Quantitative Analysis of Supraspinatus Tendon Pathologies via T2/T2* Mapping Techniques with 1.5 T MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging Protocol
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin Ooi, M.W.; Fenning, L.; Dhir, V.; Basu, S. Rotator cuff assessment on imaging. J. Clin. Orthop. Trauma 2021, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, A.; Laslett, M.; Hing, W.A.; McNair, P.J.; Coates, M.H. A prospective study of shoulder pain in primary care: Prevalence of imaged pathology and response to guided diagnostic blocks. BMC Musculoskelet. Disord. 2011, 12, 119. [Google Scholar] [CrossRef]
- Awerbuch, M.S. The clinical utility of ultrasonography for rotator cuff disease, shoulder impingement syndrome and subacromial bursitis. Med. J. Aust. 2008, 188, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Rumie, C.; Vasquez, A.; Abreu, J.A.; Guarnizo, A.P.; Rivero, O.; Ocampo, J. MRI evaluation of the shoulder: Beyond rotator cuff. In Proceedings of the ECR 2015—European Congress of Radiology, Vienna, Austria, 4–8 March 2015; pp. 1–12. [Google Scholar]
- Anz, A.W.; Lucas, E.P.; Fitzcharles, E.K.; Surowiec, R.K.; Millett, P.J.; Ho, C.P. MRI T2 mapping of the asymptomatic supraspinatus tendon by age and imaging plane using clinically relevant subregions. Eur. J. Radiol. 2014, 83, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Ganal, E.; Ho, C.P.; Wilson, K.J.; Surowiec, R.K.; Smith, W.S.; Dornan, G.J.; Millett, P.J. Quantitative MRI characterization of arthroscopically verified supraspinatus pathology: Comparison of tendon tears, tendinosis and asymptomatic supraspinatus tendons with T2 mapping. Knee Surg. Sport Traumatol. Arthrosc. 2016, 24, 2216–2224. [Google Scholar] [CrossRef]
- De Jesus, J.O.; Parker, L.; Frangos, A.J.; Nazarian, L.N. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: A meta-analysis. Am. J. Roentgenol. 2009, 192, 1701–1707. [Google Scholar] [CrossRef]
- Brockmeyer, M.; Schmitt, C.; Haupert, A.; Kohn, D.; Lorbach, O. Limited diagnostic accuracy of magnetic resonance imaging and clinical tests for detecting partial-thickness tears of the rotator cuff. Arch. Orthop. Trauma Surg. 2017, 137, 1719–1724. [Google Scholar] [CrossRef]
- Lenza, M.; Buchbinder, R.; Christensen, R.; Nca, H.; Faloppa, F. Magnetic Resonance Imaging versus Ultrasonography for Assessing Rotator Cuff Tears in Patients with Shoulder Pain for Whom Surgery Is Being Considered; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Shady, K.L.; Totty, W.G. MR Diagnosis of Rotator Cuff Tears of the Shoulder: Value of Using. Imaging 1995, 164, 1451–1455. [Google Scholar]
- Robertson, P.L.; Schweitzer, M.E.; Mitchell, D.G.; Schlesinger, F.; Epstein, R.E.; Frieman, B.G.; Fenlin, J.M. Rotator cuff disorders: Interobserver and intraobserver variation in diagnosis with MR imaging. Radiology 1995, 194, 831–835. [Google Scholar] [CrossRef]
- Balich, S.M.; Sheley, R.C.; Brown, T.R.; Sauser, D.D.; Quinn, S.F. MR imaging of the rotator cuff tendon: Interobserver agreement and analysis of interpretive errors. Radiology 1997, 204, 191–194. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, H.-J.; Kwon, H.-J.; Kim, M.S.; Choi, S.H.; Choi, Y.J.; Kim, E. T2 relaxation times of the glenohumeral joint at 3.0 T MRI in patients with and without primary and secondary osteoarthritis. Acta Radiol. 2015, 56, 1388–1395. [Google Scholar] [CrossRef]
- Potter, H.G.; Black, B.R.; Chong, L.R. New Techniques in Articular Cartilage Imaging. Clin. Sport. Med. 2009, 28, 77–94. [Google Scholar] [CrossRef]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A.; et al. Articular cartilage in the knee: Current MR imaging techniques and applications in clinical practice and research. Radiographics 2011, 31, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Juras, V.; Zbyn, S.; Pressl, C.; Valkovic, L.; Szomolanyi, P.; Frollo, I.; Trattnig, S. Regional variations of T2* in healthy and pathologic achilles tendon in vivo at 7 Tesla: Preliminary results. Magn. Reason. Med. 2012, 68, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Welsch, G.H.; Apprich, S.; Zbyn, S.; Mamisch, T.C.; Mlynarik, V.; Scheffler, K.; Bieri, O.; Trattnig, S. Biochemical (T2, T2* and magnetisation transfer ratio) MRI of knee cartilage: Feasibility at ultra-high field (7T) compared with high field (3T) strength. Eur. Radiol. 2011, 21, 1136–1143. [Google Scholar] [CrossRef]
- Apprich, S.; Mamisch, T.C.; Welsch, G.H.; Stelzeneder, D.; Albers, C.; Totzke, U.; Trattnig, S. Quantitative T2 mapping of the patella at 3.0 T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur. J. Radiol. 2012, 81, e438–e443. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.; Carballido-Gamio, J.; Tang, S.; Lai, A.; Krug, R. Quantitative assessment of morphology, T1ρ, and T2 of shoulder cartilage using MRI. Eur. Radiol. 2016, 26, 4656–4663. [Google Scholar] [CrossRef][Green Version]
- Hesper, T.; Schleich, C.; Buchwald, A.; Hosalkar, H.S.; Antoch, G.; Krauspe, R.; Zilkens, C.; Bittersohl, B. T2* Mapping of the Hip in Asymptomatic Volunteers with Normal Cartilage Morphology: An Analysis of Regional and Age-Dependent Distribution. Cartilage 2018, 9, 30–37. [Google Scholar] [CrossRef]
- Kim, P.K.; Hong, Y.J.; Im, D.J.; Suh, Y.J.; Park, C.H.; Kim, J.Y.; Chang, S.; Lee, H.J.; Hur, J.; Kim, Y.J.; et al. Myocardial T1 and T2 mapping: Techniques and clinical applications. Korean J. Radiol. 2017, 18, 113–131. [Google Scholar] [CrossRef]
- Krepkin, K.; Bruno, M.; Raya, J.G.; Adler, R.S.; Gyftopoulos, S. Quantitative assessment of the supraspinatus tendon on MRI using T2/T2* mapping and shear-wave ultrasound elastography: A pilot study. Skelet. Radiol. 2017, 46, 191–199. [Google Scholar] [CrossRef]
- Wilson, K.J.; Surowiec, R.K.; Johnson, N.S.; Lockard, C.A.; Clanton, T.O.; Ho, C.P. T2* Mapping of Peroneal Tendons Using Clinically Relevant Subregions in an Asymptomatic Population. Foot Ankle Int. 2017, 38, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.H.; Waldman, A.D.; Barker, P.B. Clinical MR Neuroimaging: Physiological and Functional Techniques; Cambridge University Press: Cambridge, UK, 2010; Available online: http://admin.cambridge.org/academic/subjects/medicine/medical-imaging/clinical-mr-neuroimaging-physiological-and-functional-techniques-2nd-edition (accessed on 1 December 2022).
- Mochizuki, T.; Sugaya, H.; Uomizu, M.; Maeda, K.; Matsuki, K.; Sekiya, I.; Muneta, T.; Akita, K. Humeral insertion of the supraspinatus and infraspinatus. J. Bone Jt. Surg.—Ser. A 2008, 90, 962–969. [Google Scholar] [CrossRef]
- Rudez, J.; Zanetti, M. Normal anatomy, variants and pitfalls on shoulder MRI. Eur. J. Radiol. 2008, 68, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.W.; Helms, C.A. Magnetic resonance imaging of the shoulder: A review of potential sources of diagnostic errors. Skelet. Radiol. 2002, 31, 373–383. [Google Scholar]
- Tuite, M.J.; Asinger, D.; Orwin, J.F. Angled oblique sagittal MR imaging of rotator cuff tears: Comparison with standard oblique sagittal images. Skelet. Radiol. 2001, 30, 262–269. [Google Scholar] [CrossRef]
- Bryant, L.; Shnier, R.; Bryant, C.; Murrell, G.A.C. A comparison of clinical estimation, ultrasonography, magnetic resonance imaging, and arthroscopy in determining the size of rotator cuff tears. J. Shoulder Elb. Surg. 2002, 11, 219–224. [Google Scholar] [CrossRef]
- Matsuki, K.; Watanabe, A.; Ochiai, S.; Kenmoku, T.; Ochiai, N.; Obata, T.; Toyone, T.; Wada, Y.; Okubo, T. Quantitative evaluation of fatty degeneration of the supraspinatus and infraspinatus muscles using T2 mapping. J. Shoulder Elb. Surg. 2014, 23, 636–641. [Google Scholar] [CrossRef]
- Iijima, Y.; Matsuki, K.; Hoshika, S.; Ueda, Y.; Onishi, K.; Tokai, M.; Takahashi, N.; Sugaya, H.; Dougo, K.; Watanabe, A. Differences in fatty degeneration of rotator cuff muscles at different sites, as quantified by T2 mapping. J. Orthop. Sci. 2017, 22, 281–284. [Google Scholar] [CrossRef]
- Deniz, G.; Kose, O.; Tugay, A.; Guler, F.; Turan, A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: Does it improve, halt or deteriorate? Arch. Orthop. Trauma Surg. 2014, 134, 985–990. [Google Scholar] [CrossRef]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures: Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Fuchs, B.; Weishaupt, D.; Zanetti, M.; Hodler, J.; Gerber, C. Fatty degeneration of the muscles of the rotator cuff: Assessment by computed tomography versus magnetic resonance imaging. J. Shoulder Elb. Surg. 1999, 8, 599–605. [Google Scholar] [CrossRef]
- Hersche, O.; Gerber, C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: A preliminary report. J. Shoulder Elb. Surg. 1998, 7, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Pingel, J.; Fredberg, U.; Qvortrup, K.; Larsen, J.O.; Schjerling, P.; Heinemeier, K.; Kjaer, M.; Langberg, H. Local biochemical and morphological differences in human Achilles tendinopathy: A case control study. BMC Musculoskelet. Disord. 2012, 13, 53. [Google Scholar]
- Zumstein, M.A.; Lädermann, A.; Raniga, S.; Schär, M.O. The biology of rotator cuff healing. Orthop. Traumatol. Surg. Res. 2017, 103, S1–S10. [Google Scholar] [CrossRef]
- Miura, Y.; Endo, K.; Sekiya, I. Histological and biochemical changes in a rat rotator cuff tear model with or without the subacromial bursa. Res. Square 2023. preprint. [Google Scholar] [CrossRef]
- Von Knobelsdorff-Brenkenhoff, F.; Prothmann, M.; Dieringer, M.A.; Wassmuth, R.; Greiser, A.; Schwenke, C.; Niendorf, T.; Schulz-Menger, J. Myocardial T1 and T2 mapping at 3 T: Reference values, influencing factors and implications. J. Cardiovasc. Magn. Reson. 2013, 15, 53. [Google Scholar] [CrossRef]
- Baeßler, B.; Schaarschmidt, F.; Stehning, C.; Schnackenburg, B.; Maintz, D.; Bunck, A.C. A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3T in healthy volunteers. Eur. J. Radiol. 2015, 84, 2161–2170. [Google Scholar] [CrossRef]
- Östör, A.J.K.; Richards, C.A.; Tytherleigh-Strong, G.; Bearcroft, P.W.; Prevost, A.T.; Speed, C.A.; Hazleman, B.L. Validation of clinical examination versus magnetic resonance imaging and arthroscopy for the detection of rotator cuff lesions. Clin. Rheumatol. 2013, 32, 1283–1291. [Google Scholar] [CrossRef]
- Momenzadeh, O.; Gerami, M.; Sefidbakht, S.; Dehghani, S. Assessment of correlation between MRI and arthroscopic pathologic findings in the shoulder joint. Arch. Bone Jt. Surg. 2015, 3, 286–290. [Google Scholar]
- Hegedus, E.J.; Goode, A.P.; Cook, C.E.; Michener, L.; Myer, C.A.; Myer, D.M.; Wright, A.A. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br. J. Sport. Med. 2012, 46, 964–978. [Google Scholar] [CrossRef]
- Lambert, A.; Loffroy, R.; Guiu, B.; Mejean, N. Rotator cuff tears: Value of 3.0T MRI. J. Radiol. 2018, 5, 583–588. [Google Scholar]
- Magee, T. 3-T MRI of the shoulder: Is MR arthrography necessary? Am. J. Roentgenol. 2009, 192, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Timins, M.E.; Erickson, S.J.; Estkowski, L.D.; Carrera, G.F.; Komorowski, R.A. Increased signal in the normal supraspinatus tendon on MR imaging: Diagnostic pitfall caused by the magic-angle effect. Am. J. Roentgenol. 1995, 165, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mirowitz, S.A. Manifestation of magic angle phenomenon: Comparative study on effects of varying echo time and tendon orientation among various MR sequences. Magn. Reson. Imaging 2003, 21, 741–744. [Google Scholar] [CrossRef] [PubMed]

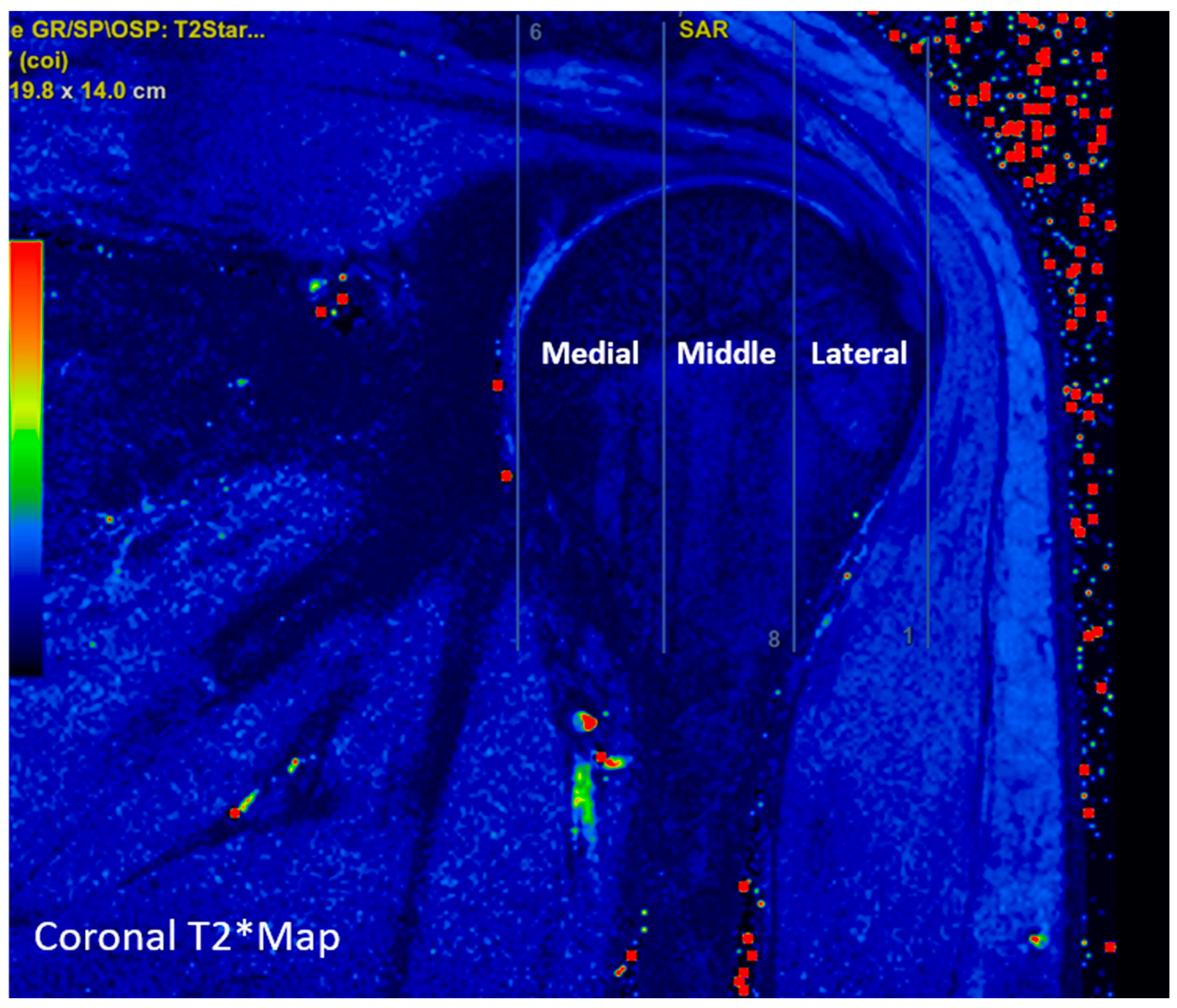
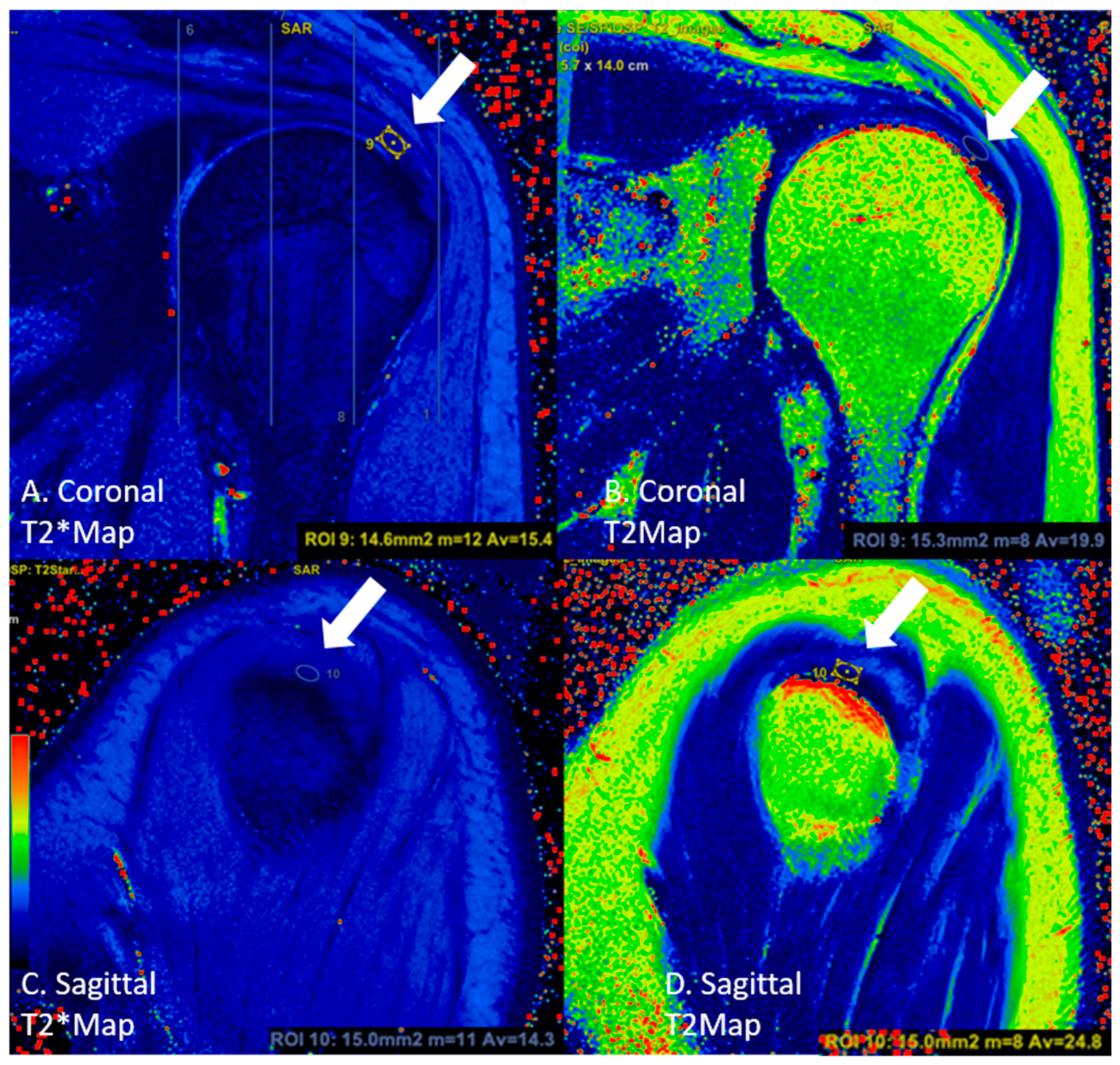
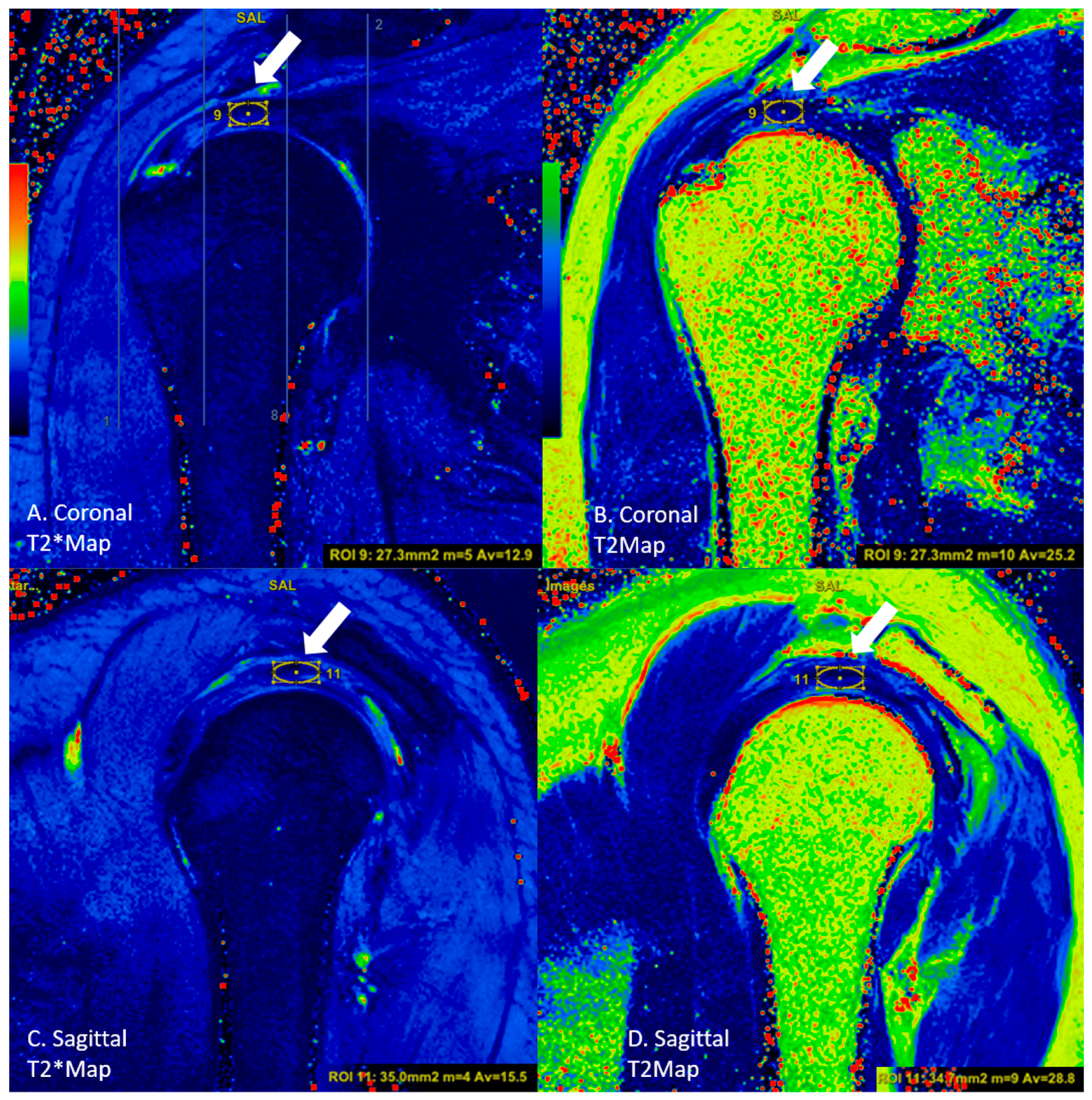
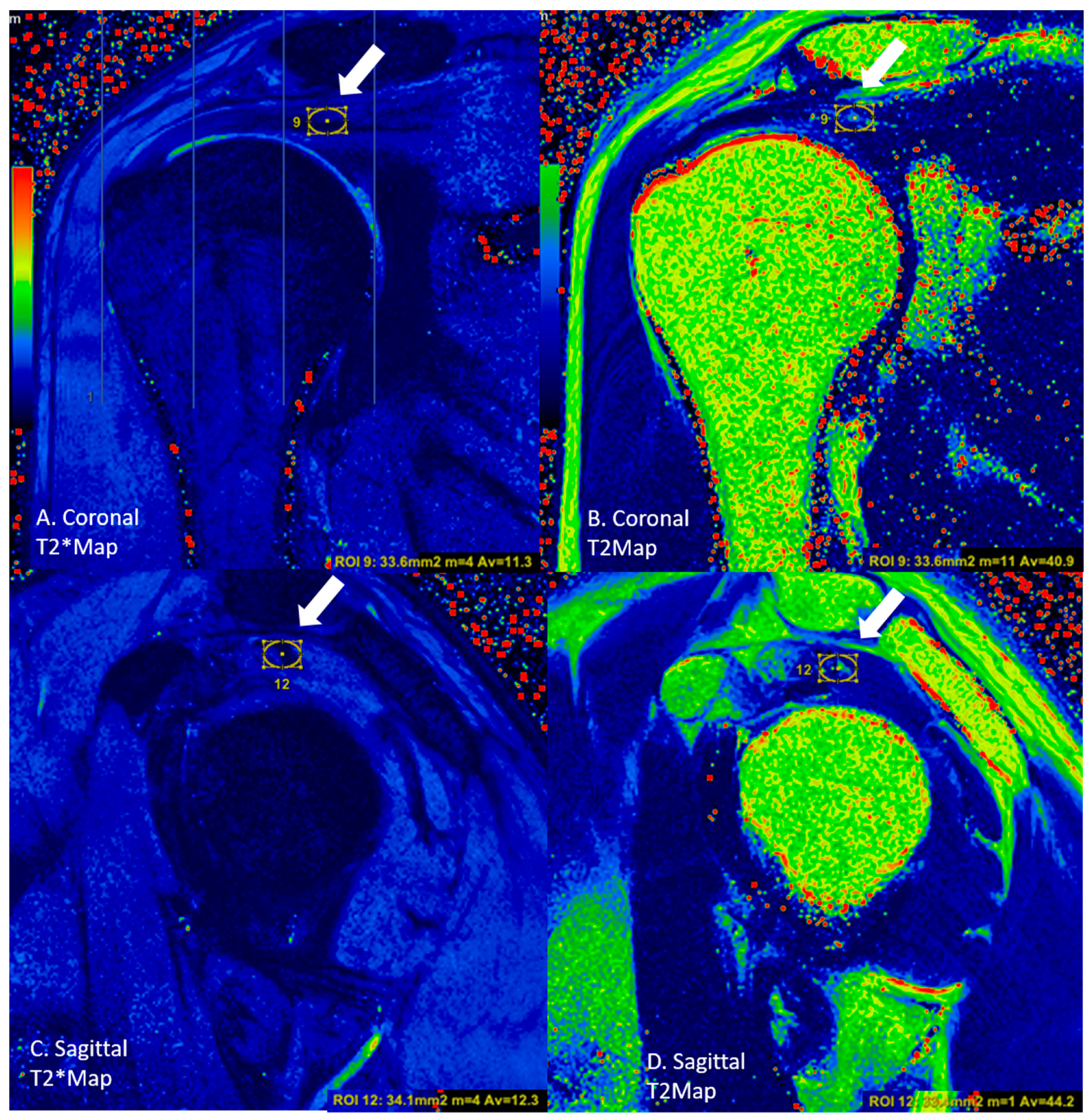
| Sequence | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Coronal T1 | Sagittal T1 | Coronal PD | Sagittal T2 | Axial PD | Coronal T2* Map | Sagittal T2* Map | Coronal T2 Map | Sagittal T2 Map |
| Repetition Time (TR) | 379 | 379 | 2200 | 3100 | 670 | 350 | 480 | 700 | 985 |
| Echo Time (TE) | 9.6 | 9.6 | 26 | 57 | 19 | 4.18 11.32 18.46 25.60 32.74 | 4.18 11.32 18.46 25.60 32.74 | 13.8 27.6 41.4 55.2 69.0 | 13.8 27.6 41.4 55.2 69.0 |
| Flip Angle | 150 | 150 | 180 | 180 | 28 | 60 | 60 | 180 | 180 |
| Field of View (mm2) | 180 mm 100% | 180 mm 100% | 160 mm 100% | 180 mm 100% | 170 mm 100% | 140 mm 100% | 140 mm 100% | 140 mm 100% | 140 mm 100% |
| Slice Thickness (mm) | 3 | 4 | 4 | 4 | 4 | 3.0 | 3.5 | 3.0 | 3.5 |
| Matrix | 384 × 269 | 320 × 240 | 384 × 276 | 256 × 230 | 320 × 224 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| Acquisition Time | 1:38 | 1:38 | 2:40 | 1:25 | 2:02 | 3:14 | 3:31 | 3:26 | 3:53 |
| Asymptomatic Group (n = 20) n (%) | Tendinosis Group (n = 20) n (%) | Tendon Rupture Group (n = 21) n (%) | * p | |
|---|---|---|---|---|
| Male | 11 (55.0) | 6 (30.0) | 10 (47.6) | 0.262 |
| Female | 9 (45.0) | 14 (70.0) | 11 (52.4) |
| Asymptomatic Group (n = 20) | Tendinosis Group (n = 20) | Tendon Rupture Group (n = 21) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age (Years) (Mean ± SD) | 33.2 ± 8.1 | 40.8 ± 10.3 | 52.9 ± 7.4 | <0.001 | |||
| Male | Female | Male | Female | Male | Female | ||
| Age (Years) (Mean ± SD) | 31.1 ± 7.4 | 35.8 ± 8.6 | 45.7 ±8.4 | 38.7 ±10.6 | 51.7 ±7.2 | 53.9 ±7.8 | |
| p Value | 0.112 | 0.179 | 0.349 | ||||
| Section | Region | Inter-Rater Reliability | Intra-Rater Reliability |
|---|---|---|---|
| Coronal | Lateral | ||
| T2* | 0.968 (0.947–0.981) | 0.984 (0.973–0.990) | |
| T2 | 0.965 (0.942–0.979) | 0.986 (0.976–0.991) | |
| Middle | |||
| T2* | 0.906 (0.843–0.944) | 0.979 (0.965–0.987) | |
| T2 | 0.702 (0.503–0.821) | 0.934 (0.891–0.961) | |
| Medial | |||
| T2* | 0.781 (0.635–0.868) | 0.832 (0.720–0.899) | |
| T2 | 0.838 (0.730–0.903) | 0.717 (0.528–0.830) | |
| Sagittal | Lateral | ||
| T2* | 0.962 (0.937–0.977) | 0.981 (0.969–0.989) | |
| T2 | 0.954 (0.924–0.973) | 0.993 (0.988–0.996) | |
| Middle | |||
| T2* | 0.909 (0.849–0.945) | 0.969 (0.948–0.981) | |
| T2 | 0.915 (0.858–0.949) | 0.918 (0.863–0.951) | |
| Medial | |||
| T2* | 0.788 (0.647–0.873) | 0.843 (0.738–0.906) | |
| T2 | 0.922 (0.871–0.954) | 0.888 (0.814–0.933) |
| Asymptomatic Group 1 (n = 20) | Tendinosis Group 2 (n = 20) | Tendon Rupture Group 3 (n = 21) | 1,2 p * | 1,3 p * | 2,3 p * | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Coronal T2* | Lateral | 14.1 ± 3.3 | 18.1 ± 3.6 | 33.4 ± 8.3 | <0.001 | <0.001 | <0.001 |
| Middle | 12.2 ± 2.1 | 13.0 ± 3.0 | 16.4 ± 5.2 | 0.957 | 0.007 | 0.028 | |
| Medial | 13.1 ± 1.9 | 12.3 ± 2.4 | 11.2 ± 1.6 | 0.256 | 0.006 | 0.188 | |
| Sagittal T2* | Lateral | 15.3 ± 2.6 | 19.1 ± 2.8 | 31.4 ± 7.3 | <0.001 | <0.001 | 0.001 |
| Middle | 12.6 ± 2.3 | 13.2 ± 3.2 | 16.2 ± 4.6 | 0.850 | 0.011 | 0.027 | |
| Medial | 13.2 ± 1.4 | 13.2 ± 2.3 | 12.2 ± 1.9 | 0.534 | 0.078 | 0.268 | |
| Coronal T2 | Lateral | 21.7 ± 3.1 | 24.6 ± 3.8 | 45.5 ± 12.3 | 0.021 | <0.001 | <0.001 |
| Middle | 23.1 ± 3.4 | 23.9 ± 4.5 | 26.5 ± 5.0 | 0.957 | 0.014 | 0.038 | |
| Medial | 33.5 ± 7.3 | 39.9 ± 7.6 | 43.4 ± 9.5 | 0.008 | 0.001 | 0.085 | |
| Sagittal T2 | Lateral | 25.2 ± 3.3 | 28.2 ± 5.0 | 46.9 ± 12.8 | 0.064 | <0.001 | <0.001 |
| Middle | 24.0 ± 4.1 | 25.5 ± 4.5 | 27.9 ± 5.4 | 0.137 | 0.012 | 0.215 | |
| Medial | 33.8 ± 5.9 | 38.9 ± 6.7 | 42.6 ± 9.8 | 0.023 | 0.002 | 0.155 | |
| Coronal | Sagittal | Coronal | Sagittal | |
|---|---|---|---|---|
| T2*Lateral | T2*Lateral | T2Lateral | T2Lateral | |
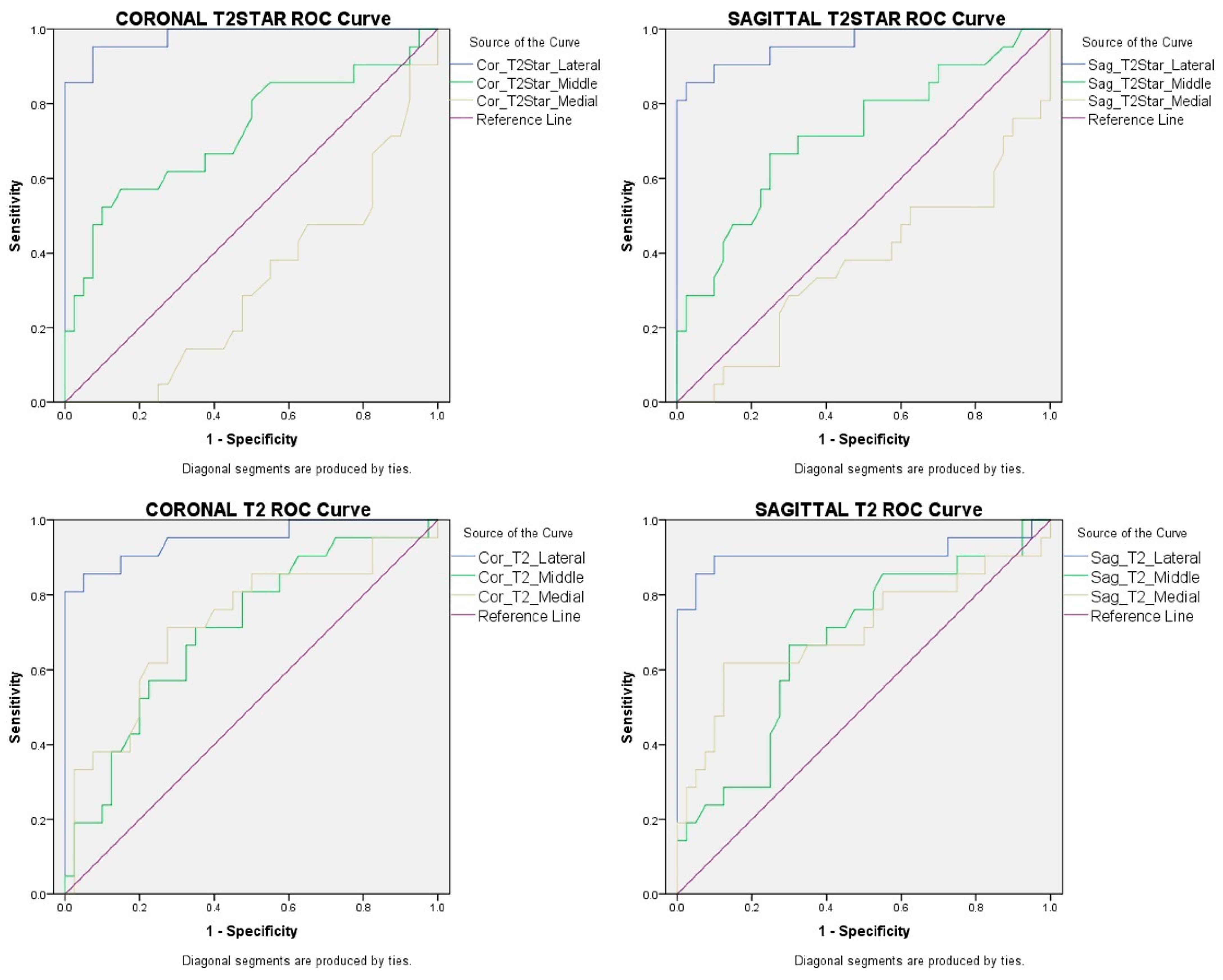
| Area Under the ROC Curve (95% Confidence Interval) | 0.980 (0–1) | 0.960 (0–1) | 0.949 (0–1) | 0.911 (0–1) |
| Standard error | 0.015 | 0.026 | 0.026 | 0.054 |
| p Value | <0.001 | <0.001 | <0.001 | <0.001 |
| Cut-off Value (ms) | 22.17 | 21.07 | 26.97 | 31.8 |
| Sensitivity | %95.2 | %90.5 | %90.5 | %90.5 |
| Specificity | %92.5 | %90 | %85 | %90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ece, B.; Yigit, H.; Ergun, E.; Koseoglu, E.N.; Karavas, E.; Aydin, S.; Kosar, P.N. Quantitative Analysis of Supraspinatus Tendon Pathologies via T2/T2* Mapping Techniques with 1.5 T MRI. Diagnostics 2023, 13, 2534. https://doi.org/10.3390/diagnostics13152534
Ece B, Yigit H, Ergun E, Koseoglu EN, Karavas E, Aydin S, Kosar PN. Quantitative Analysis of Supraspinatus Tendon Pathologies via T2/T2* Mapping Techniques with 1.5 T MRI. Diagnostics. 2023; 13(15):2534. https://doi.org/10.3390/diagnostics13152534
Chicago/Turabian StyleEce, Bunyamin, Hasan Yigit, Elif Ergun, Enver Necip Koseoglu, Erdal Karavas, Sonay Aydin, and Pinar Nercis Kosar. 2023. "Quantitative Analysis of Supraspinatus Tendon Pathologies via T2/T2* Mapping Techniques with 1.5 T MRI" Diagnostics 13, no. 15: 2534. https://doi.org/10.3390/diagnostics13152534
APA StyleEce, B., Yigit, H., Ergun, E., Koseoglu, E. N., Karavas, E., Aydin, S., & Kosar, P. N. (2023). Quantitative Analysis of Supraspinatus Tendon Pathologies via T2/T2* Mapping Techniques with 1.5 T MRI. Diagnostics, 13(15), 2534. https://doi.org/10.3390/diagnostics13152534






