalousek, D.K.; Vekemans, M. Confined placental mosaicism. J. Med. Genet. 1996, 33, 529–533. [Google Scholar] [CrossRef]
- Schreck, R.R.; Falik-Rorenstein, Z.; Hirata, G. Chromosomal Mosaicism in Chorionic Villus Sampling. Clin. Perinatol. 1990, 17, 867–888. [Google Scholar] [CrossRef]
- Kalousek, D.K.; Howard-Peebles, P.N.; Olson, S.B.; Barrett, I.J.; Dorfmann, A.; Black, S.H.; Schulman, J.D.; Wilson, R.D. Confirmation of CVS mosaicism in term placentae and high frequency of intrauterine growth retardation association with confined placental mosaicism. Prenat. Diagn. 1991, 11, 743–750. [Google Scholar] [CrossRef]
- Grati, F.R.; Bajaj, K.; Malvestiti, F.; Agrati, C.; Grimi, B.; Malvestiti, B.; Pompilii, E.; Maggi, F.; Gross, S.; Simoni, G.; et al. The type of feto-placental aneuploidy detected by cfDNA testing may influence the choice of confirmatory diagnostic procedure. Prenat. Diagn. 2015, 35, 994–998. [Google Scholar] [CrossRef]
- Samura, O.; Sekizawa, A.; Suzumori, N.; Sasaki, A.; Wada, S.; Hamanoue, H.; Hirahara, F.; Sawai, H.; Nakamura, H.; Yamada, T.; et al. Current status of non-invasive prenatal testing in Japan. J. Obstet. Gynaecol. Res. 2017, 43, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Grati, F.R.; Malvestiti, F.; Ferreira, J.C.P.B.; Bajaj, K.; Gaetani, E.; Agrati, C.; Grimi, B.; Dulcetti, F.; Ruggeri, A.M.; De Toffol, S.; et al. Fetoplacental mosaicism: Potential implications for false-positive and false-negative noninvasive prenatal screening results. Genet. Med. 2014, 16, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Samura, O.; Okamoto, A. Causes of aberrant non-invasive prenatal testing for aneuploidy: A systematic review. Taiwan J Obs. Gynecol 2020, 59, 16–20. [Google Scholar] [CrossRef]
- Kalousek, D.K.; Barrett, I.J.; McGillivray, B.C. Placental mosaicism and intrauterine survival of trisomies 13 and 18. Am. J. Hum. Genet 1989, 44, 338–343. [Google Scholar]
- Heesterbeek, C.J.; Aukema, S.M.; Galjaard, R.H.; Boon, E.M.J.; Srebniak, M.I.; Bouman, K.; Faas, B.H.W.; Govaerts, L.C.P.; Hoffer, M.J.V.; den Hollander, N.S.; et al. Noninvasive Prenatal Test Results Indicative of Maternal Malignancies: A Nationwide Genetic and Clinical Follow-Up Study. J. Clin. Oncol. 2022, 40, 2426–2435. [Google Scholar] [CrossRef]
- Pavlidis, N.A. Coexistence of Pregnancy and Malignancy. Oncol. 2002, 7, 279–287. [Google Scholar] [CrossRef]
- Dharajiya, N.G.; Namba, A.; Horiuchi, I.; Miyai, S.; Farkas, D.H.; Almasri, E.; Saldivar, J.-S.; Takagi, K.; Kamei, Y. Uterine leiomyoma confounding a noninvasive prenatal test result. Prenat. Diagn. 2015, 35, 990–993. [Google Scholar] [CrossRef]
- Levi, S. Ultrasonic assessment of the high rate of human multiple pregnancy in the first trimester. J. Clin. Ultrasound 1976, 4, 3–5. [Google Scholar] [CrossRef]
- van Eekhout, J.C.A.; Bekker, M.N.; Bax, C.J.; Galjaard, R.-J.H. Non-invasive prenatal testing (NIPT) in twin pregnancies affected by early single fetal demise: A systematic review of NIPT and vanishing twins. Prenat. Diagn. 2023, 42, 829–837. [Google Scholar] [CrossRef]
- Imudia, A.N.; Kumar, S.; Diamond, M.P.; DeCherney, A.H.; Armant, D.R. Transcervical retrieval of fetal cells in the practice of modern medicine: A review of the current literature and future direction. Fertil. Steril. 2010, 93, 1725–1730. [Google Scholar] [CrossRef] [Green Version]
- Shettles, L.B. Use of the Y chromosome in prenatal sex determination. Nature 1971, 230, 52–53. [Google Scholar] [CrossRef]
- Bobrow, M.; Lewis, B.V. Unreliability of fetal sexing using cervical material. Lancet 1971, 2, 486. [Google Scholar] [CrossRef]
- Amankwah, K.S.; Bond, E.C. Unreliability of prenatal determination of fetal sex with the use of Y-body fluorescence in midcervical smears. Am. J. Obstet. Gynecol. 1978, 130, 300–301. [Google Scholar]
- Adinolfi, M.; Davies, A.; Sharif, S.; Soothill, P.; Rodeck, C. Detection of trisomy 18 and Y-derived sequences in fetal nucleated cells obtained by transcervical flushing. Lancet 1993, 342, 403–404. [Google Scholar] [CrossRef]
- Adinolfi, M.; Sherlock, J.; Soothill, P.; Rodeck, C. Molecular evidence of fetal-derived chromosome 21 markers (STRs) in transcervical samples. Prenat. Diagn. 1995, 15, 35–39. [Google Scholar] [CrossRef]
- Adinolfi, M.; Sherlock, J.; Kemp, T.; Carritt, B.; Soothill, P.; Kingdom, J.; Rodeck, C. Prenatal detection of fetal RhD DNA sequences in transcervical samples. Lancet 1995, 345, 318–319. [Google Scholar] [CrossRef]
- Moser, G.; Gauster, M.; Orendi, K.; Glasner, A.; Theuerkauf, R.; Huppertz, B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum. Reprod. 2010, 25, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Bolnick, J.M.; Kilburn, B.A.; Bajpayee, S.; Reddy, N.; Jeelani, R.; Crone, B.; Simmerman, N.; Singh, M.; Diamond, M.P.; Armant, D.R. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil. Steril. 2014, 102, 135–142.e6. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Cioni, R.; Bussani, C.; Cirigliano, V.; Sole, F.; Costa, C.; Garcia, P.; Adinolfi, M. HLA-G positive trophoblastic cells in transcervical samples and their isolation and analysis by laser microdissection and QF-PCR. Prenat. Diagn. 2003, 23, 34–39. [Google Scholar] [CrossRef]
- Merviel, P.; Challier, J.C.; Carbillon, L.; Foidart, J.M.; Uzan, S. The Role of Integrins in Human Embryo Implantation. Fetal Diagn. Ther. 2001, 16, 364–371. [Google Scholar] [CrossRef]
- Moser, G.; Orendi, K.; Gauster, M.; Siwetz, M.; Helige, C.; Huppertz, B. The art of identification of extravillous trophoblast. Placenta 2011, 32, 197–199. [Google Scholar] [CrossRef]
- Moser, G.; Windsperger, K.; Pollheimer, J.; de Sousa Lopes, S.C.; Huppertz, B. Human trophoblast invasion: New and unexpected routes and functions. Histochem. Cell Biol. 2018, 150, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Kim, S.H.; Shim, S.H.; Jang, H.Y.; Park, H.J.; Cha, D.H. Optimization Protocol of Fixation Method for Trophoblast Retrieval from the Cervix (TRIC): A Preliminary Study. Diagnostics 2020, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; Boussata, S.; Janssen, D.; Afink, G.; Jebbink, J.; van Maarle, M.; Wortelboer, E.; Kooper, A.; Pajkrt, E. Tricky TRIC: A replication study using trophoblast retrieval and isolation from the cervix to study genetic birth defects. Prenat. Diagn. 2022, 42, 1612–1621. [Google Scholar] [CrossRef]
- Fritz, R.; Kohan-Ghadr, H.R.; Sacher, A.; Bolnick, A.D.; Kilburn, B.A.; Bolnick, J.M.; Diamond, M.P.; Drewlo, S.; Armant, D.R. Trophoblast retrieval and isolation from the cervix (TRIC) is unaffected by early gestational age or maternal obesity. Prenat. Diagn. 2015, 35, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Moser, G.; Drewlo, S.; Huppertz, B.; Armant, D.R. Trophoblast retrieval and isolation from the cervix: Origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 2018, 24, 484–496. [Google Scholar] [CrossRef]
- Imudia, A.N.; Suzuki, Y.; Kilburn, B.A.; Yelian, F.D.; Diamond, M.P.; Romero, R.; Armant, D.R. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: A pilot study. Hum. Reprod. 2009, 24, 2086–2092. [Google Scholar] [CrossRef] [Green Version]
- Jain, C.V.; Kadam, L.; van Dijk, M.; Kohan-Ghadr, H.R.; Kilburn, B.A.; Hartman, C.; Mazzorana, V.; Visser, A.; Hertz, M.; Bolnick, A.D.; et al. Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Sci. Transl. Med. 2016, 8, 363re364. [Google Scholar] [CrossRef]
- Pfeifer, I.; Benachi, A.; Saker, A.; Bonnefont, J.P.; Mouawia, H.; Broncy, L.; Frydman, R.; Brival, M.L.; Lacour, B.; Dachez, R.; et al. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta 2016, 37, 56–60. [Google Scholar] [CrossRef]
- Bourlard, L.; Manigart, Y.; Donner, C.; Smits, G.; Désir, J.; Migeotte, I.; Pichon, B. Rarity of fetal cells in exocervical samples for noninvasive prenatal diagnosis. J. Perinat. Med. 2022, 50, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Drewlo, S.; Armant, D.R. Quo vadis, trophoblast? Exploring the new ways of an old cell lineage. Placenta 2017, 60, S27–S31. [Google Scholar] [CrossRef]
- Huang, Y.; Situ, B.; Huang, L.; Cao, Y.; Sui, H.; Ye, X.; Jiang, X.; Liang, A.; Tao, M.; Luo, S.; et al. Nondestructive Identification of Rare Trophoblastic Cells by Endoplasmic Reticulum Staining for Noninvasive Prenatal Testing of Monogenic Diseases. Adv. Sci. 2020, 7, 1903354. [Google Scholar] [CrossRef] [PubMed]
- Rodeck, C.; Tutschek, B.; Sherlock, J.; Kingdom, J. Methods for the transcervical collection of fetal cells during the first trimester of pregnancy. Prenat. Diagn. 1995, 15, 933–942. [Google Scholar] [CrossRef]
- Adinolfi, M.; Sherlock, J.; Tutschek, B.; Halder, A.; Delhanty, J.; Rodeck, C. Detection of fetal cells in transcervical samples and prenatal diagnosis of chromosomal abnormalities. Prenat. Diagn. 1995, 15, 943–949. [Google Scholar] [CrossRef]
- Bussani, C.; Cioni, R.; Scarselli, B.; Barciulli, F.; Bucciantini, S.; Simi, P.; Fogli, A.; Scarselli, G. Strategies for the isolation and detection of fetal cells in transcervical samples. Prenat. Diagn. 2002, 22, 1098–1101. [Google Scholar] [CrossRef]
- Sherlock, J.; Halder, A.; Tutschek, B.; Delhanty, J.; Rodeck, C.; Adinolfi, M. Prenatal detection of fetal aneuploidies using transcervical cell samples. J. Med. Genet. 1997, 34, 302–305. [Google Scholar] [CrossRef]
- Hong, K.; Jang, H.Y.; Shim, S.H.; Cho, H.Y.; Cha, D.H. Advanced Strategy of Trophoblasts Retrieval and Isolation from the Cervix (TRIC): Comparison of Two HLA-G Antibodies for Immunomagnetic Isolation of Trophoblasts. J. Pers. Med. 2023, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Shiverick, K.T.; King, A.; Frank, H.; Whitley, G.S.; Cartwright, J.E.; Schneider, H. Cell culture models of human trophoblast II: Trophoblast cell lines—A workshop report. Placenta 2001, 22, S104–S106. [Google Scholar] [CrossRef]
- Bolnick, A.D.; Fritz, R.; Jain, C.; Kadam, L.; Bolnick, J.M.; Kilburn, B.A.; Singh, M.; Diamond, M.P.; Drewlo, S.; Armant, D.R. Trophoblast Retrieval and Isolation From the Cervix for Noninvasive, First Trimester, Fetal Gender Determination in a Carrier of Congenital Adrenal Hyperplasia. Reprod. Sci. 2016, 23, 717–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussani, C.; Cioni, R.; Mattei, A.; Fambrini, M.; Marchionni, M.; Scarselli, G. Prenatal diagnosis of common aneuploidies in transcervical samples using quantitative fluorescent-PCR analysis. Mol. Diagn. Ther. 2007, 11, 117–121. [Google Scholar] [CrossRef]
- Cirigliano, V.; Sherlock, J.; Petrou, M.; Ward, R.H.; Rodeck, C.; Adinolfi, M. Transcervical cells and the prenatal diagnosis of haemoglobin (Hb) mutations. Clin. Genet. 1999, 56, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, M.; el-Hashemite, N.; Sherlock, J.; Ward, R.H.; Petrou, M.; Rodeck, C. Prenatal detection of Hb mutations using transcervical cells. Prenat. Diagn. 1997, 17, 539–543. [Google Scholar] [CrossRef]
- Blaschitz, A.; Hutter, H.; Leitner, V.; Pilz, S.; Wintersteiger, R.; Dohr, G.; Sedlmayr, P. Reaction patterns of monoclonal antibodies to HLA-G in human tissues and on cell lines: A comparative study. Hum. Immunol. 2000, 61, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Carosella, E.D.; Rouas-Freiss, N.; Tronik-Le Roux, D.; Moreau, P.; LeMaoult, J. HLA-G: An Immune Checkpoint Molecule. Adv. Immunol. 2015, 127, 33–144. [Google Scholar] [CrossRef]
- Furukawa, A.; Meguro, M.; Yamazaki, R.; Watanabe, H.; Takahashi, A.; Kuroki, K.; Maenaka, K. Evaluation of the Reactivity and Receptor Competition of HLA-G Isoforms toward Available Antibodies: Implications of Structural Characteristics of HLA-G Isoforms. Int. J. Mol. Sci. 2019, 20, 5947. [Google Scholar] [CrossRef] [Green Version]
- Parasar, P.; Bernard, M.; Ahn, S.H.; Kshirsagar, S.K.; Nguyen, S.L.; Grzesiak, G.R.; Vettathu, M.; Martin, D.; Petroff, M.G. Isolation and characterization of uterine leukocytes collected using a uterine swab technique. Am. J. Reprod. Immunol. 2022, 88, e13614. [Google Scholar] [CrossRef]
- Manaster, I.; Mandelboim, O. The unique properties of uterine NK cells. Am. J. Reprod. Immunol. 2010, 63, 434–444. [Google Scholar] [CrossRef]
- Lindquist, A.; Poulton, A.; Halliday, J.; Hui, L. Prenatal diagnostic testing and atypical chromosome abnormalities following combined first-trimester screening: Implications for contingent models of non-invasive prenatal testing. Ultrasound Obs. Gynecol 2018, 51, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Norton, M.E.; Jelliffe-Pawlowski, L.L.; Currier, R.J. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet. Gynecol. 2014, 124, 979–986. [Google Scholar] [CrossRef]
- Petersen, O.B.; Vogel, I.; Ekelund, C.; Hyett, J.; Tabor, A. Potential diagnostic consequences of applying non-invasive prenatal testing: Population-based study from a country with existing first-trimester screening. Ultrasound Obstet. Gynecol 2014, 43, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Vogel, I.; Tabor, A.; Ekelund, C.; Lou, S.; Hyett, J.; Petersen, O.B. Population-Based Screening for Trisomies and Atypical Chromosomal Abnormalities: Improving Efficacy using the Combined First Trimester Screening Algorithm as well as Individual Risk Parameters. Fetal Diagn. Ther. 2019, 45, 424–429. [Google Scholar] [CrossRef]
- Spinillo, S.L.; Farina, A.; Sotiriadis, A.; Pozzoni, M.; Giglio, S.; Papale, M.; Candiani, M.; Cavoretto, P.I. Pregnancy outcome of confined placental mosaicism: Meta-analysis of cohort studies. Am. J. Obstet. Gynecol. 2022, 227, 714–727.e1. [Google Scholar] [CrossRef]
- Hong, K.; Kim, S.H.; Cha, D.H.; Park, H.J. Defective Uteroplacental Vascular Remodeling in Preeclampsia: Key Molecular Factors Leading to Long Term Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 11202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Han, T.; Yin, G.; Wang, X.; Yao, Y. Expression of human leukocyte antigen-G during normal placentation and in preeclamptic pregnancies. Hypertens Pregnancy 2012, 31, 252–260. [Google Scholar] [CrossRef]
- Colbern, G.T.; Chiang, M.H.; Main, E.K. Expression of the nonclassic histocompatibility antigen HLA-G by preeclamptic placenta. Am. J. Obstet. Gynecol. 1994, 170, 1244–1250. [Google Scholar] [CrossRef]
- Bolnick, J.M.; Kohan-Ghadr, H.R.; Fritz, R.; Bolnick, A.D.; Kilburn, B.A.; Diamond, M.P.; Armant, D.R.; Drewlo, S. Altered Biomarkers in Trophoblast Cells Obtained Noninvasively Prior to Clinical Manifestation of Perinatal Disease. Sci. Rep. 2016, 6, 32382. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Meta-Analysis and Systematic Review to Assess the Role of Soluble FMS-Like Tyrosine Kinase-1 and Placenta Growth Factor Ratio in Prediction of Preeclampsia. Hypertension 2018, 71, 306–316. [Google Scholar] [CrossRef]
- Bian, X.; Biswas, A.; Huang, X.; Lee, K.J.; Li, T.K.-T.; Masuyama, H.; Ohkuchi, A.; Park, J.S.; Saito, S.; Tan, K.H.; et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women With Suspected Preeclampsia. Hypertension 2019, 74, 164–172. [Google Scholar] [CrossRef]
- Andersen, L.L.T.; Helt, A.; Sperling, L.; Overgaard, M. Decision Threshold for Kryptor sFlt-1/PlGF Ratio in Women With Suspected Preeclampsia: Retrospective Study in a Routine Clinical Setting. J. Am. Heart Assoc. 2021, 10, e021376. [Google Scholar] [CrossRef]
- Neuman, R.I.; Baars, M.D.; Saleh, L.; Broekhuizen, M.; Nieboer, D.; Cornette, J.; Schoenmakers, S.; Verhoeven, M.; Koch, B.C.P.; Russcher, H.; et al. Omeprazole Administration in Preterm Preeclampsia: A Randomized Controlled Trial to Study Its Effect on sFlt-1 (Soluble Fms-Like Tyrosine Kinase-1), PlGF (Placental Growth Factor), and ET-1 (Endothelin-1). Hypertension 2022, 79, 1297–1307. [Google Scholar] [CrossRef] [PubMed]



| NIPT | TRIC | |
|---|---|---|
| Invasiveness | Non-invasive | Non-invasive |
| Approach method | Maternal serum | Maternal cervix brushing |
| Targeted fetal genetic source | Cell-free DNA | Extravillous trophoblast |
| Obtained fetal DNA | Fragmented | Whole |
| Gestational age | ≥9 weeks | ≥5 weeks |
| Maternal BMI, gestational age | Affected | Not affected |
| Purpose | Screening only | Can be diagnostic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, K.; Park, H.J.; Jang, H.Y.; Shim, S.H.; Jang, Y.; Kim, S.H.; Cha, D.H. A Novel Paradigm for Non-Invasive Prenatal Genetic Screening: Trophoblast Retrieval and Isolation from the Cervix (TRIC). Diagnostics 2023, 13, 2532. https://doi.org/10.3390/diagnostics13152532
Hong K, Park HJ, Jang HY, Shim SH, Jang Y, Kim SH, Cha DH. A Novel Paradigm for Non-Invasive Prenatal Genetic Screening: Trophoblast Retrieval and Isolation from the Cervix (TRIC). Diagnostics. 2023; 13(15):2532. https://doi.org/10.3390/diagnostics13152532
Chicago/Turabian StyleHong, Kirim, Hee Jin Park, Hee Yeon Jang, Sung Han Shim, Yoon Jang, Soo Hyun Kim, and Dong Hyun Cha. 2023. "A Novel Paradigm for Non-Invasive Prenatal Genetic Screening: Trophoblast Retrieval and Isolation from the Cervix (TRIC)" Diagnostics 13, no. 15: 2532. https://doi.org/10.3390/diagnostics13152532
APA StyleHong, K., Park, H. J., Jang, H. Y., Shim, S. H., Jang, Y., Kim, S. H., & Cha, D. H. (2023). A Novel Paradigm for Non-Invasive Prenatal Genetic Screening: Trophoblast Retrieval and Isolation from the Cervix (TRIC). Diagnostics, 13(15), 2532. https://doi.org/10.3390/diagnostics13152532






