Association of the EPAS1 rs7557402 Polymorphism with Hemodynamically Significant Patent Ductus Arteriosus Closure Failure in Premature Newborns under Pharmacological Treatment with Ibuprofen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Genetic Variant Analysis
2.3. DNA Sequencing
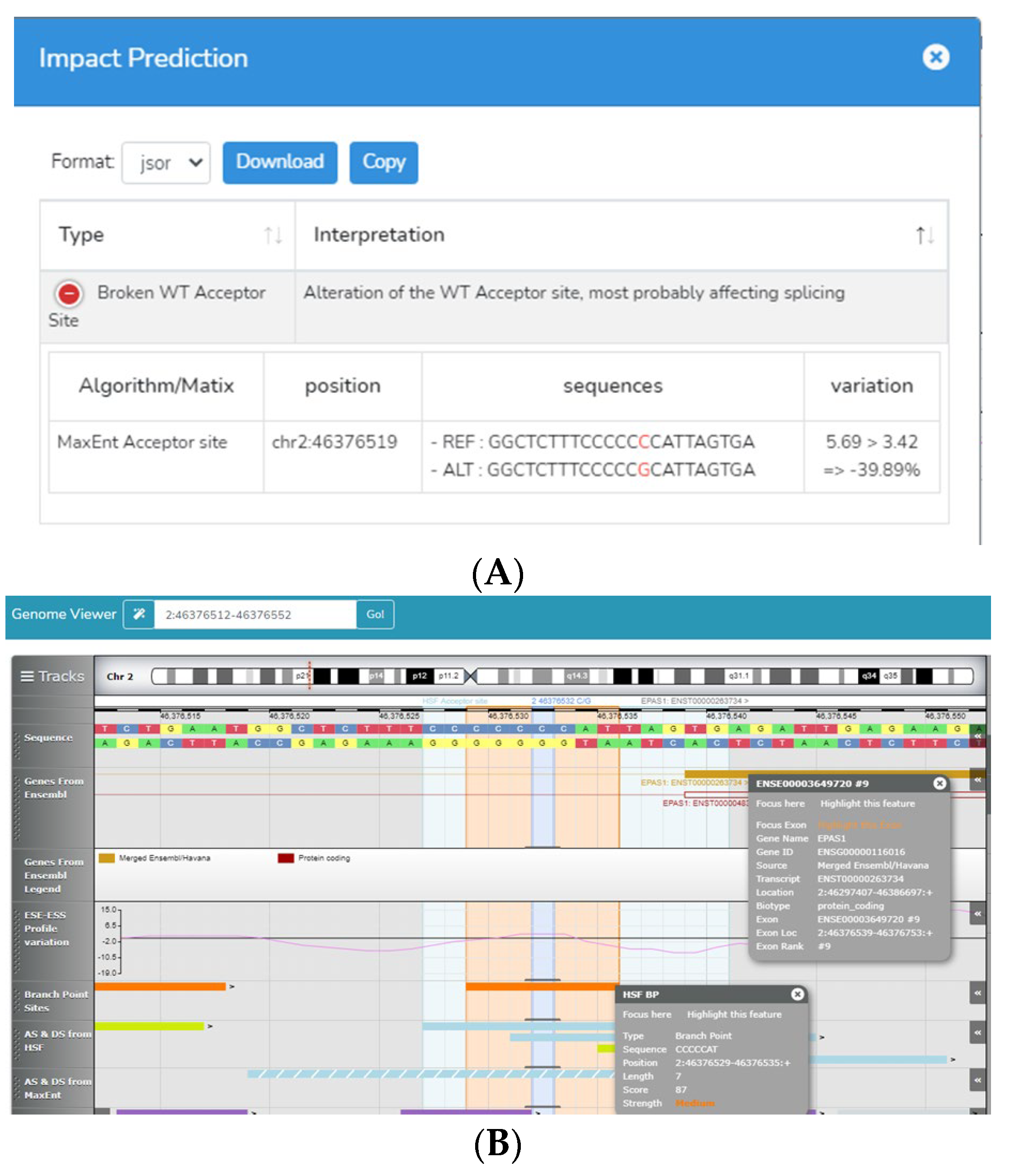
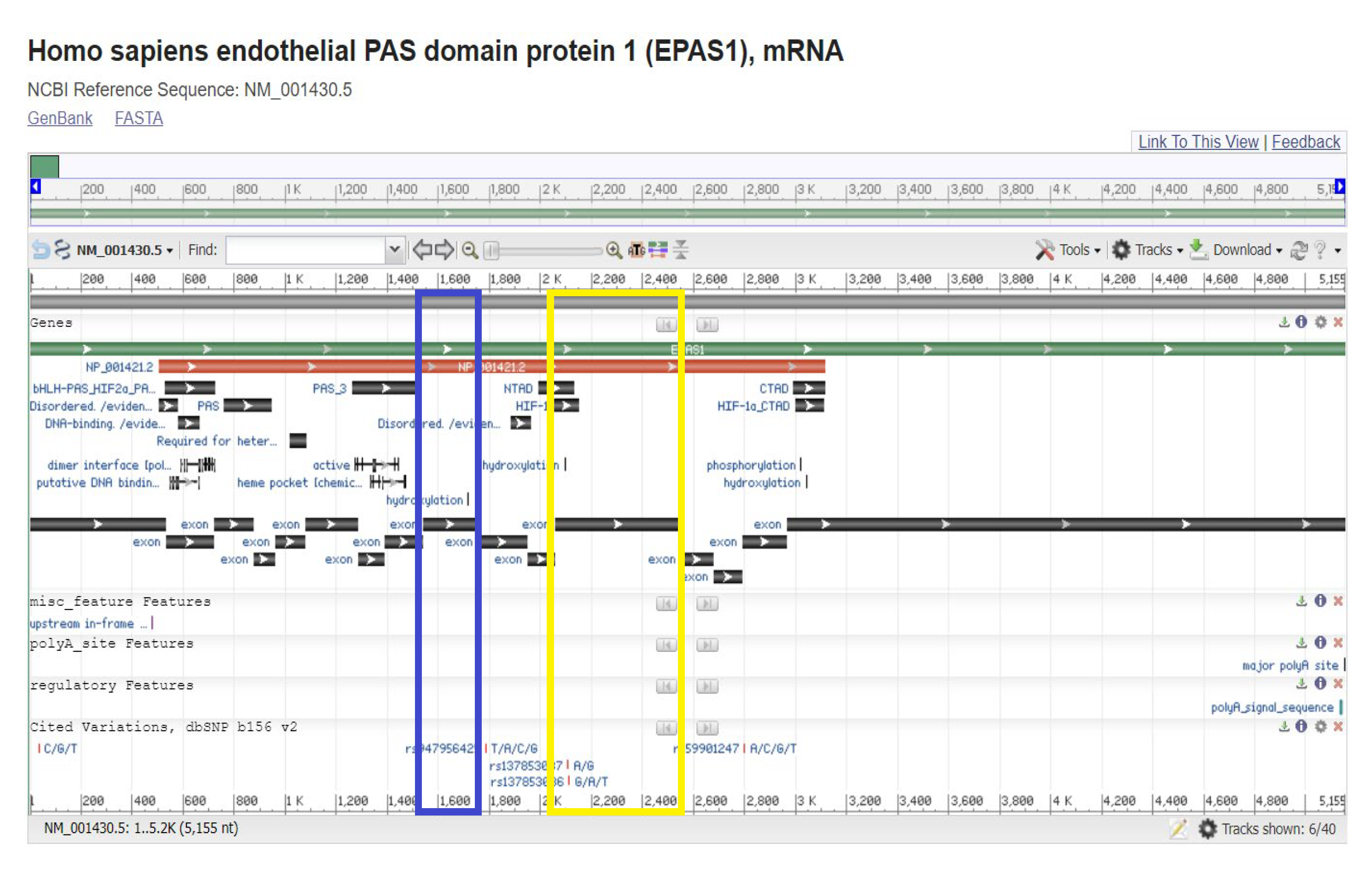
2.4. Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Population Sample Characteristics
3.2. Genetic Variant Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| PDA | Patent ductus arteriosus |
| EPAS1 | Endothelial PAS domain protein 1 |
| SNP | Single-nucleotide polymorphism |
| OR | Odds ratio |
| HRM | High-resolution melting |
| HSF | Human splicing finder |
| ACGM | American College of Medical Genetics and Genomics |
| COX | Cyclooxygenase |
| TFAP2B | Transcription factor AP-2 beta |
| HIF | Hypoxia-inducible factor |
| PHD | Prolyl hydroxylase |
References
- Gillam-Krakauer, M.; Reese, J. Diagnosis and Management of Patent Ductus Arteriosus. Neoreviews 2018, 19, e394–e402. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.G.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146, e20201209. [Google Scholar] [CrossRef] [PubMed]
- Cordero-González, G.; Gómez-Tamayo, T.; Santillán-Briceño, V.; Machuca-Vacac, A.; Fernández-Carrocera, L.A. Experience with ibuprofen in treatment of patent ductus arteriosus in a tertiary hospital in Mexico City. Perinatol. Reprod. Hum. 2016, 30, 115–121. [Google Scholar]
- Parkerson, S.; Philip, R.; Talati, A.; Sathanandam, S. Management of Patent Ductus Arteriosus in Premature Infants in 2020. Front. Pediatr. 2021, 8, 590578. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Luis, G.E.; Borges-Lujan, M.; Villamor, E. Association between endotypes of prematurity and pharmacological closure of patent ductus arteriosus: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1078506. [Google Scholar] [CrossRef] [PubMed]
- Polania-Rodríquez, M.H.; Rodríguez-Terán, G.; Zamorano-Jiménez, C.; ASánchez-Velázquez, L.D. Diagnóstico ecocardiográfico de persistencia del conducto arterioso en recién nacidos hospitalizados en la Unidad de Cuidados Intensivos Neonatales. An. Médicos 2015, 60, 185–190. [Google Scholar]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607. [Google Scholar] [CrossRef]
- Vargas Dornelles, L.; Lúcia-Corso, A.; de-Cássia-Silveira, R.; Soibelmann-Procianoy, R. Comparison of two dose regimens of ibuprofen for the closure of patent ductus arteriosus in preterm newborns. J. Pediatr. 2016, 92, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Backes, C.H.; Hill, K.D.; Shelton, E.L.; Slaughter, J.L.; Lewis, T.R.; Weisz, D.E.; Mah, M.L.; Bhombal, S.; Smith, C.V.; McNamara, P.J.; et al. Patent Ductus Arteriosus: A Contemporary Perspective for the Pediatric and Adult Cardiac Care Provider. J Am Heart Assoc. 2022, 11, e025784. [Google Scholar] [CrossRef]
- Mitra, S.; de Boode, W.P.; Weisz, D.E.; Shah, P.S. Interventions for patent ductus arteriosus (PDA) in preterm infants: An overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2023, 4, CD013588. [Google Scholar] [CrossRef]
- Mitra, S.; Gardner, C.E.; MacLellan, A.; Disher, T.; Styranko, D.M.; Campbell-Yeo, M.; Kuhle, S.; Johnston, B.C.; Dorling, J. Prophylactic cyclo-oxygenase inhibitor drugs for the prevention of morbidity and mortality in preterm infants: A network meta-analysis. Cochrane Database Syst. Rev. 2022, 4, CD013846. [Google Scholar]
- Katsaras, D.N.; Katsaras, G.N.; Chatziravdeli, V.I.; Papavasileiou, G.N.; Touloupaki, M.; Mitsiakos, G.; Doxani, C.; Stefanidis, I.; Dardiotis, E. Comparative safety and efficacy of paracetamol versus non-steroidal anti-inflammatory agents in neonates with patent ductus arteriosus: A systematic review and meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2022, 88, 3078–3100. [Google Scholar] [CrossRef]
- Dagle, J.M.; Lepp, N.T.; Cooper, M.E.; Schaa, L.; Kelsey, K.J.P.; Orr, K.L.; Murray, J.C. Determination of genetic predisposition to patent ductus arteriosus in preterm infants. NIH Public Access 2009, 123, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Weisz, D.; Jain, A.; Jong, G.’t. Management of the patent ductus arteriosus in preterm infants. Paediatr. Child Health 2022, 27, 63–64. [Google Scholar] [CrossRef]
- Engbers, A.G.J.; Völler, S.; Flint, R.B.; Goulooze, S.C.; de Klerk, J.; Krekels, E.H.J.; van Dijk, M.; Willemsen, S.P.; Reiss, I.K.M.; Knibbe, C.A.J.; et al. The Effect of Ibuprofen Exposure and Patient Characteristics on the Closure of the Patent Ductus Arteriosus in Preterm Infants. Clin. Pharmacol. Ther. 2022, 112, 307–315. [Google Scholar] [CrossRef]
- Ivey, N.K.; Sutcliffe, D.; Richardson, J.; Clyman, R.I.; Garcia, J.A.; Srivastava, D. Transcriptional Regulation During Development of the Ductus Arteriosus. Circ. Res. 2008, 103, 388–395. [Google Scholar] [CrossRef]
- Parikh, P.; Bai, H.; Swartz, M.F.; Alfieris, G.M.; Dean, D.A. Identification of differentially regulated genes in human patent ductus arteriosus. Exp. Biol. Med. 2016, 241, 2112–2118. [Google Scholar] [CrossRef]
- Chen, Y.W.; Zhao, W.; Zhang, Z.F.; Fu, Q.; Shen, J.; Zhang, Z.; Ji, W.; Wang, J.; Li, F. Familial nonsyndromic patent ductus arteriosus caused by mutations in TFAP2B. Pediatr. Cardiol. 2011, 32, 958. [Google Scholar] [CrossRef]
- Illumina Clinical Services Laboratory (Illumina), ICSL. 2018. Available online: https://www.ncbi.nlm.nih.gov/clinvar/submitters/504895/ (accessed on 22 July 2022).
- Perrotta SStiehl, P.; Punzo, F.; Scianguetta, S.; Borriello ABencivenga, D.; Casale, M.; Nobili, B.; Fasoli, S.; Balduzzi, A.; Cro, A.; Nytko, K.; et al. Congenital erythrocytosis associated with gain-of-function HIF2A gene mutations and erythropoietin levels in the normal range. Haematologica 2013, 98, 1624. [Google Scholar] [CrossRef]
- Hajj, H.; Dagle, J.M. Genetics of Patent Ductus Arteriosus Susceptibility and Treatment to PDA Gene Discovery. YSPER 2012, 36, 98–104. [Google Scholar]
- Tanaka, T.; Akiyama, H.; Kanai, H.; Sato, M.; Takeda, S.; Sekiguchi, K.; Yokoyama, T.; Kurabayashi, M. Endothelial PAS Domain Protein 1 (EPAS1) Induces Adrenomedullin Gene Expression in Cardiac Myocytes: Role of EPAS1 in an Inflammatory Response in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2002, 34, 748. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Yeh, J.L.; Liou, S.F.; Dai, Z.K.; Wu, B.N.; Hsu, J.H. Gamma-secretase inhibitor prevents proliferation and migration of ductus arteriosus smooth muscle cells through the Notch3-HES1/2/5 pathway. Int. J. Biol. Sci. 2016, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Primer Blast Platform. 2012. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 22 July 2022).
- NCBI Reference Sequence Database. 2000. Available online: https://www.ncbi.nlm.nih.gov/refseq/ (accessed on 22 July 2022).
- NCBI. Nucleotide Blast platform. 2022. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastx&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome (accessed on 22 July 2022).
- Translate Tool. ExPASy Bioinformatics Resource Portal. 1993. Available online: https://web.expasy.org/translate/ (accessed on 22 July 2022).
- NCBI. Single-Nucleotide Polymorphism Database (dbSNP). 1998. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 22 July 2022).
- NCBI. ClinVar Database. 2012. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 22 July 2022).
- Mutalyzer Website. 2021. Available online: https://mutalyzer.nl/webservices (accessed on 22 July 2022).
- PolyPhen-2 (Polymorphism Phenotyping v2). 2010. Available online: http://genetics.bwh.harvard.edu/pph2/ (accessed on 22 July 2022).
- The Uniprot Consortium. 2002. Available online: https://www.uniprot.org/ (accessed on 22 July 2022).
- RCSB PDB. 2000. Available online: https://www.rcsb.org (accessed on 22 July 2022).
- Human Splicing Finder. 2009. Available online: http://www.umd.be/HSF3/ (accessed on 22 July 2022).
- Quanto Software. 2007. Available online: http://hydra.usc.edu/GxE/ (accessed on 22 July 2022).
- Haploview Software. 2005. Available online: https://www.broadinstitute.org/haploview/haploview (accessed on 22 July 2022).
- Practice Guidelines of the American College of Medical Genetics and Genomics. 2007. Available online: https://www.acmg.net/ACMG/Medical-Genetics-Practice-Resources/Practice-Guidelines.aspx (accessed on 22 July 2022).
- Martínez-Roque, A.M. Repercusión hemodinámica en pacientes neonatos con conducto arterioso persistente: Factores asociados. Arch Cardiol Mex. 2017, 87, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Benson, M.A.; Bhattacharya, S.; Chen, Y.; Hu, J.; Li, F. Characterization of transcription factor AP-2 beta mutations involved in familial isolated patent ductus arteriosus suggests haploinsufficiency. J. Surg. Res. 2014, 188, 466–472. [Google Scholar]
- Hamrick, S.E.G.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2010, 125, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 407, cm8. [Google Scholar] [CrossRef]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous? Cells 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Ensembl Genome Browser. 1999. Available online: https://www.ensembl.org/Homo_sapiens/Variation/Explore?db=core;r=2:46376032-;v=rs7557402;vdb=variation;vf=18484625 (accessed on 13 July 2023).
- Regulome DB v.2. 2012. Available online: https://regulomedb.org/regulome-search?regions=chr2%3A46376531-46376532&genome=GRCh38 (accessed on 13 July 2023).
| Control | Case | p | ||
|---|---|---|---|---|
| Mother | ||||
| Hypertension n (%) | 9 (32.1) | 7 (36.8) | 0.763 | |
| Preeclampsia n (%) | 9 (32.1) | 5 (26.3) | 0.753 | |
| T2D n (%) | 4 (14.3) | 0 | 0.137 | |
| Prenatal infections n (%) | 12(42.9) | 9 (47.4) | 0.775 | |
| Prenatal treatment | Indomethacin n (%) | 0 | 1 (5.3) | 0.404 |
| Steroid n (%) | 20 (76.9) | 13 (68.4) | 0.734 | |
| Newborn | ||||
| Sex F/M n | 9/19 | 8/11 | 0.546 | |
| Gestational age | 30 ± 2.3 | 28.8 ± 2.8 | 0.112 | |
| Birth weight (g) | 1229 ± 349 | 928 ± 154 | <0.0001 | |
| Ductal diameter (mm) | 2.9 ± 0.9 | 2.9 ± 0.8 | 0.765 | |
| Sepsis n (%) | 16 (57.1) | 1 (5.3) | <0.0001 | |
| Early n (%) | 7 (25) | 0 | 0.032 | |
| Late n (%) | 10 (35.7) | 0 | 0.003 |
| Genotype Frequency (%) | TFAP2B | Allelic Frequency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9757AT | A/A | A/T | T/T | p * | A | T | p ** | ORc | (IC 95%) | p HW |
| Control (n = 28) | 23 (82) | 5 (18) | 0 (0) | 51 (91) | 5 (9) | 1 | ||||
| Case (n = 19) | 19 (100) | 0 (0) | 0 (0) | 0.018 | 38 (100) | 0 (0) | 0.0032 | - | - | 1 |
| 29461AT | A/A | A/T | T/T | A | T | |||||
| Control (n = 28) | 24 (85.7) | 4 (14.3) | 0 (0) | 52 (93) | 4 (7) | 1 | ||||
| Case (n = 19) | 17 (89.5) | 2 (10.5) | 0 (0) | 0.70 | 36 (95) | 2 (5) | 0.767 | 0.699 | 0.21–2.28 | 1 |
| EPAS1 | ||||||||||
| rs7557402 | C/C | C/G | G/G | C | G | |||||
| Control (n = 28) | 24 (85.7) | 4 (14.3) | 0 (0) | 52 (93) | 4 (7) | 1 | ||||
| Case (n = 19) | 14 (73.7) | 2 (10.5) | 3 (15.8) | 0.05 | 30 (79) | 8 (21) | 0.007 | 3.53 | 1.42–8.74 | 0.0096 |
| 54455C>T | C/C | C/T | T/T | C | T | |||||
| Control (n = 28) | 24 (85.7) | 4 (14.3) | 0 (0) | 52 (93) | 4 (7) | 1 | ||||
| Case (n = 19) | 17 (89.5) | 1 (5.3) | 1 (5.3) | 0.25 | 35 (92) | 3 (8) | 1 | 1.15 | 0.402–3.31 | 0.08 |
| 54522G>A | G/G | G/A | A/A | G | A | |||||
| Control (n = 28) | 24 (85.7) | 1 (3.6) | 3 (10.7) | 49 (88) | 7 (12) | 0.0007 | ||||
| Case (n = 19) | 16 (84.2) | 0 (0) | 3 (15.8) | 0.53 | 32 (84) | 6 (16) | 0.541 | 1.39 | 0.62–3.12 | 0.0003 |
| rs769385664 | G/G | G/A | A/A | G | A | |||||
| Control (n = 28) | 26 (92.6) | 1 (3.6) | 1 (3.6) | 53 (95) | 3 (5) | 0.055 | ||||
| Case (n = 19) | 16 (84.2) | 3 (15.8) | 0 (0) | 0.21 | 35 (92) | 3 (8) | 0.567 | 1.65 | 0.521–5.23 | 1 |
| 54699C>A | C/C | C/A | A/A | C | A | |||||
| Control (n = 28) | 21 (75) | 5 (17.9) | 2 (7.1) | 47 (84) | 9 (16) | 0.11 | ||||
| Case (n = 19) | 13 (68.4) | 4 (21.1) | 2 (10.5) | 0.87 | 30 (79) | 8 (21) | 0.466 | 1.39 | 0.679–2.86 | 0.14 |
| 54700A>C | A/A | A/C | C/C | A | C | |||||
| Control (n = 28) | 22 (78.6) | 5 (17.9) | 1 (3.6) | 49 (88) | 7 (12) | 0.35 | ||||
| Case (n = 19) | 14 (73.7) | 4 (21.1) | 1 (5.3) | 0.92 | 32 (84) | 6 (16) | 0.541 | 1.39 | 0.623–3.127 | 0.37 |
| 54701G>C | G/G | G/C | C/C | G | C | |||||
| Control (n = 28) | 19 (67.9) | 8 (28.6) | 1 (3.6) | 46 (82) | 10 (18) | 1 | ||||
| Case (n = 19) | 14 (73.7) | 5 (26.3) | 0 (0) | 0.57 | 33 (87) | 5 (13) | 0.434 | 0.68 | 0.313–1.476 | 1 |
| rs1286681984 | C/C | C/A | A/A | C | A | |||||
| Control (n = 28) | 24 (85.7) | 4 (14.3) | 0 (0) | 52 (93) | 4 (7) | 1 | ||||
| Case (n = 19) | 16 (84.2) | 2 (10.5) | 1 (5.3) | 0.38 | 34 (89) | 4 (11) | 0.459 | 1.64 | 0.61–4.42 | 0.16 |
| 54703C>G | C/C | C/G | G/G | C | G | |||||
| Control (n = 28) | 24 (85.7) | 4 (14.3) | 0 (0) | 52 (93) | 4 (7) | 1 | ||||
| Case (n = 19) | 17 (89.5) | 2 (10.5) | 0 (0) | 0.7 | 36 (95) | 2 (5) | 0.767 | 0.699 | 0.21–2.28 | 1 |
| 54707C>G | C/C | C/G | G/G | C | G | |||||
| Control (n = 28) | 25 (89.3) | 3 (10.7) | 0 (0) | 53 (95) | 3 (5) | 1 | ||||
| Case (n = 19) | 17 (89.5) | 2 (10.5) | 0 (0) | 0.98 | 36 (95) | 2 (5) | 1 | 1 | 0.28–3.56 | 1 |
| rs762559920 | G/G | G/C | C/C | G | C | |||||
| Control (n = 28) | 27 (96.4) | 1 (3.6) | 0 (0) | 55 (98) | 1 (2) | 1 | ||||
| Case (n = 19) | 16 (84.2) | 3 (15.8) | 0 (0) | 0.14 | 35 (92) | 3 (8) | 0.1 | 4.26 | 0.88–20.59 | 1 |
| Model | Genotype | Case | Control | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| Codominant | C/C | 14 (73.7%) | 24 (85.7%) | 0.056 | |
| C/G | 2 (10.5%) | 4 (14.3%) | 1.17 (0.19–7.21) | ||
| G/G | 3 (15.8%) | 0 (0%) | 0.00 (0.00-NA) | ||
| Dominant | C/C | 14 (73.7%) | 24 (85.7%) | 0.31 | |
| C/G-G/G | 5 (26.3%) | 4 (14.3%) | 0.47 (0.11–2.03) | ||
| Recessive | C/C-C/G | 16 (84.2%) | 28 (100%) | 0.017 | |
| G/G | 3 (15.8%) | 0 (0%) | 0.00 (0.00-NA) | ||
| Overdominant | C/C-G/G | 17 (89.5%) | 24 (85.7%) | 1 | 0.7 |
| C/G | 2 (10.5%) | 4 (14.3%) | 1.42 (0.23–8.64) | ||
| Additive | - | - | - | 0.41 (0.13–1.26) | 0.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogel-Ayala, D.G.; Muñoz-Medina, J.E.; Vicente-Juárez, V.D.; Grether-González, P.; Morales-Barquet, D.A.; Martínez-García, A.d.J.; Echaniz-Aviles, M.O.L.; Sevilla-Montoya, R.; Martínez-Juárez, A.; Artega-Vázquez, J.; et al. Association of the EPAS1 rs7557402 Polymorphism with Hemodynamically Significant Patent Ductus Arteriosus Closure Failure in Premature Newborns under Pharmacological Treatment with Ibuprofen. Diagnostics 2023, 13, 2558. https://doi.org/10.3390/diagnostics13152558
Rogel-Ayala DG, Muñoz-Medina JE, Vicente-Juárez VD, Grether-González P, Morales-Barquet DA, Martínez-García AdJ, Echaniz-Aviles MOL, Sevilla-Montoya R, Martínez-Juárez A, Artega-Vázquez J, et al. Association of the EPAS1 rs7557402 Polymorphism with Hemodynamically Significant Patent Ductus Arteriosus Closure Failure in Premature Newborns under Pharmacological Treatment with Ibuprofen. Diagnostics. 2023; 13(15):2558. https://doi.org/10.3390/diagnostics13152558
Chicago/Turabian StyleRogel-Ayala, Diana G., José Esteban Muñoz-Medina, Valeria Dejanira Vicente-Juárez, Patricia Grether-González, Deneb Algedi Morales-Barquet, Alfonso de Jesús Martínez-García, María Olga Leticia Echaniz-Aviles, Rosalba Sevilla-Montoya, Alejandro Martínez-Juárez, Jazmin Artega-Vázquez, and et al. 2023. "Association of the EPAS1 rs7557402 Polymorphism with Hemodynamically Significant Patent Ductus Arteriosus Closure Failure in Premature Newborns under Pharmacological Treatment with Ibuprofen" Diagnostics 13, no. 15: 2558. https://doi.org/10.3390/diagnostics13152558






